Abstract
Background: Natural products are key sources of anti-inflammatory agents, yet the potential of fish visceral extracts remains largely unexplored. This study evaluated the anti-inflammatory activity of a spleen extract from rainbow trout (Oncorhynchus mykiss). Methods: A crude spleen extract and its four solvent fractions were tested in LPS-stimulated RAW264.7 macrophages. Nitric oxide production and expression of iNOS, COX-2, and cytokines were assessed by qRT-PCR and Western blotting. The most active fraction, OSB (n-butanol layer), was further analyzed for its effects on NF-κB signaling, macrophage polarization, and ROS generation. Results: The crude spleen extract significantly reduced NO production and downregulated iNOS and COX-2 expression at both the transcriptional and translational levels. Among the four fractions, the OSB fraction exhibited the most potent and consistent anti-inflammatory effects. OSB markedly suppressed LPS-induced expression of iNOS, COX-2, and pro-inflammatory cytokines, while enhancing anti-inflammatory cytokines. Mechanistic analyses demonstrated that OSB inhibited NF-κB activation by preventing the nuclear translocation of the p65 subunit. Additionally, OSB attenuated LPS-induced ROS production and reduced the expression of M1 macrophage markers, indicating inhibition of M1 polarization. Conclusions: The OSB fraction from rainbow trout spleen exhibits potent anti-inflammatory activity by modulating the NF-κB pathway and suppressing M1 macrophage polarization, suggesting its potential as a natural therapeutic agent.
1. Introduction
Inflammation is a crucial component of the innate immune response triggered by microbial infections, tissue injury, or antigenic invasion. It is typically characterized by symptoms such as pain, swelling, itching, and fever, which accompany the elimination of pathogens and damaged cells from affected tissues [1]. Although the inflammatory response is generally self-limiting, its dysregulation or persistence can lead to chronic inflammatory conditions and contribute to the development and progression of various diseases, including cancer, diabetes, inflammatory bowel disease, asthma, and other autoimmune disorders [2]. Conventional anti-inflammatory agents, which primarily act by modulating inflammation-related cytokine expression, remain the standard treatment for these conditions [3]. However, their prolonged use is frequently associated with adverse effects such as gastrointestinal ulceration, mucosal perforation, and renal toxicity. These limitations underscore the urgent need for safer and more effective therapeutic alternatives with a reduced risk of drug resistance [4,5].
Macrophages, as key effectors of the innate immune system, play a central role in orchestrating inflammatory responses via activation of the Toll-like receptor 4 (TLR4)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway upon lipopolysaccharide (LPS) stimulation [6,7]. Dysregulation of this pathway is implicated in numerous inflammatory diseases, including rheumatoid arthritis, pneumonia, and inflammatory bowel disease, making it a critical therapeutic target [8,9,10,11].
In recent years, natural product-derived compounds have gained significant attention for their ability to modulate inflammation-related signaling pathways [12,13]. The development of anti-inflammatory agents from natural sources is considered a promising alternative to synthetic drugs due to their favorable safety profiles and cost-effectiveness [14,15]. Notably, approximately 25% of anti-inflammatory drugs approved by the U.S. Food and Drug Administration (FDA) are derived from natural products, highlighting their therapeutic potential [16]. Fish processing by-products, such as heads, viscera, and bones—often comprising up to 50% of the total body mass depending on the species—are typically discarded through landfilling or incineration, contributing to environmental pollution, including greenhouse gas emissions, water contamination, and ecosystem disruption [17,18,19]. Repurposing these by-products offers not only a sustainable waste management strategy, but also a valuable opportunity to discover novel bioactive compounds from marine sources [20].
Previous studies have reported anti-inflammatory effects from various fish by-products. For example, cutlassfish head peptone was shown to modulate the mitogen-activated protein kinase (MAPK) signaling pathway in macrophages [21], and extracts from olive flounder heads suppressed iNOS and COX-2 expression [22]. Similarly, salmon bone extracts inhibited nitric oxide production, suggesting anti-inflammatory potential [23]. Despite these findings, the anti-inflammatory properties of fish visceral organ extracts remain poorly characterized and underexplored.
Among fish species, rainbow trout (O. mykiss) has gained attention for its high content of polydeoxyribonucleotide (PDRN)—a DNA-derived compound with molecular weights ranging from 50 kDa to 1500 kDa [24]. PDRN has been shown to attenuate LPS-induced acute lung injury in rats by inhibiting both the NF-κB and MAPK pathways [25], and to promote wound healing through the upregulation of vascular endothelial growth factor (VEGF) in diabetic mouse models [26]. Additionally, PDRN enhances osteoblast proliferation [27], increases collagen synthesis in incisional wound models [28], and exerts anti-inflammatory effects by modulating cytokine expression in osteoarthritic cell systems [29]. These findings collectively highlight the therapeutic potential of PDRN in treating inflammatory conditions [30]. However, commercial PDRN production typically relies on sperm- or milt-derived extraction from rainbow trout, which is limited by high production costs and the seasonal availability of spermatozoa [31].
