Placental Expression of Sirtuins in Women with Gestational Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. RNA Isolation
2.3. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction
- B2M forward: 5′-AATGCGGCATCTTCAAACCT-3′
- B2M reverse: 5′-TGACTTTGTCACAGCCCAAGA-3′
- SIRT1 forward: 5′-ACGCTGGAACAGGTTGCGGG-3′
- SIRT1 reverse: 5′-AGCGGTTCATCAGCTGGGCAC-3′
- SIRT3 forward: 5′-AAGTGTTGTTGGAAGTGGAG-3′
- SIRT3 reverse: 5′-TGTGAAAGAAGAATGGGAGT-3′
- SIRT4 forward: 5′-AGACTCCTTGTGATGACTGG-3′
- SIRT4 reverse: 5′-AGTACAGCTTTCCGAGTTTC-3′
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Szukiewicz, D. The Role of Sirtuins in Aging and Age-Related Diseases. Adv. Med. Sci. 2016, 61, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, L.; Du, P.; Liu, X.; Xia, Y. Effects of Sirtuin 1 Deficiency on Trophoblasts and Its Implications in the Pathogenesis of Pre-Eclampsia. J. Obstet. Gynaecol. 2023, 43, 2282103. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Fajardy, I.; Moitrot, E.; Vambergue, A.; Vandersippe-Millot, M.; Deruelle, P.; Rousseaux, J. Time Course Analysis of RNA Stability in Human Placenta. BMC Mol. Biol. 2009, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Meller, M.; Vadachkoria, S.; Luthy, D.A.; Williams, M.A. Evaluation of Housekeeping Genes in Placental Comparative Expression Studies. Placenta 2005, 26, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Karahoda, R.; Robles, M.; Marushka, J.; Stranik, J.; Abad, C.; Horackova, H.; Tebbens, J.D.; Vaillancourt, C.; Kacerovsky, M.; Staud, F. Prenatal Inflammation as a Link between Placental Expression Signature of Tryptophan Metabolism and Preterm Birth. Hum. Mol. Genet. 2021, 30, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, F.; Peng, Y.; Chen, R.; Zhou, W.; Wang, H.; OuYang, J.; Yu, B.; Xu, Z. Transcriptomic Profiling of Human Placenta in Gestational Diabetes Mellitus at the Single-Cell Level. Front. Endocrinol. 2021, 12, 679582. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Yamada, J.; Beauharnais, C.; Wenger, J.B.; Thadhani, R.I.; Wexler, D.; Roberts, D.J.; Bentley-Lewis, R. Type 1, Type 2 and Gestational Diabetes Mellitus Differentially Impact Placental Pathologic Characteristics of Uteroplacental Malperfusion. Placenta 2015, 36, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Aldahmash, W.M.; Alwasel, S.H.; Aljerian, K. Gestational Diabetes Mellitus Induces Placental Vasculopathies. Environ. Sci. Pollut. Res. 2022, 29, 19860–19868. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Grabowska, M.; Mikołajek-Bedner, W.; Tarnowski, M. Sirtuins and Their Implications in the Physiopathology of Gestational Diabetes Mellitus. Pharmaceuticals 2025, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Kahmini, F.R.; Ghaleh, H.D.; Shahgaldi, S. Sirtuins: Subtle Regulators Involved in Convoluted Mechanisms of Pregnancy. Cell Physiol. Biochem. 2022, 56, 644–662. [Google Scholar] [PubMed]
- Joaquim, V.H.A.; da Silva, J.G.; Jannig, P.R. Understanding the role of sirtuin 1 in muscle physiology during androgen deprivation. J. Physiol. 2023, 601, 4659–4660. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Moyce, B.L.; Dolinsky, V.W. Maternal beta-Cell Adaptations in Pregnancy and Placental Signalling: Implications for Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3467. [Google Scholar] [CrossRef] [PubMed]
- Tsiotra, P.C.; Halvatsiotis, P.; Patsouras, K. Circulating adipokines and mRNA expression in adipose tissue and the placenta in womenwith gestational diabetes mellitus. Peptides 2018, 101, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Herrin, M.A.; Pitruzzello, M.C.; Mulla, M.J.; Werner, E.F.; Pettker, C.M.; Flannery, C.A.; Abrahams, V.M. Glucose and metformin modulate human first trimester trophoblast function: A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am. J. Reprod. Immunol. 2015, 73, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Liu, X.; Huang, H. Advances in the role of silence information regulator family in pathological pregnancy. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021, 50, 335–344. [Google Scholar] [CrossRef] [PubMed]
- De Kreutzenberg, S.V.; Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Semplicini, A.; Man, C.D.; Cobelli, C.; Fadini, G.P.; Avogaro, A. Downregulation of the Longevity-Associated Protein Sirtuin 1 in Insulin Resistance and Metabolic Syndrome: Potential Biochemical Mechanisms. Diabetes 2010, 59, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Kojima, J.; Dai, Y.; Suzuki, T.; Ono, M.; Nishi, H. Sirtuin 1 Is a Potential Therapeutic Candidate Gene for Fetal Growth Restriction via Insulin-like 4. J. Matern.-Fetal Neonatal Med. 2023, 36, 2253486. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Ye, X.; Chen, Z.; Fu, H.; Li, S.; Xu, P.; Yu, J.; Wen, L.; Gao, R.; Fu, Y.; et al. Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial−Mesenchymal Transition through an Increase in Vimentin Acetylation. Aging Cell 2021, 20, e13491. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Y.; Liu, M.; Liao, L.; Wei, X.; Zhou, R. SIRT3 Deficiency Affects the Migration, Invasion, Tube Formation and Necroptosis of Trophoblast and Is Implicated in the Pathogenesis of Preeclampsia. Placenta 2022, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; An, X.; Fan, D. Histone Deacetylase Sirtuin 2 Enhances Viability of Trophoblasts Through P65-Mediated MicroRNA-146a/ACKR2 Axis. Reprod. Sci. 2021, 28, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Turek, I.; Wozniak, L.; Cypryk, K.; Nadel, I.; Wojcik, M. Evaluation of Leukocyte SIRT1 Expression in Women with Gestational Diabetes Mellitus (GDM) in the Third Trimester of Pregnancy. Diabetol. Klin. 2014, 3, 3–11. [Google Scholar]
- Zhang, Q.; Ye, X.; Xu, X.; Yan, J. Placenta-derived Exosomal miR-135a-5p Promotes Gestational Diabetes Mellitus Pathogenesis by Activating PI3K/AKT Signalling Pathway via SIRT1. J. Cell. Mol. Med. 2023, 27, 3729–3743. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Chang, X.; Yuan, Z.; Wang, Y. Expression and Correlation Analysis of Silent Information Regulator 1 (SIRT1), Sterol Regulatory Element-Binding Protein-1 (SREBP1), and Pyroptosis Factor in Gestational Diabetes Mellitus. J. Matern.-Fetal Neonatal Med. 2024, 37, 2311809. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Szewczyk, G.; Szukiewicz, D. The Role of Sirtuin-1 (SIRT1) in the Physiology and Pathophysiology of the Human Placenta. IJMS 2023, 24, 16210. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Massa, V.; Terraneo, L.; Samaja, M.; Doi, P.; Bulfamante, G.P.; Marconi, A.M. Gestational Diabetes Affects Fetal Autophagy. Placenta 2017, 55, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, D.; Liu, J.; Qiu, D.; Wang, J.; Cheng, X.; Yang, X.; Li, R.; Wang, G. Gestational Diabetes Mellitus in Women Increased the Risk of Neonatal Infection via Inflammation and Autophagy in the Placenta. Medicine 2020, 99, e22152. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, D.; Tang, D.; Shangguan, Y.; Cao, Y.; Guo, R.; Luan, S.; Yun, C.; Morgera, S.; Hocher, B.; et al. Integrated Proteome and Acetylome Analyses Unveil Protein Features of Gestational Diabetes Mellitus and Preeclampsia. Proteomics 2022, 22, 2200124. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Alessio, N.; Falone, S.; Pietrangelo, L.; Lanuti, P.; Cordone, V.; Santini, S.J.; Di Pietrantonio, N.; Marchisio, M.; Protasi, F.; et al. Endothelial Cells from Umbilical Cord of Women Affected by Gestational Diabetes: A Suitable in Vitro Model to Study Mechanisms of Early Vascular Senescence in Diabetes. FASEB J. 2021, 35, e21662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Q.; Chen, B.; Zeng, J.; Dou, X.; Pan, Z.; Xiao, J.; Li, M.; Wang, F.; Chen, C.; et al. Cardamonin Protects against Iron Overload Induced Arthritis by Attenuating ROS Production and NLRP3 Inflammasome Activation via the SIRT1/p38MAPK Signaling Pathway. Sci. Rep. 2023, 13, 13744. [Google Scholar] [CrossRef] [PubMed]
- Chou, X.; Li, X.; Min, Z.; Ding, F.; Ma, K.; Shen, Y.; Sun, D.; Wu, Q. Sirtuin-1 Attenuates Cadmium-Induced Renal Cell Senescence through P53 Deacetylation. Ecotoxicol. Environ. Saf. 2022, 245, 114098. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Dong, L.; Li, Y.; Liu, G. SIRT1 and HIF1α Signaling in Metabolism and Immune Responses. Cancer Lett. 2018, 418, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bouras, T.; Fu, M.; Sauve, A.A.; Wang, F.; Quong, A.A.; Perkins, N.D.; Hay, R.T.; Gu, W.; Pestell, R.G. SIRT1 Deacetylation and Repression of P300 Involves Lysine Residues 1020/1024 within the Cell Cycle Regulatory Domain 1. J. Biol. Chem. 2005, 280, 10264–10276. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J. SIRT1 Regulates Autoacetylation and Histone Acetyltransferase Activity of TIP60. J. Biol. Chem. 2010, 285, 11458–11464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New Emerging Biological Function and Therapeutic Target. Theranostics 2020, 10, 8315–8342. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ding, Y.; Dai, G.; Zhang, Y.; Xu, M.; Shen, J.; Chen, T.; Chen, Y.; Meng, G. Sirtuin 3 Deficiency Exacerbates Diabetic Cardiomyopathy via Necroptosis Enhancement and NLRP3 Activation. Acta Pharmacol. Sin. 2021, 42, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Fritz, K.S.; Galligan, J.J.; Smathers, R.L.; Roede, J.R.; Shearn, C.T.; Reigan, P.; Petersen, D.R. 4-Hydroxynonenal Inhibits SIRT3 via Thiol-Specific Modification. Chem. Res. Toxicol. 2011, 24, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Giralt, A.; Hondares, E.; Villena, J.A.; Ribas, F.; Díaz-Delfín, J.; Giralt, M.; Iglesias, R.; Villarroya, F. Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α Controls Transcription of the Sirt3 Gene, an Essential Component of the Thermogenic Brown Adipocyte Phenotype. J. Biol. Chem. 2011, 286, 16958–16966. [Google Scholar] [CrossRef] [PubMed]
