Abstract
Background/Objectives: Mutations in the BRCA1 and BRCA2 genes are well-known risk factors for ovarian cancer. They are also associated with response to platinum-based chemotherapy; however, their definitive impact on patient prognosis remains not fully understood. This study aimed to investigate the influence of BRCA mutation status on the age of ovarian cancer onset and on treatment outcomes in patients with high-grade serous ovarian cancer. Methods: This single-center retrospective analysis included newly diagnosed FIGO stage III and IV HGSOC patients treated between June 2018 and April 2023. Patients’ age, tumor histology, CA125 levels, BRCA mutation status, type of treatment (neoadjuvant or adjuvant chemotherapy), and surgical outcomes were collected and analyzed. Survival analyses were performed using the Kaplan–Meier method and log-rank test. Results: Pathogenic mutations were identified in 25 patients (15 in BRCA1, 10 in BRCA2). Patients with a BRCA mutation were diagnosed at a significantly younger age (median 58.78 years) compared to non-carriers (66.81 years; p < 0.001), with BRCA1 carriers being diagnosed the youngest (median 46.52 years). The study found no statistically significant difference in progression-free survival (PFS) between BRCA carriers and non-carriers. However, a significant improvement in overall survival (OS) was observed for patients with a BRCA1 mutation (p = 0.036). No significant OS difference was found for BRCA2 carriers. Conclusions: BRCA mutations, particularly in the BRCA1 gene, are associated with an earlier onset ovarian cancer. BRCA1 mutation appears to be a favorable prognostic factor for overall survival in patients with HGSOC. Our findings demonstrate the clinical implications of different BRCA mutations and support the need for further research in larger cohorts to confirm their influence on prognostic effects.
1. Introduction
Despite modern treatment methods, ovarian cancer remains the leading cause of death in women with gynecological cancer. The majority of patients are diagnosed at advanced stages when the cancer has already metastasized, which contributes to its high mortality rate [1,2]. BRCA1 and BRCA2 mutations were identified as ovarian cancer risk factors. Patients diagnosed with a BRCA1 mutation are estimated to have a lifetime risk of ovarian cancer equal to 40–60% by the age of 80. In comparison, in the BRCA2 population, the lifetime risk is estimated to be between 11 and 27% [3,4,5]. Multiple prognostic factors were determined that impact patients’ prognosis during ovarian cancer treatment, including patient age, FIGO staging [6], the extent of residual disease after cytoreductive surgical treatment [7,8], tumor histology and grading [6], ECOG status, and response to platinum-based chemotherapy.
Several predictive factors were also selected to determine the groups of patients likely to benefit from particular therapies. Homologous recombination deficiency is the leading predictive factor associated with response to platinum-based chemotherapy and maintenance treatment with PARP inhibitors. BRCA1 and BRCA2 mutations were also found to be associated with an improved response to platinum-based chemotherapy [6,9,10]. However, there is still conflicting evidence regarding the association between BRCA mutation and a patient’s prognosis. While studies demonstrate the association between BRCA mutation, response to platinum-based chemotherapy, and progression-free survival, the specific impact of a BRCA mutation may vary depending on additional risk factors such as tumor histology, staging, and the treatment regimens used as a part of the therapy [11,12,13,14,15,16]
In this study, we aim to investigate the differences in patient prognosis with regard to BRCA mutation status and its influence on the age of disease onset.
2. Materials and Methods
This retrospective analysis involved 123 patients newly diagnosed with high-grade serous carcinoma of the ovary (HGSOC), fallopian tube, or primary peritoneum, classified as FIGO stage III or IV. For all patients, first-line surgical and chemotherapy treatment was performed in the Department of Oncological Gynecology between June 2018 and April 2023. The final follow-up was completed at the end of February 2024. Individuals with missing or incomplete clinicopathological information were excluded from the study. Collected data included patient age, tumor histology, CA125 levels at diagnosis, KELIM score (CA-125 Elimination Rate Constant K), BRCA mutation status (assessed using Next Generation Sequencing via Illumina Miseq), type of chemotherapy (neoadjuvant or adjuvant), type of surgery (primary cytoreductive surgery or interval cytoreductive surgery), and surgical outcomes (optimal, suboptimal cytoreduction, or no surgery). The alternations in the BRCA1 or BRCA2 genes were classified as pathogenic according to the ClinVar report.