In this study, we aimed to elucidate the molecular mechanisms underlying the anti-inflammatory effects of visceral extracts derived from rainbow trout using an LPS-stimulated macrophage model. Our findings provide novel insights into the bioactivity of fish by-product-derived visceral organ extracts and their potential as sustainable sources of anti-inflammatory therapeutics.
2. Materials and Methods
2.1. Preparation of Crude Extract and Fractionation Samples
Thirteen-month-old female rainbow trout were obtained from Woori Trout Farm (Chuncheon, Gangwon-do, Republic of Korea). Following dissection, their liver, gallbladder, spleen, and kidneys were harvested and extracted with 70% ethanol at 60 °C for 16 h. The resulting crude extracts were lyophilized, reconstituted in 70% ethanol, and subsequently diluted in a culture medium for use in in vitro experiments. The crude spleen extract was suspended in distilled water and sequentially partitioned with n-hexane, ethyl acetate, and n-butanol to yield solvent-soluble fractions. Initially, n-hexane was added to the aqueous suspension to obtain the n-hexane-soluble fraction. The remaining aqueous layer was subsequently extracted with ethyl acetate and then with n-butanol to yield the respective ethyl acetate- and n-butanol-soluble fractions. The final residual aqueous layer was collected as the water-soluble fraction. As a result, the crude extract was separated into four distinct fractions.
2.2. Cell Culture
RAW264.7 murine macrophage cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. The cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S). The cells were gently detached using a cell scraper without trypsinization. Freeze-dried extracts from the rainbow trout liver, gallbladder, spleen, and kidneys were dissolved in 70% ethanol and diluted in the culture medium to the desired concentrations. Lipopolysaccharide (LPS) was dissolved in triple-distilled water and diluted to a final concentration of 1 μg/mL in DMEM.
2.3. Nitric Oxide Assay
RAW264.7 cells were seeded in 96-well plates and incubated for 24 h. The cells were then treated with crude extracts from the liver, gallbladder, spleen, or kidneys of the rainbow trout in combination with 1 μg/mL LPS. RAW264.7 cells were treated with each extract at concentrations of 10, 20, 40, 50, 100, and 200 μg/mL. After 24 h, the culture supernatant was collected and transferred to a new 96-well plate for nitric oxide measurement using the NO Plus Detection Kit (iNtRON Biotechnology, Seongnam, Republic of Korea), according to the manufacturer’s protocol.
2.4. Quantitative Reverse Transcription PCR (qRT-PCR)
RAW264.7 cells were seeded in culture dishes and incubated for 24 h, followed by treatment with OSH, OSE, OSB, or OSW in the presence of 1 μg/mL LPS for an additional 24 h. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and cDNA synthesis was performed using oligodT primers and M-MLV reverse transcriptase (Promega, Madison, WI, USA). qRT-PCR was conducted using the TOPreal™ qPCR 2X PreMIX (Enzynomics, Daejeon, Republic of Korea). Relative gene expression levels were calculated using the 2−ΔΔCt method, with normalization to GAPDH. The primer sequences are listed below (Table 1):

Table 1.
qRT-PCR Primer Sequences.
2.5. Western Blotting
RAW264.7 cells were seeded into 100 mm dishes and treated 24 h later according to experimental conditions. After treatment, the cells were harvested using a scraper, and total protein was extracted using a RIPA lysis buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA, 140 mM NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, and 0.1% SDS) containing a cOmplete™ Protease Inhibitor Cocktail (Roche, Basel, Switzerland). Protein concentration was measured using the Bradford assay (Thermo Fisher Scientific, Waltham, MA, USA). Proteins were separated via SDS-PAGE and transferred to 0.45 μm PVDF membranes. The membranes were blocked with 5% skim milk for 30 min at room temperature and then incubated overnight at 4 °C with primary antibodies diluted in 1× TBST containing 1% BSA. After washing, the membranes were incubated with HRP-conjugated secondary antibodies diluted in 5% skim milk for 2 h at room temperature. Protein bands were visualized using an ECL detection kit (GE Healthcare, Chicago, IL, USA). iNOS (13120S), COX-2 (12282S), IκBα (9242S), p-p65 (3033S), and CD86 (19589S) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA), and p65 (ab32536) and Lamin B1 (ab16048) antibodies were obtained from Abcam (Cambridge, UK).
2.6. Immunofluorescence
RAW264.7 cells were seeded onto coverslips in 6-well plates and treated 24 h after seeding. The cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 in PBS for 3 min, and blocked with 5% skim milk in PBS for 1 h at room temperature. The cells were incubated with primary antibodies (1:200 dilution) for 2 h, followed by Alexa Fluor 488-conjugated goat anti-mouse IgG (1:200; Abcam, Boston, MA, USA) for 1 h. Nuclei were stained with DAPI (1:2000 in PBS). Fluorescence images were acquired using a confocal microscope (Nikon, Tokyo, Japan).