- Neeli, P.K.; Gollavilli, P.N.; Mallappa, S.; Hari, S.G.; Kotamraju, S. A Novel metadherinΔ7 Splice Variant Enhances Triple Negative Breast Cancer Aggressiveness by Modulating Mitochondrial Function via NFĸB-SIRT3 Axis. Oncogene 2020, 39, 2088–2102. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and Sirtuin 3: Physiological Modulators of Metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; O’Callaghan, C.; Chang, E.D.; Jiang, H.; Vassilopoulos, A. Context-Dependent Roles for SIRT2 and SIRT3 in Tumor Development Upon Calorie Restriction or High Fat Diet. Front. Oncol. 2020, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Someya, S. Maintaining Good Hearing: Calorie Restriction, Sirt3, and Glutathione. Exp. Gerontol. 2013, 48, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.S.; Dittenhafer-Reed, K.E.; Yu, W.; Bailey, D.J.; Selen, E.S.; Boersma, M.D.; Carson, J.J.; Tonelli, M.; Balloon, A.J.; Higbee, A.J.; et al. Calorie Restriction and SIRT3 Trigger Global Reprogramming of the Mitochondrial Protein Acetylome. Mol. Cell 2013, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wang, Y.; Zhang, Z.; Yang, D.; Gu, D.; He, B.; Zhang, X.; He, D.; Wang, H.; Jose, P.A.; et al. Calorie Restriction Protects against Contrast-Induced Nephropathy via SIRT1/GPX4 Activation. Oxidative Med. Cell. Longev. 2021, 2021, 2999296. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Velilla, M.; Herrero, L.; Cervela, L.; Ribeiro, M.L.; Simó, R.; Villena, J.A. Calorie Restriction and SIRT1 Overexpression Induce Different Gene Expression Profiles in White Adipose Tissue in Association with Metabolic Improvement. Mol. Nutr. Food Res. 2021, 65, 2000672. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, G.; Li, Q.; Li, W.; Thamotharan, S.; Tosevska, A.; Morselli, M.; Sung, K.; Janzen, C.; Zhou, X.; Pellegrini, M.; et al. Cell-Free DNA Methylation and Transcriptomic Signature Prediction of Pregnancies with Adverse Outcomes. Epigenetics 2021, 16, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kuang, A.; Bain, J.R.; Hayes, M.G.; Muehlbauer, M.J.; Ilkayeva, O.R.; Newgard, C.B.; Powe, C.E.; Hivert, M.-F.; Scholtens, D.M.; et al. Metabolomic and Genetic Architecture of Gestational Diabetes Subtypes. Diabetologia 2024, 67, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Khambule, L.; George, J.A. The Role of Inflammation in the Development of GDM and the Use of Markers of Inflammation in GDM Screening. In Reviews on Biomarker Studies of Metabolic and Metabolism-Related Disorders; Guest, P.C., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1134, pp. 217–242. ISBN 978-3-030-12667-4. [Google Scholar]