3. Results
3.1. Population Characteristics
In the study group, 51 patients (41.5%) received neoadjuvant chemotherapy, while the remaining 72 patients (58.5%) were treated with adjuvant therapy. Most of the patients (101 individuals) were diagnosed with stage III disease according to the FIGO classification, and 22 were identified as having stage IV disease. Next-generation sequencing (NGS) did not detect pathogenic BRCA mutations in most of the participants. However, 15 patients carried a pathogenic BRCA1 mutation, and 10 had a pathogenic BRCA2 mutation. One patient had a previous history of breast cancer. The average age at the time of diagnosis was around 63 years, although there was a broad age range from 25 to nearly 84 years. In the entire cohort, the mean progression-free survival was 1.77 years, and the mean overall survival was 2.33 years (Table 1).

Table 1.
Descriptive statistics of quantitative research variables (N = 123).
In the group of patients diagnosed with a BRCA1 mutation, the most common mutation variant was nonsense (seven patients). Four patients were found to have a frameshift mutation, three had missense mutations, and one had a splice acceptor mutation. Among BRCA2 mutations, the most common was a frameshift mutation (five patients), while four patients had a missense mutation and one had a nonsense mutation.
The distribution of qualitative demographic variables was compared between the patients identified as having pathogenic BRCA1 and/or BRCA2 mutations vs. those in which the mutation was not found. There was a significant difference in the age of the patients at the time of ovarian cancer diagnosis. Patients with a BRCA mutation were diagnosed at a significantly younger age than the non-BRCA-mutated population. The study did not show significant differences between the treatment groups for CA125 at diagnosis and the KELIM constant (Table 2).

Table 2.
Comparison of the assessed variables between patients with BRCA1 and/or BRCA2 mutation vs. no BRCA mutation.
When calculated separately for BRCA1 and BRCA2 mutations, there were also significant differences in patients’ age at diagnosis onset, with a median age of 46.52 for the BRCA1 population and 63.83 for BRCA2 patients (p < 0.001). Using a multiple comparisons test, the differences were statistically significant between the BRCA1 and non-BRCA-mutated populations (mean rank difference [D] = −38.30; p = 0.016), as well as for the BRCA1 and BRCA2 populations (D = −39.84; p < 0.001). There were no significant differences between the BRCA1 and BRCA2 patient groups (D = −1.54, p = 0.456).
3.2. Comparison of BRCA Mutation vs. Non-BRCA Population
The distribution of qualitative demographic variables was compared between patients with BRCA1 or BRCA2 mutations and non-BRCA carriers. Variables such as type of surgery (ICS/PCS), FIGO stage, presence of tumor residues after cytoreductive surgery (R0 or <1 cm vs. >1 cm), ascites, and hydrothorax were evaluated. There were no significant differences in the assessed variables between the different groups of patients (Table 3).

Table 3.
Comparison of qualitative demographic variables between patients with BRCA mutation and the non-BRCA population.
3.3. Survival Analysis
Survival analyses were performed using Kaplan–Meier analysis and the log-rank test. Two main time variables, progression-free and overall survival, were analyzed and expressed in years. The log-rank test was used to compare the curves between the assessed groups and, where appropriate, point survival estimates (e.g., 4- and 5-year) were created.
Figure 1 demonstrates the relationship between progression-free survival and BRCA mutation status (regardless of mutation type). The analysis did not reveal any statistically significant differences between patients with and without the mutation (χ2 (1) = 2.65; p = 0.103), although there was a trend towards a more favorable prognosis in the group with the mutation. The 3-year probability of maintaining a progression-free status was 22% (95% CI [7.3–65.1%]) in the group with the mutation and 14% (95% CI [6.8–27.4%]) in the group without the mutation; however, the differences did not reach statistical significance.
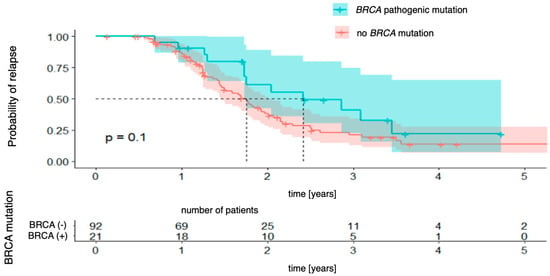
Figure 1.