2.7. Reactive Oxygen Species (ROS) Analysis
RAW264.7 cells were seeded in 60 mm dishes and treated according to experimental protocols 24 h after seeding. The cells were incubated with 10 μM carboxy-H2DCFDA (Invitrogen, Carlsbad, CA, USA) at 37 °C for 30 min. After washing twice with PBS, intracellular ROS levels were measured using a flow cytometer (FACSymphony, Bergen County, NJ, USA) with excitation at 488 nm and emission at 525 nm.
2.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 8.0 and ImageJ v1.54d. Data are presented as the mean ± standard deviation (SD) from at least three independent experiments. Comparisons between two groups were evaluated using Student’s t-test. A p-value < 0.05 was considered statistically significant, with the following notations: p ≤ 0.05 (*) and p ≤ 0.01 (**).
3. Results
3.1. Anti-Inflammatory Effect of Rainbow Trout Spleen Crude Extract in LPS-Stimulated RAW264.7 Cells
To investigate the bioactivity of organ-derived extracts from rainbow trout, crude extracts were prepared from the liver, gallbladder, spleen, and kidneys using 70% ethanol (Figure 1A). The anti-inflammatory potential of each extract was evaluated by treating RAW264.7 macrophages with LPS in the presence of each crude extract. NO production—a key marker of inflammation—was measured to assess the inflammatory response. LPS stimulation induced a nearly 10-fold increase in NO production compared to that in the untreated controls. Importantly, none of the crude extracts alone triggered an inflammatory response. Among the organ extracts tested, only the spleen crude extract markedly reduced LPS-induced NO production in a concentration-dependent manner, whereas the liver, gallbladder, and kidney extracts had no significant effect (Figure 1B). To further examine the anti-inflammatory activity of the spleen extract, we analyzed the expression levels of inflammation-related genes. Quantitative real-time PCR demonstrated that the spleen extract significantly downregulated the LPS-induced expression of iNOS and COX-2 mRNA in a dose-dependent manner (Figure 1C). Consistent with the mRNA findings, a Western blot analysis revealed that spleen extract treatment also reduced the protein expression levels of iNOS and COX-2 induced by LPS (Figure 1D). Collectively, these results indicate that the spleen crude extract of rainbow trout exerts potent anti-inflammatory effects by suppressing NO production and downregulating the expression of key pro-inflammatory mediators in LPS-stimulated RAW264.7 macrophages.
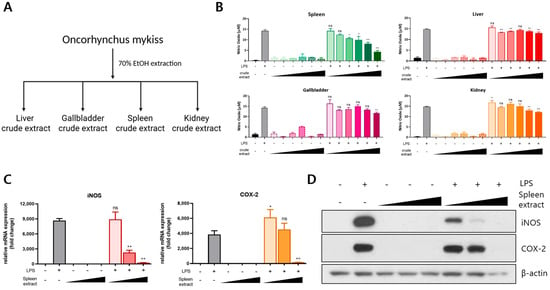
Figure 1.
Anti-inflammatory effects of rainbow trout spleen crude extract in LPS-stimulated RAW264.7 cells. (A) Crude extracts were prepared from the liver, gallbladder, spleen, and kidneys of rainbow trout via 70% ethanol extraction at 60 °C for 16 h. (B) RAW264.7 cells were treated with each extract (10–200 μg/mL) in the presence of 1 μg/mL LPS, and nitric oxide (NO) production was quantified. (C) The mRNA expression levels of iNOS and COX-2 were assessed via qRT-PCR following treatment with the spleen crude extract (100–400 μg/mL). (D) The protein expression of iNOS and COX-2 was analyzed through Western blotting under the same treatment conditions. All treatments were performed for 24 h. The data are presented as mean ± SD (n = 3). A statistical analysis was conducted using one-way ANOVA followed by multiple comparisons. p ≤ 0.05 (*) and p ≤ 0.01 (**). ns: not significant.
3.2. Fractionation of Rainbow Trout Spleen Crude Extract and Anti-Inflammatory Effects of Each Fraction
To identify the bioactive anti-inflammatory components within the spleen crude extract, Solvent partitioning based on polarity was employed to obtain four fractions: OSH (O. mykiss Spleen crude extract n-Hexane layer), OSE (O. mykiss Spleen crude extract Ethyl acetate layer), OSB (O. mykiss Spleen crude extract n-Butanol layer), and OSW (O. mykiss Spleen crude extract Water layer) (Figure 2A). The chemical composition of each fraction was analyzed through ultra-performance liquid chromatography-mass spectrometry (UPLC-MS), revealing distinct profiles among the fractions (Figure 2B). To evaluate and compare the anti-inflammatory activity of each fraction, RAW264.7 cells were treated with 1 μg/mL LPS in the presence of either the crude spleen extract or individual fractions (OSH, OSE, OSB, or OSW) at a concentration of 200 μg/mL for 24 h. A quantitative PCR analysis was performed to assess the mRNA expression of iNOS, COX-2, and the pro-inflammatory cytokines IL-6 and TNF-α. LPS stimulation significantly increased the expression of all four genes. Co-treatment with the spleen crude extract, OSH, OSE, or OSB attenuated this upregulation to varying degrees. Among the tested fractions, OSH, OSE, and OSB exhibited stronger inhibitory effects on inflammatory gene expression than the unfractionated crude extract. However, microscopic examination revealed that OSH and OSE induced cytotoxic effects, as indicated by the presence of floating and detached cells. In contrast, OSB treatment significantly suppressed the LPS-induced expression of iNOS, COX-2, IL-6, and TNF-α without causing observable cytotoxicity (Figure 2C,D). These results suggest that the principal anti-inflammatory activity of the spleen crude extract is enriched in the OSB fraction, which exerts potent anti-inflammatory effects while maintaining cell viability.