- Chen, J.; Wang, Q.; Li, R.; Li, Z.; Jiang, Q.; Yan, F.; Ye, J. The role of sirtuins in the regulatin of oxidative stress during the progress and therapy of type 2 diabetes mellitus. Life Sci. 2023, 333, 122187. [Google Scholar] [CrossRef] [PubMed]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; German, N.J.; Saha, A.K.; de Boer, V.C.; Davies, M.; Koves, T.R.; Dephoure, N.; Fischer, F.; Boanca, G.; Vaitheesvaran, B.; et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 2013, 50, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Zhang, G.; Li, L.; Li, H.; Jin, X.; Wang, Y.; Li, B. Sirt4 deficiency promotes the development of atherosclerosis by activating the NF-κB/IκB/CXCL2/3 pathway. Atherosclerosis 2023, 373, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Potthast, A.; Rohrbach, A.; Borns, K.; Das, A.M.; von Versen-Höynck, F. Gestational diabetes induces alterations of sirtuins in fetal endothelial cells. Pediatr. Res. 2016, 79, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Zaganjor, E.; Vyas, S.; Haigis, M.C. SIRT4 Is a Regulator of Insulin Secretion. Cell Chem. Biol. 2017, 24, 656–658. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Emamgholipour, S.; Aliakbar, S.; Amini, M.; Gorgani-Firuzjaee, S.; Hossein-Nezhad, A. Differential expressions of SIRT1, SIRT3, and SIRT4 in peripheral blood mononuclear cells from patients with type 2 diabetic retinopathy. Arch. Physiol. Biochem. 2020, 126, 363–368. [Google Scholar] [CrossRef] [PubMed]

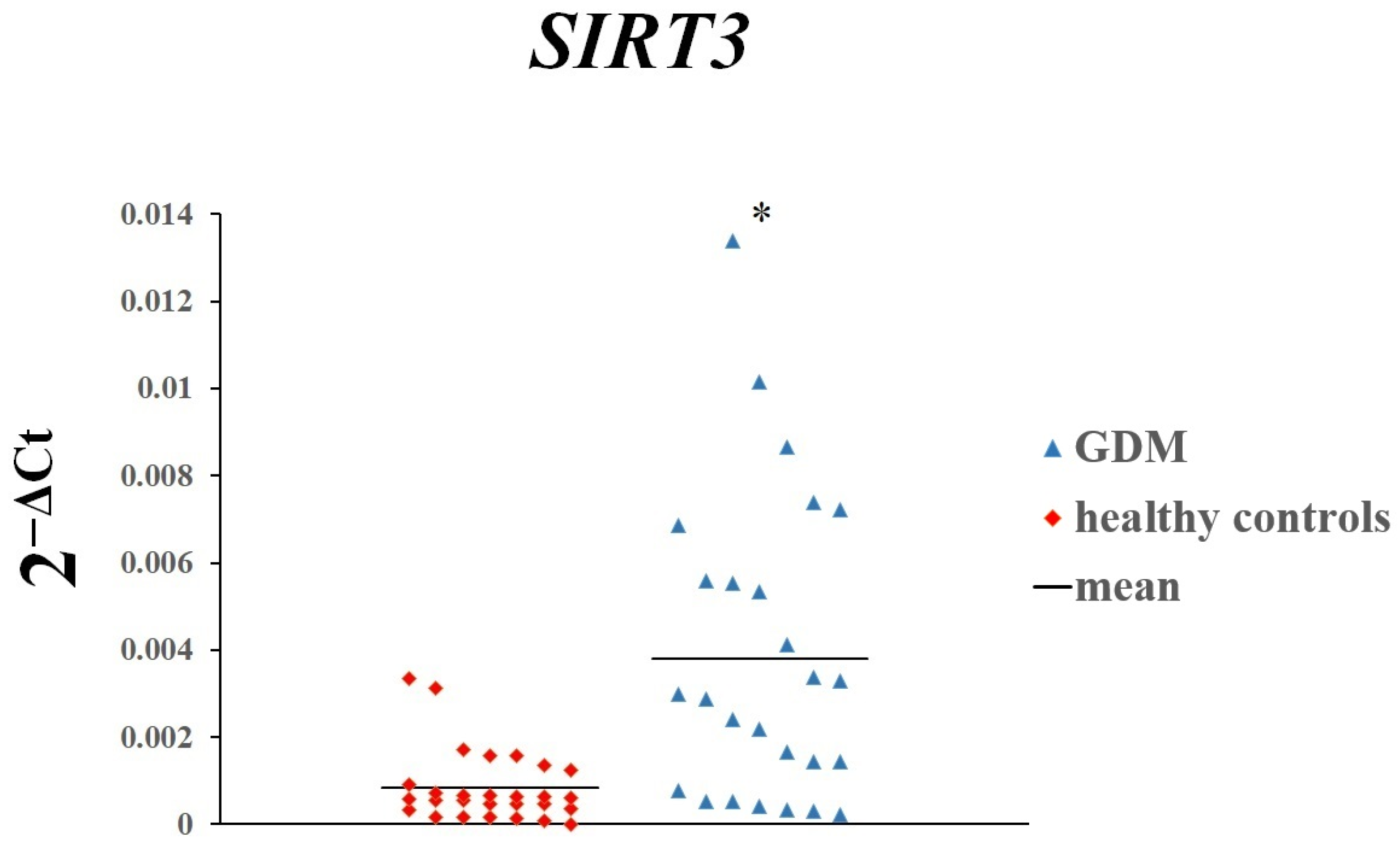
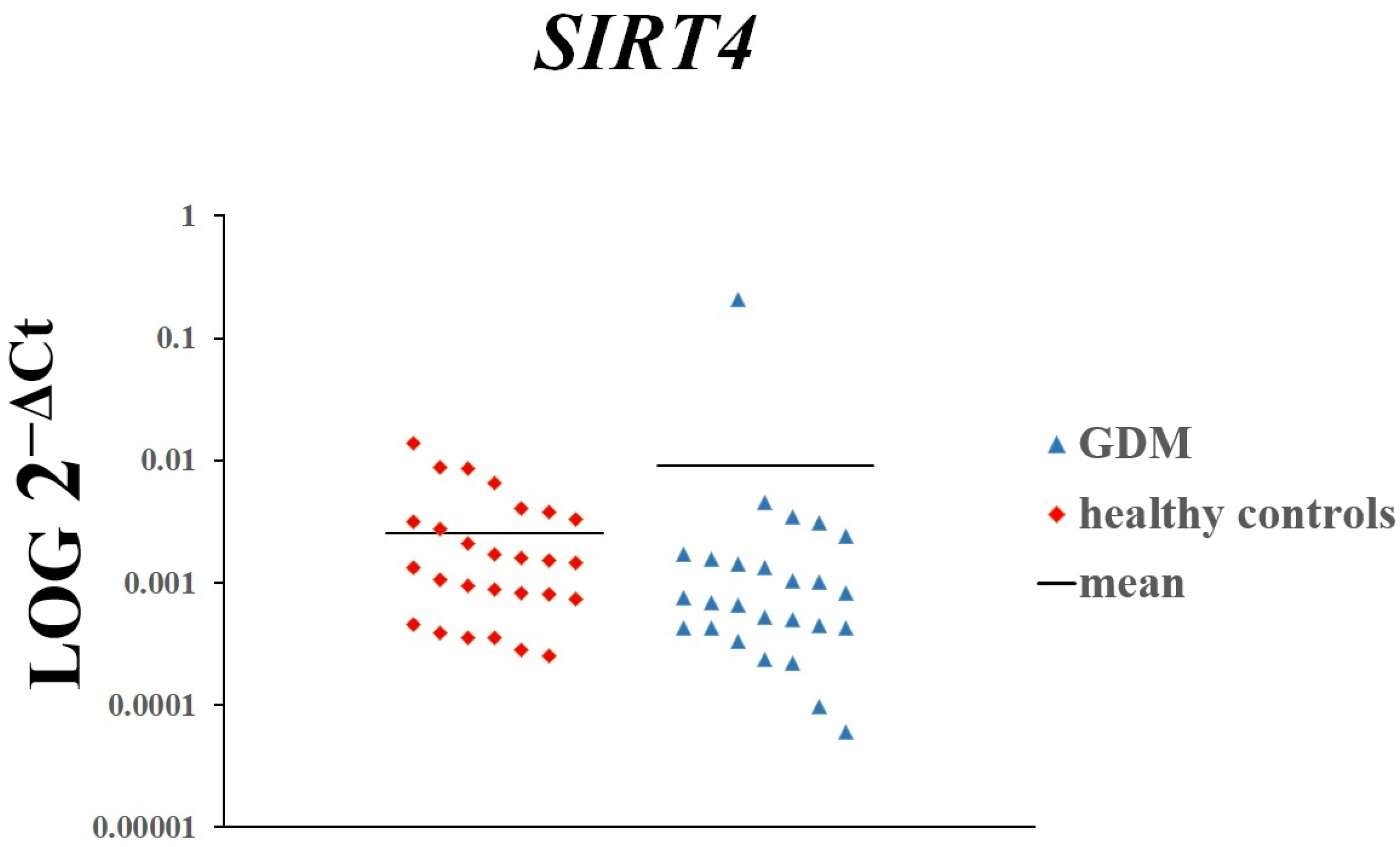
| Parameters | GDM | Healthy Controls |
|---|---|---|
| Mean ± SD | ||
| Age (years) | 32.3 ± 4.3 | 30.2 ± 4.8 |
| Body mass before pregnancy (kg) | 70.9 ± 16.3 | 69.4 ± 18.5 |
| Body mass at birth (kg) | 83.2 ± 15.7 | 81.1 ± 14.7 |
| Body mass increase during pregnancy (kg) | 12.3 ± 6.0 | 11.7 ± 7.6 |
| BMI before pregnancy (kg/m2) | 25.9 ± 5.5 | 24.7 ± 5.2 |
| BMI at birth (kg/m2) | 30.4 ± 5.4 | 29.1 ± 4.3 |
| BMI increase during pregnancy (kg/m2) | 4.5 ± 2.2 | 4.4 ± 2.6 |
| Daily insulin requirement (unit) | 13.7 ± 15.8 | 0 ± 0 |
| Newborn body mass (g) | 3401.9 ± 594.3 | 3318.6 ± 378.0 |
| APGAR (0–10) | 9.3 ± 0.8 | 9.5 ± 0.7 |
| Clinical Parameters | Rs | p |
|---|---|---|
| Age [years] | 0.28 | 0.16 |
| APGAR [0–10] | 0.03 | 0.87 |
| BMI at birth [kg/m2] | −0.05 | 0.81 |
| BMI before pregnancy [kg/m2] | −0.05 | 0.80 |
| BMI increase during pregnancy [kg/m2] | 0.03 | 0.87 |