Progression-free survival stratified by BRCA mutation status.
Next, the Kaplan–Meier curves were created for progression-free survival concerning the presence of BRCA1 and BRCA2 mutations, which were analyzed separately. For BRCA1 mutations (Figure 2), the analysis did not reveal any statistically significant differences between the groups (χ2 (1) = 2.30; p = 0.130). The 3-year probability of maintaining a progression-free state was almost identical in both groups, being 15% (95% CI [8.2–28.6%]) in the group without the mutation and 15% (95% CI [3.0–80.6%]) in the group with the mutation, with the vast confidence interval in the latter indicating a considerable uncertainty in the estimate. For BRCA2 mutations (Figure 3), there was also no significant difference (χ2 (1) = 0.01; p = 0.990). The 3-year progression-free survival was 25% (95% CI [16.7–37.2%]) in the wild-type group and 31% (95% CI [10.1–96.2%]) in the mutation-positive group.
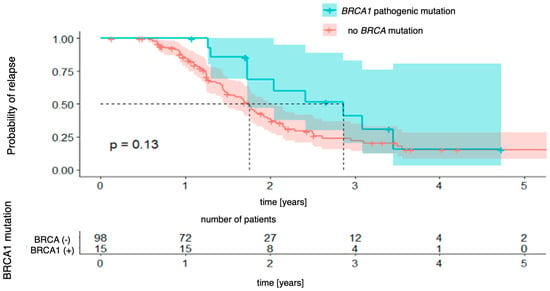
Figure 2.
Progression-free survival for BRCA1-mutation patients vs. patients with no pathogenic BRCA mutation.
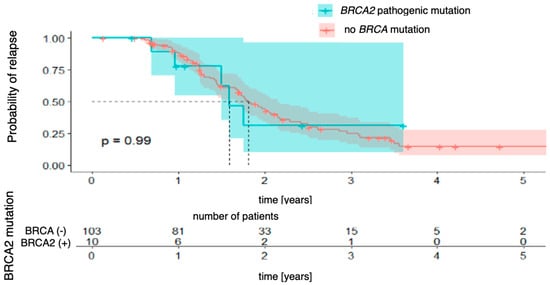
Figure 3.
Progression-free survival for BRCA2-mutation patients vs. patients with no pathogenic BRCA mutation.
Subsequent Kaplan–Meier survival curves were generated for BRCA mutation status (BRCA pathogenic mutation vs. no BRCA pathogenic mutation). The analysis did not reveal a statistically significant difference in overall survival between patients with and without the mutation [χ2 (1) = 1.92; p = 0.088]. However, there was a clear trend toward a better prognosis in the mutation-positive group. The 4-year survival probability in this group was 68% (95% CI [48.0–96.8%]), whereas in patients without the mutation, it was estimated at 31% (95% CI [19.7–48.0%]). This difference did not reach statistical significance, but the divergence of the curves suggests that BRCA mutation status may be associated with better long-term survival (Figure 4).
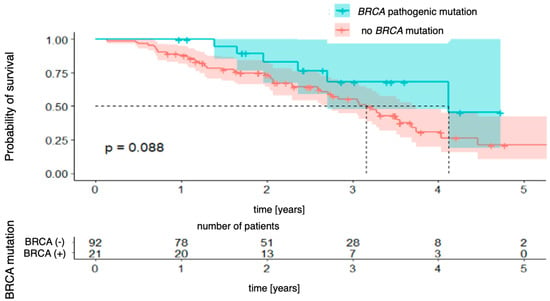
Figure 4.
Overall survival stratified by BRCA mutation status.
The relationships with the occurrence of BRCA1 and BRCA2 mutations were analyzed successively. In the case of the BRCA1 mutation (Figure 5), a significant difference in overall survival was observed between patients with and without the mutation [χ2 (1) = 4.41; p = 0.036]. The four-year probability of survival in patients with a BRCA1 mutation was as high as 84% (95% CI [64.9–100.0%]), while in the group without a mutation, it was estimated at 30% (95% CI [19.6–47.2%]). For the BRCA2 mutation (Figure 6), the differences did not reach the level of statistical significance [χ2(1) = 2.73; p = 0.099], but the direction of the effect indicated a worse prognosis in patients with this mutation.