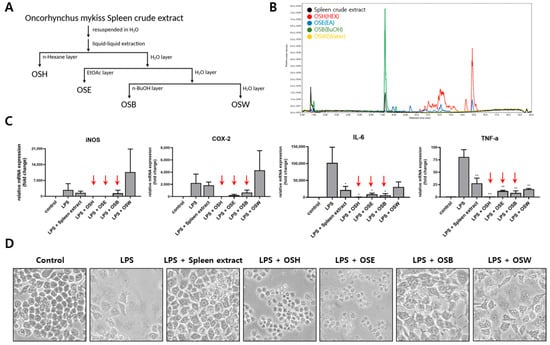
Figure 2.
Fractionation of rainbow trout spleen extract and evaluation of anti-inflammatory activity of each fraction. (A) The spleen crude extract was fractionated into four solvent layers (OSH, OSE, OSB, and OSW) through polarity-based liquid–liquid extraction and lyophilized. (B) Major constituents of each fraction were characterized via a UPLC-MS analysis. (C) RAW264.7 cells were treated with the crude extract or each fraction (200 μg/mL) in the presence of 1 μg/mL LPS, and the mRNA expression of iNOS, COX-2, IL-6, and TNF-α was quantified through qRT-PCR. The red arrows indicate the fractions that showed stronger anti-inflammatory effects than the crude extract. (D) Morphological changes in the RAW264.7 cells were observed via phase-contrast microscopy following treatment. The data are presented as mean ± SD (n = 3). Statistical significance was determined using one-way ANOVA with multiple comparisons. p ≤ 0.05 (*) and p ≤ 0.01 (**).
3.3. Regulatory Effect of OSB on iNOS and COX-2 Expression
Among the spleen-derived fractions, OSB exhibited the most potent and consistent anti-inflammatory activity, suggesting that the key bioactive constituents are predominantly enriched in this fraction. To further elucidate its mechanism of action, we examined the effects of OSB on iNOS and COX-2 protein expression in LPS-stimulated RAW264.7 cells. A Western blot analysis revealed that OSB treatment markedly suppressed the LPS-induced upregulation of iNOS and COX-2 protein levels in a dose-dependent manner (Figure 3A). In addition, a time-course experiment demonstrated that OSB effectively inhibited the time-dependent induction of both proteins following LPS stimulation (Figure 3B). Consistent with these results, an immunofluorescence analysis showed a marked decrease in the cytoplasmic fluorescence signals for iNOS and COX-2 following OSB and LPS co-treatment. In contrast, the cells treated with LPS alone exhibited strong fluorescence intensity, indicating robust protein expression (Figure 3C,D). Therefore, these results demonstrate that OSB effectively attenuates LPS-induced inflammatory responses by suppressing the expression of key pro-inflammatory enzymes—namely, iNOS and COX-2—in RAW264.7 macrophages.
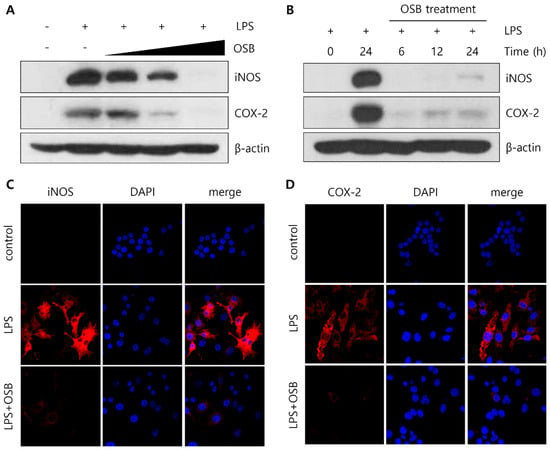
Figure 3.
Regulatory effects of the OSB fraction on iNOS and COX-2 expression in LPS-stimulated RAW264.7 cells. (A) The dose-dependent effects of OSB (100–400 μg/mL) on iNOS and COX-2 protein expression were analyzed through Western blotting. (B) Time-course analysis of iNOS and COX-2 expression following treatment with OSB (200 μg/mL) for 6–24 h in the presence of LPS. (C,D) Immunofluorescence analysis of iNOS and COX-2 protein expression after treatment with OSB (200 μg/mL) for 24 h. All experiments were conducted with LPS stimulation (1 μg/mL, 24 h). In the immunofluorescence analysis, cell nuclei were stained with DAPI (blue), and iNOS and COX-2 were visualized with red fluorescence.