| Body mass at birth [kg] | −0.08 | 0.71 |
| Body mass before pregnancy [kg] | 0.04 | 0.83 |
| Body mass increase during pregnancy [kg] | 0.08 | 0.70 |
| Daily insulin requirement [unit] | 0.19 | 0.33 |
| Newborn body mass [g] | −0.04 | 0.86 |
| Rs—Spearman rank correlation coefficient. |
| Clinical Parameters | Rs | p |
|---|---|---|
| Age [years] | 0.33 | 0.09 |
| APGAR [0–10] | −0.21 | 0.30 |
| BMI at birth [kg/m2] | −0.08 | 0.70 |
| BMI before pregnancy [kg/m2] | −0.12 | 0.56 |
| BMI increase during pregnancy [kg/m2] | 0.08 | 0.71 |
| Body mass at birth [kg] | −0.11 | 0.58 |
| Body mass before pregnancy [kg] | −0.05 | 0.82 |
| Body mass increase during pregnancy [kg] | 0.11 | 0.57 |
| Daily insulin requirement [unit] | 0.15 | 0.46 |
| Newborn body mass [g] | −0.08 | 0.69 |
| Rs—Spearman rank correlation coefficient. |
| Clinical Parameters | Rs | p |
|---|---|---|
| Age [years] | 0.11 | 0.58 |
| APGAR [0–10] | 0.09 | 0.67 |
| BMI at birth [kg/m2] | −0.20 | 0.31 |
| BMI before pregnancy [kg/m2] | −0.21 | 0.30 |
| BMI increase during pregnancy [kg/m2] | 0.07 | 0.73 |
| Body mass at birth [kg] | −0.25 | 0.21 |
| Body mass before pregnancy [kg] | −0.17 | 0.41 |
| Body mass increase during pregnancy [kg] | 0.09 | 0.66 |
| Daily insulin requirement [unit] | 0.02 | 0.90 |
| Newborn body mass [g] | 0.04 | 0.85 |
| Rs—Spearman rank correlation coefficient. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerewaty, M.; Ustianowski, Ł.; Kiełbowski, K.; Bakinowska, E.; Safranow, K.; Tarnowski, M.; Sroczyński, T.; Pawlik, A. Placental Expression of Sirtuins in Women with Gestational Diabetes. Genes 2025, 16, 844. https://doi.org/10.3390/genes16070844
Czerewaty M, Ustianowski Ł, Kiełbowski K, Bakinowska E, Safranow K, Tarnowski M, Sroczyński T, Pawlik A. Placental Expression of Sirtuins in Women with Gestational Diabetes. Genes. 2025; 16(7):844. https://doi.org/10.3390/genes16070844
Chicago/Turabian StyleCzerewaty, Michał, Łukasz Ustianowski, Kajetan Kiełbowski, Estera Bakinowska, Krzysztof Safranow, Maciej Tarnowski, Tomasz Sroczyński, and Andrzej Pawlik. 2025. "Placental Expression of Sirtuins in Women with Gestational Diabetes" Genes 16, no. 7: 844. https://doi.org/10.3390/genes16070844
APA StyleCzerewaty, M., Ustianowski, Ł., Kiełbowski, K., Bakinowska, E., Safranow, K., Tarnowski, M., Sroczyński, T., & Pawlik, A. (2025). Placental Expression of Sirtuins in Women with Gestational Diabetes. Genes, 16(7), 844. https://doi.org/10.3390/genes16070844