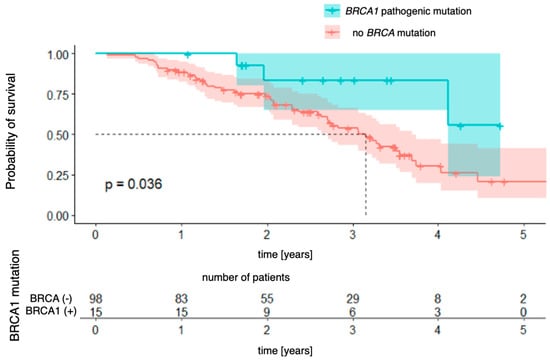
Figure 5.
Overall survival for BRCA1-mutation patients vs. patients with no pathogenic BRCA mutation.
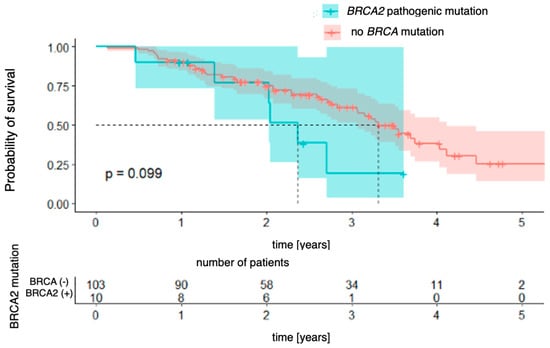
Figure 6.
Overall survival for BRCA2-mutation patients vs. patients with no pathogenic BRCA mutation.
4. Discussion
BRCA mutations significantly increase the risk of ovarian cancer, and the cumulative risk increases with a patient’s age. Even though the age of cancer diagnosis varies, patients with BRCA mutations seem to have a lower age of cancer diagnosis when compared to the general population of patients, with the mean age of cancer diagnosis being around 51 years for BRCA1 patients and 61 years for BRCA2 patients [17]. In our study, patients with a BRCA mutation were diagnosed at a significantly younger age when compared to the non-BRCA-mutated population (with a median of 58.78 years for BRCA1/2 and 66.81 for non-BRCA patients). When calculated separately for BRCA1 and BRCA2 mutations, there were also significant differences in patient age at diagnosis, with a median age of 46.52 for the BRCA1 population and 63.83 for BRCA2 patients. The results are similar to those in the literature; however, several factors may influence a patient’s age at diagnosis, including BRCA mutation type (e.g., nonsense, frameshift) [17], family history of breast or ovarian cancer [18,19], or exposure to certain environmental factors [20]. Although the BRCA1 and BRCA2 genes have been sequenced in millions of women, some of the mutation variants still cause difficulties in their clinical interpretation. The problem is driven by the large number of variants of uncertain significance (VUS), as there are hundreds of possible single-nucleotide variants (SNVs) that have received conflicting interpretations [21]. A study on saturation genome editing was performed to accurately classify BRCA1 variants in 13 exons encoding functionally critical domains of BRCA1. The study results showed an almost perfectly concordant distribution of functional effects for almost 4000 SNVs with the previously established assessments of pathogenicity [22]. A similar large-scale saturation mutagenesis evaluation was performed for the BRCA2 gene, generating functional scores for 6551 SNVs [23]. Sequencing maps serve as valuable resources for interpreting previously unidentified variants of BRCA mutations and provide additional knowledge to the ClinVar data, allowing easier interpretation regarding the pathogenic/non-pathogenic variants of BRCA1/2 mutations and allowing us to better predict the overall survival of individual patients.
The literature shows conflicting data regarding the influence of BRCA mutation on patient prognosis; moreover, the relative prognoses of BRCA1/2 carriers remains unclear. Sims et al. showed patients with BRCA/HRD− tumors to have worse PFS and OS when compared to germline BRCA+ or somatic BRCA/HRD+ status [24]. A favorable prognosis among BRCA carriers was also demonstrated by Chetrit et al. [25], Cass et al. [9], and Boyd et al. [26]. On the other hand, Yang et al. demonstrated a more favorable treatment outcome only for BRCA2 mutation carriers and no significant difference for BRCA1 mutation carriers when compared to non-carriers [27].