3.4. Regulatory Effect of OSB on the NF-κB Signaling Pathway
To investigate the mechanism by which OSB regulates inflammatory responses, we examined its effects on the NF-κB signaling pathway, a key upstream regulator of iNOS and COX-2 expression that is activated through LPS stimulation. In RAW264.7 macrophages, OSB treatment inhibited the LPS-induced phosphorylation of IκBα, thereby preventing its degradation. This inhibition subsequently suppressed the phosphorylation of the NF-κB subunit p65, which is dependent on IκBα activation (Figure 4A). A Western blot analysis further demonstrated that the OSB-mediated suppression of p65 phosphorylation resulted in reduced nuclear translocation of p65 following LPS stimulation (Figure 4B). Supporting these findings, an immunofluorescence analysis showed that p65 was translocated to the nucleus upon LPS stimulation, while OSB co-treatment blocked this translocation (Figure 4C). These results indicate that OSB attenuates LPS-induced inflammatory signaling by modulating the NF-κB pathway, thereby inhibiting the activation and nuclear translocation of p65.
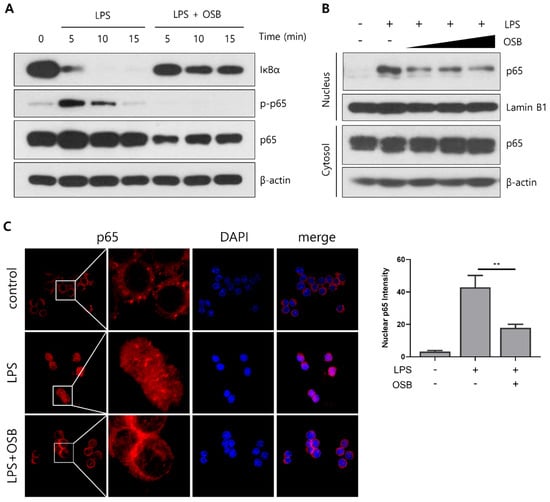
Figure 4.
Inhibitory effects of OSB on NF-κB signaling pathway activation. (A) Western blot analysis of IκBα and p65 phosphorylation in RAW264.7 cells treated with OSB (200 μg/mL) in the presence of LPS. (B) The nuclear translocation of p65 was examined after treatment with OSB (100–400 μg/mL) for 2 h. (C) Immunofluorescence analysis of p65 localization following OSB treatment (400 μg/mL, 2 h). The nuclear p65 fluorescence intensity was quantified using ImageJ. All experiments included LPS stimulation (1 μg/mL). In the immunofluorescence analysis, cell nuclei were stained with DAPI (blue), and p65 was visualized with red fluorescence. p ≤ 0.01 (**).
3.5. Regulatory Effect of OSB on M1 Macrophage Polarization
The effects of OSB on macrophage polarization were assessed by analyzing the mRNA expression levels of pro- (IL-6, TNF-α, IL-23p19, and IL-12p40) and anti-inflammatory (IL-13 and TGF-β) cytokines using quantitative PCR. OSB treatment significantly suppressed the LPS-induced upregulation of the pro-inflammatory cytokines, while simultaneously enhancing the expression of the anti-inflammatory cytokines IL-13 and TGF-β. Furthermore, the expression of the M1 macrophage surface markers CD40 and CD86 was evaluated. LPS-induced increases in CD40 and CD86 mRNA levels were notably attenuated by the OSB treatment (Figure 5A). Protein-level changes in CD86 expression were confirmed through immunofluorescence staining, which demonstrated marked upregulation of CD86 on RAW264.7 cell surfaces following LPS stimulation; this increase was effectively inhibited by OSB (Figure 5B). Additionally, intracellular reactive oxygen species (ROS) levels were measured using the fluorescent probe H2DCFDA. In the graph, the x-axis represents fluorescence intensity detected in the FITC channel, displayed on a logarithmic scale. This intensity corresponds to the relative intracellular ROS levels, with higher values indicating greater ROS accumulation. The y-axis indicates the number of cells falling within each fluorescence intensity bin. OSB treatment dose-dependently reduced LPS-induced ROS production in RAW264.7 cells (Figure 5C). Collectively, these results indicate that OSB effectively suppresses LPS-induced M1 macrophage polarization by modulating cytokine expression, surface marker levels, and oxidative stress.
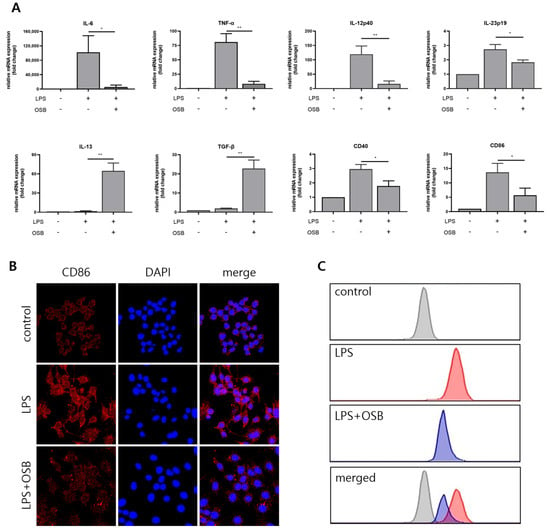
Figure 5.