Several studies have shown no influence of BRCA mutation on treatment outcomes. A study by Liontos et al. showed no influence of BRCA1/2 mutation (even considering the different locations of the genes) on survival outcomes in HGSOC patients regarding PFS and OS. The authors suggested the potential effect of co-contributing risk factors and other genetic abnormalities to further influence patient prognosis [28]. There are higher rates of concurrent TP53 mutations among patients with BRCA germline or somatic mutations. TP53 mutations were associated with primary platinum sensitivity in high-grade serous cancer patients, even when adjusted for covariates such as BRCA mutation status [29]. In our study, we only stratified the patients for BRCA mutation status and did not consider the presence of any other concurrent mutations. The lack of survival differences was also demonstrated in the studies conducted by Lee et al. [30], Buller et al. [31], and the United Kingdom Coordinating Committee for Cancer Research (UKCCCR) Familial Ovarian Cancer Study Group [32].
A ten-year survival analysis showed that despite the initial survival advantage of patients with a BRCA mutation, there is no difference in long-term survival. The authors suggested the possible correlation of BRCA mutation with a higher initial sensitivity of BRCA carriers to chemotherapy. However, they found that the surgical status of no residual disease was the strongest predictor of long-term survival [33]. The same group of researchers demonstrated a short-term survival advantage to be associated with carrying inherited BRCA1 or BRCA2 mutations and that there was a lower annual mortality rate during the first two years post-diagnosis among mutation carriers compared to non-carriers, with the trend reversed from year three onwards [34]. Also, a retrospective analysis of the multicenter MITO trial showed R = 0 status as the only predictor of longer overall survival [35].
Our study did not reveal any statistically significant differences in PFS between patients with and without the BRCA mutation. There were also no differences in separate analyses differentiating for BRCA1 and BRCA2 mutations compared to the non-BRCA population. As for overall survival, the study did not reveal a statistically significant difference in overall survival between patients with and without the mutation. Upon separate analysis, in the case of the BRCA1 mutation, a significant difference in overall survival was observed between patients with and without the mutation (p = 0.036). The four-year probability of survival in patients with a BRCA1 mutation was as high as 84% (95% CI [64.9–100.0%]), while in the group without a mutation, it was estimated at 30% (95% CI [19.6–47.2%]). For the BRCA2 mutation, the differences did not reach statistical significance.
The strength of our study was the homogenous distribution of patient characteristics and prognostic variables among the different groups. Our results show no differences between BRCA and non-BRCA populations concerning the type of performed surgery (ICS/PCS), FIGO stage, presence of tumor residues after cytoreductive surgery (<1 vs. >1 cm), ascites, and hydrothorax. However, our study had some limitations, including the retrospective design of the study and the relatively limited sample size of the BRCA-mutated population. Moreover, the database included only patients diagnosed with high-grade serous ovarian cancer. This allowed us to perform survival analysis on a relatively homogenous population of patients. On the other hand, it might have affected the analysis regarding patients’ age at ovarian cancer diagnosis as, for example, BRCA mutation carriers are sometimes incidentally diagnosed at the time of prophylactic surgery. During the study, our center did not have the ability to perform HRD testing for the non-BRCA population of patients. As HRD status significantly influences patient prognosis, the survival outcomes might have been different for the HRD and HRP patients that we were unable to categorize. Also, the survival outcome might have been influenced by the maintenance therapy and subsequent therapies used as a part of ovarian cancer treatment. At the time of the study, only BRCAm patients were able to receive maintenance Olaparib treatment, and on 1 January 2022, niraparib treatment was introduced a first-line treatment for advanced ovarian cancer patients regardless of BRCA mutation status. Among the studied population, 20 patients received niraparib maintenance, 10 olaparib monotherapy, and 5 patients underwent combined olaparib plus bevacizumab treatment.
Despite the literature data showing a superior response to platinum-based chemotherapy and a potential initial short-term survival benefit among the BRCA-mutated population of patients, there is no clear answer if the results translate to long-term survival [10,34,36]. Further long-term survival studies are needed, taking into consideration other prognostic factors such as tumor histology, staging, BRCA and HRD status, and the chemotherapy scheme used, as well as the residual disease after cytoreductive surgery. HRD testing, especially among the BRCA1/2 negative population, seems to be an important predictor for patients’ treatment response and overall survival and a strong prognostic marker, independent of its role in predicting PARP inhibitor sensitivity [37]. While BRCA1 and BRCA2 mutations are the most common cause of homologous recombination deficiency, HRD can also be caused by mutations in different homologous recombination repair (HRR) pathway genes including ATM, CHECK2, PALB2, and RAD51C [38] or epigenetic silencing of HRR genes including promoter methylation of the BRCA1 gene [39]. BRCA1 promoter methylation is reported to occur in approximately 10-15% of high-grade serous ovarian cancer tumors [39,40,41,42]. As BRCA1-methylated ovarian cancer shows similar clinicopathological characteristics to BRCA1m ovarian cancer, researchers have begun investigations on its influence on patient survival. A recent meta-analysis of BRCA1 promoter methylation showed no survival differences between BRCA1-methylated and non-BRCA-methylated ovarian cancer [39]. However, there is still limited data regarding the impact of methylation status on patient prognosis.
5. Conclusions
This study provides an additional basis for further research using larger patient series. It underlines the different aspects of germline BRCA1/2 mutations not only on the age of disease onset but also on patient prognosis. Our results suggest the possible influence of BRCA1 mutation status on overall patients survival.
Author Contributions
Conceptualization, K.M. and A.C.-G.; methodology, K.M.; formal analysis, K.M.; investigation, K.M., A.M., M.R.; resources, K.M., B.M.; data curation, K.M., A.M., M.R.; writing—original draft preparation, K.M.; writing—review and editing, K.M., A.C.-G., J.M.; visualization, K.M.; supervision, A.C.-G., J.M.; project administration, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to its retrospective nature (KB.006.139.2025, Pomeranian Medical University Bioethics Comitee).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef]
- Søgaard, M.; Kjær, S.K.; Gayther, S. Ovarian cancer and genetic susceptibility in relation to the BRCA1 and BRCA2 genes. Occurrence, clinical importance and intervention. Acta Obstet. Gynecol. Scand. 2006, 85, 93–105. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B. Breast and Ovarian Cancer Risks Due to Inherited Mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef]
- Shao, C.; Guo, H.; Chen, L.; Chen, J.; Wang, L.; Wang, H. Prognostic factors and clinic-pathologic characteristics of ovarian tumor with different histologic subtypes—A SEER database population study of 41,376 cases. Transl. Cancer Res. 2023, 12, 1937–1950. [Google Scholar] [CrossRef]
- Clark, T.G.; Stewart, M.E.; Altman, D.G.; Gabra, H.; Smyth, J.F. A prognostic model for ovarian cancer. Br. J. Cancer 2001, 85, 944–952. [Google Scholar] [CrossRef]
- Berman, M.L. Future directions in the surgical management of ovarian cancer. Gynecol. Oncol. 2003, 90, S33–S39. [Google Scholar] [CrossRef]
- Cass, I.; Baldwin, R.L.; Varkey, T.; Moslehi, R.; Narod, S.A.; Karlan, B.Y. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 2003, 97, 2187–2195. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, L.; et al. Association between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382–389. [Google Scholar] [CrossRef]
- Artioli, G.; Borgato, L.; Cappetta, A.; Wabersich, J.; Mocellin, S.; Palma, M.D.; Nicoletto, O. Overall survival in BRCA-associated ovarian cancer: Case-control study of an Italian series. Eur. J. Gynaecol. Oncol. 2010, 31, 658–661. [Google Scholar]
- Liu, J.; Cristea, M.C.; Frankel, P.; Neuhausen, S.L.; Steele, L.; Engelstaedter, V.; Matulonis, U.; Sand, S.; Tung, N.; Garber, J.E.; et al. Clinical characteristics and outcomes of BRCA-associated ovarian cancer: Genotype and survival. Cancer Genet. 2012, 205, 34–41. [Google Scholar] [CrossRef]
- Gallagher, D.J.; Konner, J.A.; Bell-McGuinn, K.M.; Bhatia, J.; Sabbatini, P.; Aghajanian, C.A.; Offit, K.; Barakat, R.R.; Spriggs, D.R.; Kauff, N.D. Survival in epithelial ovarian cancer: A multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann. Oncol. 2011, 22, 1127–1132. [Google Scholar] [CrossRef]
- Rudaitis, V.; Zvirblis, T.; Kanopiene, D.; Janulynaite, D.; Griskevicius, L.; Janavicius, R. BRCA1/2 mutation status is an independent factor of improved survival for advanced (stage III-IV) ovarian cancer. Int. J. Gynecol. Cancer 2014, 24, 1395–1400. [Google Scholar] [CrossRef]