Effects of OSB on LPS-induced M1 macrophage polarization. (A) qRT-PCR analysis of pro-inflammatory cytokines (IL-6, TNF-α, IL-12p40, and IL-23p19), anti-inflammatory cytokines (IL-13 and TGF-β), and M1 surface markers (CD40 and CD86) following OSB treatment (200 μg/mL, 24 h). (B) Immunofluorescence analysis of CD86 expression in RAW264.7 cells treated with OSB (200 μg/mL). In the immunofluorescence analysis, cell nuclei were stained with DAPI (blue), and CD86 was visualized with red fluorescence. (C) The intracellular reactive oxygen species (ROS) levels were measured using H2DCFDA after OSB treatment (400 μg/mL, 24 h). All cells were stimulated with 1 μg/mL LPS. The data are expressed as mean ± SD (n = 3), and statistical significance was assessed through one-way ANOVA with multiple comparisons. p ≤ 0.05 (*) and p ≤ 0.01 (**).
4. Discussion
The development of therapeutics derived from natural products for inflammatory diseases has garnered increasing attention in recent years [32,33,34]. A critical aspect of anti-inflammatory drug development involves elucidating the regulatory mechanisms that govern inflammation-associated signaling pathways [35]. Among these pathways, the TLR4/NF-κB pathway has been identified as a central target in the treatment of inflammatory disorders [36]. While several studies have explored the anti-inflammatory potential of fish by-product extracts, including those from the heads, bones, and fins, the biological activity of viscera-derived extracts remains largely underexplored [21,22,23]. Notably, extracts from the viscera of marine organisms such as Turbo cornutus and abalone have demonstrated anti-inflammatory activity by suppressing MAPK signaling components, including JNK and p38 phosphorylation [35,37].
Rainbow trout viscera have traditionally been considered industrial waste; however, recent efforts have focused on repurposing these by-products as valuable sources of bioactive compounds [38]. In this study, we investigated the anti-inflammatory effects of crude extracts from the liver, gallbladder, spleen, and kidneys of rainbow trout, with a focus on identifying active constituents with therapeutic potential. The initial evaluation was conducted via NO assays, as macrophages produce NO as a hallmark of LPS-induced inflammatory responses. Among the organ extracts tested, the spleen extract exhibited a dose-dependent inhibition of NO production. Furthermore, this extract significantly reduced both the mRNA and protein expression levels of iNOS as well as COX-2, another key inflammatory mediator. These results suggest that the spleen extract exerts a more pronounced anti-inflammatory effect relative to other tissue-derived extracts.
To isolate the bioactive constituents responsible for these effects, the spleen crude extract underwent solvent fractionation via liquid–liquid extraction based on differential polarity. This process yielded four fractions: OSH, OSE, OSB, and OSW. UPLC-MS analysis revealed distinct differences in the abundance of major components among the spleen crude extract and its fractions (OSH, OSE, OSB, and OSW). These findings indicate that the crude extract was effectively fractionated based on polarity, resulting in compositional variations across the different fractions. Each fraction, along with the crude extract, was evaluated for its ability to modulate the mRNA expression of inflammatory mediators (iNOS, COX-2, IL-6, and TNF-α). The OSH, OSE, and OSB fractions exhibited greater inhibitory activity than the crude extract. However, cytotoxic effects, including cell detachment and lysis, were observed following treatment with OSH and OSE. In contrast, the OSB fraction demonstrated strong and consistent anti-inflammatory activity without cytotoxicity, suggesting that it contains enriched bioactive compounds from the rainbow trout spleen.
A subsequent analysis confirmed that OSB treatment suppressed the LPS-induced expression of iNOS and COX-2 in a dose-dependent manner at the protein level. Time-course experiments revealed that although OSB suppressed these inflammatory proteins, their levels gradually increased over time, indicating partial rather than complete inhibition. These effects were validated through both Western blotting and immunofluorescence microscopy. Notably, OSB treatment reduced the cytoplasmic fluorescence intensity of iNOS and COX-2 induced by LPS, consistent with the biochemical results. These results align with the reported anti-inflammatory effects of natural compounds, such as resveratrol and quercetin [39,40], which similarly downregulate iNOS and COX-2 expression. Taken together, our findings provide mechanistic evidence supporting the anti-inflammatory activity of the OSB fraction.
Given the pivotal role of NF-κB signaling in regulating the transcription of iNOS, COX-2, and other pro-inflammatory genes, we further investigated the upstream mechanisms modulated by OSB. LPS stimulation leads to IκBα phosphorylation and degradation, facilitating the nuclear translocation of the NF-κB p65 subunit. OSB treatment inhibited both IκBα phosphorylation/degradation and p65 activation, thereby preventing its translocation to the nucleus. Western blotting and immunofluorescence analyses confirmed this suppression, demonstrating a reduction in nuclear p65 localization upon OSB treatment. Notably, this mode of action mirrors that of curcumin—a well-characterized natural compound known to suppress NF-κB activation [41]. Collectively, these findings suggest that OSB exerts its anti-inflammatory effects by preventing NF-κB activation and nuclear translocation, thereby downregulating the transcription of pro-inflammatory mediators.