- Vencken, P.M.L.H.; Kriege, M.; Hoogwerf, D.; Beugelink, S.; van der Burg, M.E.; Hooning, M.J.; Berns, E.M.; Jager, A.; Collee, M.; Burger, C.W.; et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann. Oncol. 2011, 22, 1346–1352. [Google Scholar] [CrossRef]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Gronwald, J.; Karlan, B.; Rosen, B.; Huzarski, T.; Moller, P.; Lynch, H.T.; Singer, C.F.; Senter, L.; Neuhausen, S.L.; et al. Age-specific ovarian cancer risks among women with a BRCA1 or BRCA2 mutation. Gynecol. Oncol. 2018, 150, 85–91. [Google Scholar] [CrossRef]
- Biglia, N.; Sgandurra, P.; Bounous, V.E.; Maggiorotto, F.; Piva, E.; Pivetta, E.; Ponzone, R.; Pasini, B. Ovarian cancer in BRCA1 and BRCA2 gene mutation carriers: Analysis of prognostic factors and survival. Ecancermedicalscience 2016, 10, 639. [Google Scholar] [CrossRef][Green Version]
- Boyd, J. Specific keynote: Hereditary ovarian cancer: What we know. Gynecol. Oncol. 2003, 88, S8–S10. [Google Scholar] [CrossRef]
- Jasiewicz, A.; Rudnicka, H.; Kluźniak, W.; Gronwald, W.; Kluz, T.; Cybulski, C.; Jakubowska, A.; Lubiński, J.; Gronwald, J. Frequency of BRCA1 and BRCA2 mutations in ovarian cancer patients in South-East Poland. Hered. Cancer Clin. Pract. 2022, 20, 12. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Hoover, J.; et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016, 44, D862–D868. [Google Scholar] [CrossRef]
- Findlay, G.M.; Daza, R.M.; Martin, B.; Zhang, M.D.; Leith, A.P.; Gasperini, M.; Janizek, J.D.; Huang, X.; Starita, L.M.; Shendure, J. Accurate classification of BRCA1 variants with saturation genome editing. Nature 2018, 562, 217–222. [Google Scholar] [CrossRef]
- Sahu, S.; Galloux, M.; Southon, E.; Caylor, D.; Sullivan, T.; Arnaudi, M.; Zanti, M.; Geh, J.; Chari, R.; Michailidou, K.; et al. Saturation genome editing-based clinical classification of BRCA2 variants. Nature 2025, 638, 538–545. [Google Scholar] [CrossRef]
- Sims, T.; Floyd, J.; Sood, A.; Westin, S.; Fellman, B.; Unke, J.; Rangel, K.; Hilton, T.; Fleming, N. Correlation of BRCA and HRD status with clinical and survival outcomes in patients with advanced-stage ovarian cancer in the age of PARPi maintenance therapy (187). Gynecol. Oncol. 2022, 166, S107–S108. [Google Scholar] [CrossRef]
- Chetrit, A.; Hirsh-Yechezkel, G.; Ben-David, Y.; Lubin, F.; Friedman, E.; Sadetzki, S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: The National Israeli Study of Ovarian Cancer. J. Clin. Oncol. 2008, 26, 20–25. [Google Scholar] [CrossRef]
- Boyd, J.; Sonoda, Y.; Federici, M.G.; Bogomolniy, F.; Rhei, E.; Maresco, D.L.; Saigo, P.E.; Almadrones, L.A.; Barakat, R.R.; Brown, C.L.; et al. Clinicopathologic Features of BRCA-Linked and Sporadic Ovarian Cancer. JAMA 2000, 283, 2260–2265. [Google Scholar] [CrossRef]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef]
- Liontos, M.; Zografos, E.; Zoumpourlis, P.; Andrikopoulou, A.; Svarna, A.; Fiste, O.; Kunadis, E.; Papatheodoridi, A.M.; Kaparelou, M.; Koutsoukos, K.; et al. Brca1/2 mutation types do not affect prognosis in ovarian cancer patients. Curr. Oncol. 2021, 28, 4446–4456. [Google Scholar] [CrossRef]