Macrophage polarization is another critical determinant of inflammatory responses [42]. Upon LPS stimulation, macrophages differentiate into the pro-inflammatory M1 phenotype, characterized by the increased expression of surface markers (CD40 and CD86), the elevated production of pro-inflammatory cytokines, and enhanced ROS generation [43]. Our results demonstrated that OSB significantly downregulated the LPS-induced mRNA expression of IL-6, TNF-α, IL-12p40, and IL-23p19. In parallel, it upregulated anti-inflammatory cytokines, such as IL-13 and TGF-β. OSB also suppressed the expression of M1 surface markers CD40 and CD86 at both the mRNA and protein levels. The immunofluorescence analysis further confirmed reduced CD86 surface expression. Moreover, OSB effectively diminished LPS-induced ROS generation, suggesting inhibition of oxidative stress—a hallmark of M1 polarization. These results collectively support the notion that OSB suppresses M1 polarization in LPS-stimulated RAW264.7 macrophages.
This study evaluated the anti-inflammatory activity of the OSB fraction under in vitro conditions. Although OSB effectively modulated iNOS and COX-2 expression through inhibition of the NF-κB pathway, the in vitro nature of this work limits direct conclusions regarding its therapeutic efficacy in complex physiological systems. Therefore, future studies employing in vivo animal models are necessary to assess the anti-inflammatory potency and safety profile of OSB under biologically relevant conditions. Moreover, OSB remains a crude extract, and the relatively high concentrations used in this study (up to 400 μg/mL) may not be feasible for clinical application. Accordingly, further work is required to chemically characterize the OSB fraction and isolate its active constituents. The identification of these bioactive components will be essential for elucidating the mechanisms of action and evaluating their pharmacological relevance in vivo.
Nevertheless, the anti-inflammatory properties demonstrated by OSB in this study are consistent with those reported for natural compounds such as resveratrol, quercetin, and curcumin [39,40,41]. These findings support the potential of OSB as a promising bioactive candidate and provide a strong rationale for further investigation.
5. Conclusions
This study demonstrated that the OSB fraction, derived from the spleen extract of rainbow trout (O. mykiss), exhibits potent anti-inflammatory activity without inducing cytotoxic effects. OSB effectively attenuated LPS-induced M1 macrophage polarization by downregulating the expression of iNOS, COX-2, pro-inflammatory cytokines, M1 surface markers, and intracellular ROS levels, primarily via inhibition of the NF-κB signaling pathway. However, as the current findings are based on in vitro experiments, further in vivo studies are required to validate the therapeutic efficacy and safety of OSB in physiological contexts. Additionally, the chemical characterization of OSB is necessary to identify its active constituents and to further elucidate their mechanisms of action. Collectively, these results provide mechanistic insight into the anti-inflammatory potential of fish viscera-derived bioactive compounds and suggest that the OSB fraction may serve as a promising candidate for the development of natural anti-inflammatory therapeutics.
Author Contributions
Conceptualization: D.-Y.K. and K.-C.K. Methodology: D.-Y.K., H.Y. and K.-C.K. Investigation: D.-Y.K., W.-S.C. and J.-H.P. Resources: S.K., H.-H.N., H.Y. and K.-C.K. Data curation: D.-Y.K., J.P. and W.S. Original draft preparation: D.-Y.K. Writing review and editing: D.-Y.K. and K.-C.K. Supervision: K.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the National Research Foundation of Korea (2016R1D1A3B02006754) Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wallach, D.; Kang, T.-B.; Kovalenko, A. Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat. Rev. Immunol. 2014, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- James, D.S. The multisystem adverse effects of NSAID therapy. J. Am. Osteopath. Assoc. 1999, 99, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, Y.H.; Zhu, Y.T.; Li, M.X.; Pei, H.H. Requirement of Rab21 in LPS-induced TLR4 signaling and pro-inflammatory responses in macrophages and monocytes. Biochem. Biophys. Res. Commun. 2019, 508, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Huang, J.; Zhou, P.; Li, L.; Zheng, Q.; Fu, H. The role of TLR4/NF-kB signaling axis in pneumonia: From molecular mechanisms to regulation by phytochemicals. Naunyn-Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef] [PubMed]