- Ghezelayagh, T.S.; Pennington, K.P.; Norquist, B.M.; Khasnavis, N.; Radke, M.R.; Kilgore, M.R.; Garcia, R.L.; Lee, M.; Katz, R.; Leslie, K.K.; et al. Characterizing TP53 mutations in ovarian carcinomas with and without concurrent BRCA1 or BRCA2 mutations. Gynecol. Oncol. 2021, 160, 786–792. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.M.; Lee, Y.H.; Chong, G.O.; Lee, N.Y.; Lee, I.H.; Park, J.Y.; Hong, D.G. Comparison of Survival Outcomes According to BRCA1/2 Variant Type in High-grade Serous Ovarian Cancer. In Vivo 2022, 36, 1903–1910. [Google Scholar] [CrossRef]
- Buller, R.E.; Shahin, M.S.; Geisler, J.P.; Zogg, M.; De Young, B.R.; Davis, C.S. Failure of BRCA1 dysfunction to alter ovarian cancer survival. Clin. Cancer Res. 2002, 8, 1196–1202. [Google Scholar]
- Pharoah, P.D.P.; Easton, D.F.; Stockton, D.L.; Gayther, S.; Ponder, B.A.J. Survival in familial, BRCA1-associated, and BRCA2-associated epithelial ovarian cancer. Cancer Res. 1999, 59, 868–871. [Google Scholar]
- Kotsopoulos, J.; Rosen, B.; Fan, I.; Moody, J.; McLaughlin, J.R.; Risch, H.; May, T.; Sun, P.; Narod, S.A. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol. Oncol. 2016, 140, 42–47. [Google Scholar] [CrossRef]
- McLaughlin, J.R.; Rosen, B.; Moody, J.; Pal, T.; Fan, I.; Shaw, P.A.; Risch, H.A.; Sellers, T.A.; Sun, P.; Narod, S.A. Long-term ovarian cancer survival associated with mutation in BRCA1 or BRCA2. J. Natl. Cancer Inst. 2013, 105, 141–148. [Google Scholar] [CrossRef]
- Artioli, G.; Giannone, G.; Valabrega, G.; Maggiorotto, F.; Genta, S.; Pignata, S.; Lorusso, D.; Cormio, G.; Scalone, S.; Nicoletto, M.O.; et al. Characteristics and outcome of BRCA mutated epithelial ovarian cancer patients in Italy: A retrospective multicenter study (MITO 21). Gynecol. Oncol. 2021, 161, 755–761. [Google Scholar] [CrossRef]
- Gigerenzer, G.; Wegwarth, O. Five year survival rates can mislead. BMJ 2013, 346, f548. [Google Scholar] [CrossRef]
- Stewart, M.D.; Vega, D.M.; Arend, R.C.; Baden, J.F.; Barbash, O.; Beaubier, N.; Collins, G.; French, T.; Ghahramani, N.; Hinson, P.; et al. Homologous Recombination Deficiency: Concepts, Definitions, and Assays. Oncologist 2022, 27, 167–174. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hirasawa, A. Homologous recombination deficiencies and hereditary tumors. Int. J. Mol. Sci. 2022, 23, 348. [Google Scholar] [CrossRef]
- Kalachand, R.D.; Stordal, B.; Madden, S.; Chandler, B.; Cunningham, J.; Goode, E.L.; Ruscito, I.; I Braicu, E.; Sehouli, J.; Ignatov, A.; et al. BRCA1 Promoter Methylation and Clinical Outcomes in Ovarian Cancer: An Individual Patient Data Meta-Analysis. JNCI J. Natl. Cancer Inst. 2020, 112, 1190–1203. [Google Scholar] [CrossRef]
- Geisler, J.P.; Hatterman-Zogg, M.A.; Rathe, J.A.; Buller, R.E. Frequency of BRCA1 dysfunction in ovarian cancer. J. Natl. Cancer Inst. 2002, 94, 61–67. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Nemeth, E.; Tran, H.; Shvartsman, H.; Cass, I.; Narod, S.; Karlan, B.Y. BRCA1 promoter region hypermethylation in ovarian carcinoma: A population-based study. Cancer Res. 2000, 60, 5329–5333. [Google Scholar]
- Abkevich, V.; Timms, K.M.; Hennessy, B.T.; Potter, J.; Carey, M.S.; Meyer, L.A.; Smith-McCune, K.; Broaddus, R.; Lu, K.H.; Chen, J.; et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br. J. Cancer 2012, 107, 1776–1782. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).