- Dejban, P.; Nikravangolsefid, N.; Chamanara, M.; Dehpour, A.; Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother. Res. 2021, 35, 835–845. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. Primer: Toll-like receptor signaling pathways--what do rheumatologists need to know? Nat. Clin. Pract. Rheumatol. 2008, 4, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Winand, L.; Sester, A.; Nett, M. Bioengineering of Anti-Inflammatory Natural Products. ChemMedChem 2021, 16, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-X.; Zhou, M.; Ma, H.-L.; Qiao, Y.-B.; Li, Q.-S. The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products. ChemMedChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Jachak, S.M. Recent developments in anti-inflammatory natural products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. Review on Anti-Inflammatory Activity of Natural Products. ChemistrySelect 2025, 10, e05885. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Liceaga, A.M. Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates. Food Bioprocess. Technol. 2017, 10, 582–591. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Lee, S.-J.; Im, J.; Marasinghe, S.D.; Jo, E.; Bandara, M.S.; Lee, Y.; Lee, J.; Park, G.-H.; Oh, C. Antioxidant and Anti-Inflammatory Activities of Cutlassfish Head Peptone in RAW 264.7 Macrophages. Antioxidants 2025, 14, 286. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.A.C.K.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.-G.; Jeon, Y.-J.; Kang, S.I. Pepsin Hydrolysate from Surimi Industry-Related Olive Flounder Head Byproducts Attenuates LPS-Induced Inflammation and Oxidative Stress in RAW 264.7 Macrophages and In Vivo Zebrafish Model. Mar. Drugs 2024, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Saisavoey, T.; Sangtanoo, P.; Reamtong, O.; Karnchanatat, A. Free radical scavenging and anti-inflammatory potential of a protein hydrolysate derived from salmon bones on RAW 264.7 macrophage cells. J. Sci. Food Agric. 2019, 99, 5112–5121. [Google Scholar] [CrossRef] [PubMed]
- Belmontesi, M. Polydeoxyribonucleotide for the improvement of a hypertrophic retracting scar—An interesting case report. J. Cosmet. Dermatol. 2020, 19, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.-G.; Hwang, J.J.; Chang, B.S.; Kim, S.-H.; Jin, J.-J.; Hwang, L.; Kim, C.-J.; Choi, C.W. Polydeoxyribonucleotide ameliorates lipopolysaccharide-induced acute lung injury via modulation of the MAPK/NF-κB signaling pathway in rats. Int. Immunopharmacol. 2020, 83, 106444. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Kim, M.; Kim, S.H.; Cho, S.-R.; Kim, H.J. Anti-inflammatory Effect of DNA Polymeric Molecules in a Cell Model of Osteoarthritis. Inflammation 2018, 41, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Heo, S.Y.; Oh, G.W.; Heo, S.J.; Jung, W.K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Mar. Drugs 2021, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Wang, S.-L.; Nguyen, V.B. Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, W.; Chen, Y.; Wan, X.; Wang, J. Fucoxanthin: A promising compound for human inflammation-related diseases. Life Sci. 2020, 255, 117850. [Google Scholar] [CrossRef] [PubMed]
- Almutary, A.G.; Begum, M.Y.; Kyada, A.K.; Gupta, S.; Jyothi, S.R.; Chaudhary, K.; Sharma, S.; Sinha, A.; Abomughaid, M.M.; Imran, M.; et al. Inflammatory signaling pathways in Alzheimer’s disease: Mechanistic insights and possible therapeutic interventions. Ageing Res. Rev. 2025, 104, 102548. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Kang, N.; Kim, J.; Yang, H.W.; Ahn, G.; Heo, S.J. Anti-Inflammatory Effect of Turbo cornutus Viscera Ethanolic Extract against Lipopolysaccharide-Stimulated Inflammatory Response via the Regulation of the JNK/NF-kB Signaling Pathway in Murine Macrophage RAW 264.7 Cells and a Zebrafish Model: A Preliminary Study. Foods 2022, 11, 364. [Google Scholar] [CrossRef]
- Cao, J.; Li, Q.; Shen, X.; Yao, Y.; Li, L.; Ma, H. Dehydroepiandrosterone attenuates LPS-induced inflammatory responses via activation of Nrf2 in RAW264.7 macrophages. Mol. Immunol. 2021, 131, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Ryu, B.; Sun Park, W.; Choi, I.L.W.; Jung, W.-K. Inhibitory Effects and Molecular Mechanism of an Anti-inflammatory Peptide Isolated from Intestine of Abalone, Haliotis Discus Hannai on LPS-Induced Cytokine Production via the p-p38/p-JNK Pathways in RAW264.7 Macrophages. J. Food Nutr. Res. 2025, 4, 690–698. [Google Scholar]
- Nikoo, M.; Benjakul, S.; Yasemi, M.; Ahmadi Gavlighi, H.; Xu, X. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-product with different pretreatments: Antioxidant activity and their effect on lipid and protein oxidation of raw fish emulsion. LWT 2019, 108, 120–128. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-F.; Chen, G.-W.; Chen, Y.-C.; Shen, C.-K.; Lu, D.-Y.; Yang, L.-Y.; Chen, J.-H.; Yeh, W.-L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. Nutrients 2022, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Dehnavi, S.; Asadirad, A.; Xu, S.; Majeed, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Curcumin and chemokines: Mechanism of action and therapeutic potential in inflammatory diseases. Inflammopharmacology 2023, 31, 1069–1093. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Dickinson, A.; Nakuleswaran, P.; Maghami, S.; Alagoda, S.; Hook, A.L.; Ghaemmaghami, A.M. Targeting Macrophage Polarization for Reinstating Homeostasis following Tissue Damage. Int. J. Mol. Sci. 2024, 25, 7278. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 2946. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).