Clinician-Based Functional Scoring and Genomic Insights for Prognostic Stratification in Wolf–Hirschhorn Syndrome
Abstract
1. Introduction
2. Materials and Methods
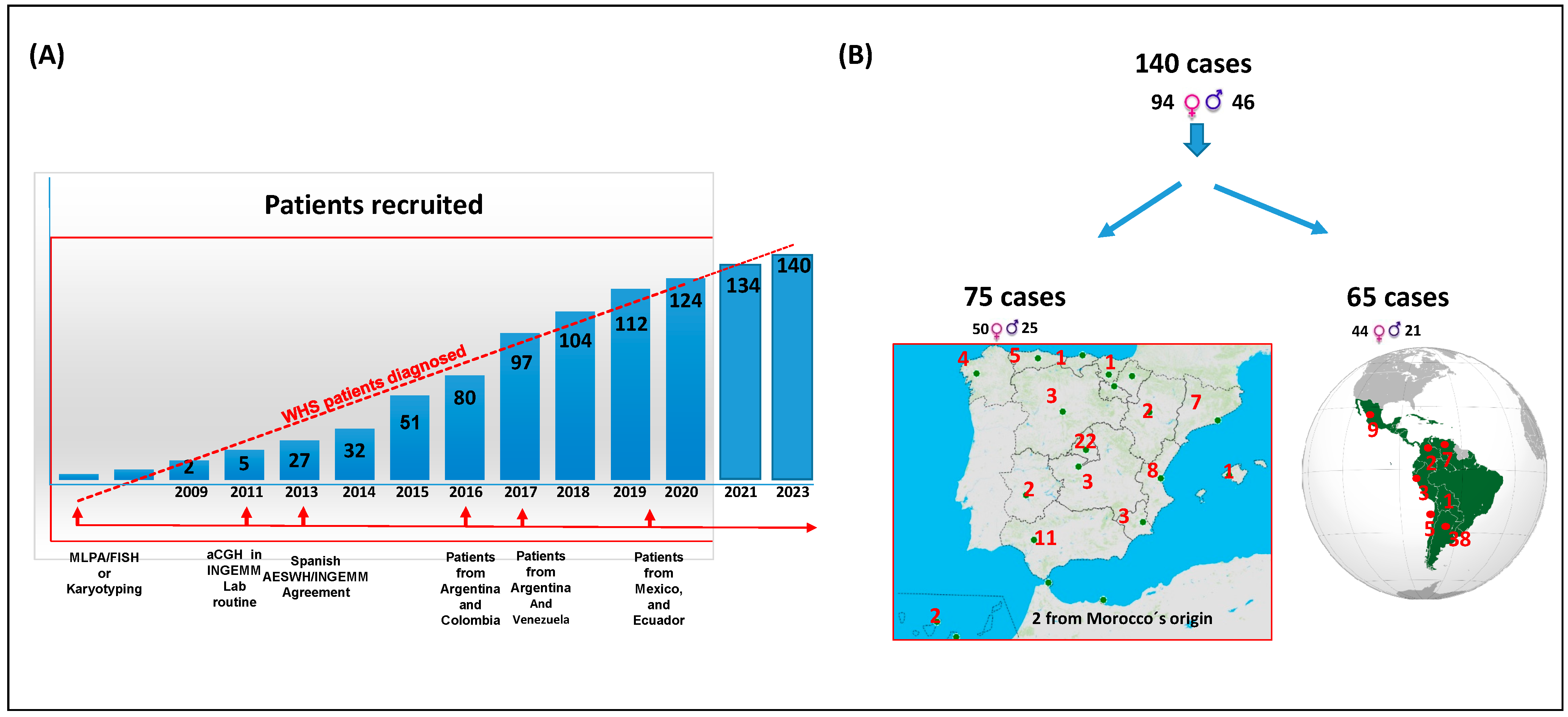
2.1. Cohort Description
2.2. Clinical Data Collection
2.3. Genetic Characterization
2.4. Construction of the GFAP Score
2.5. Statistical Analyses
3. Results
3.1. Cohort Characteristics
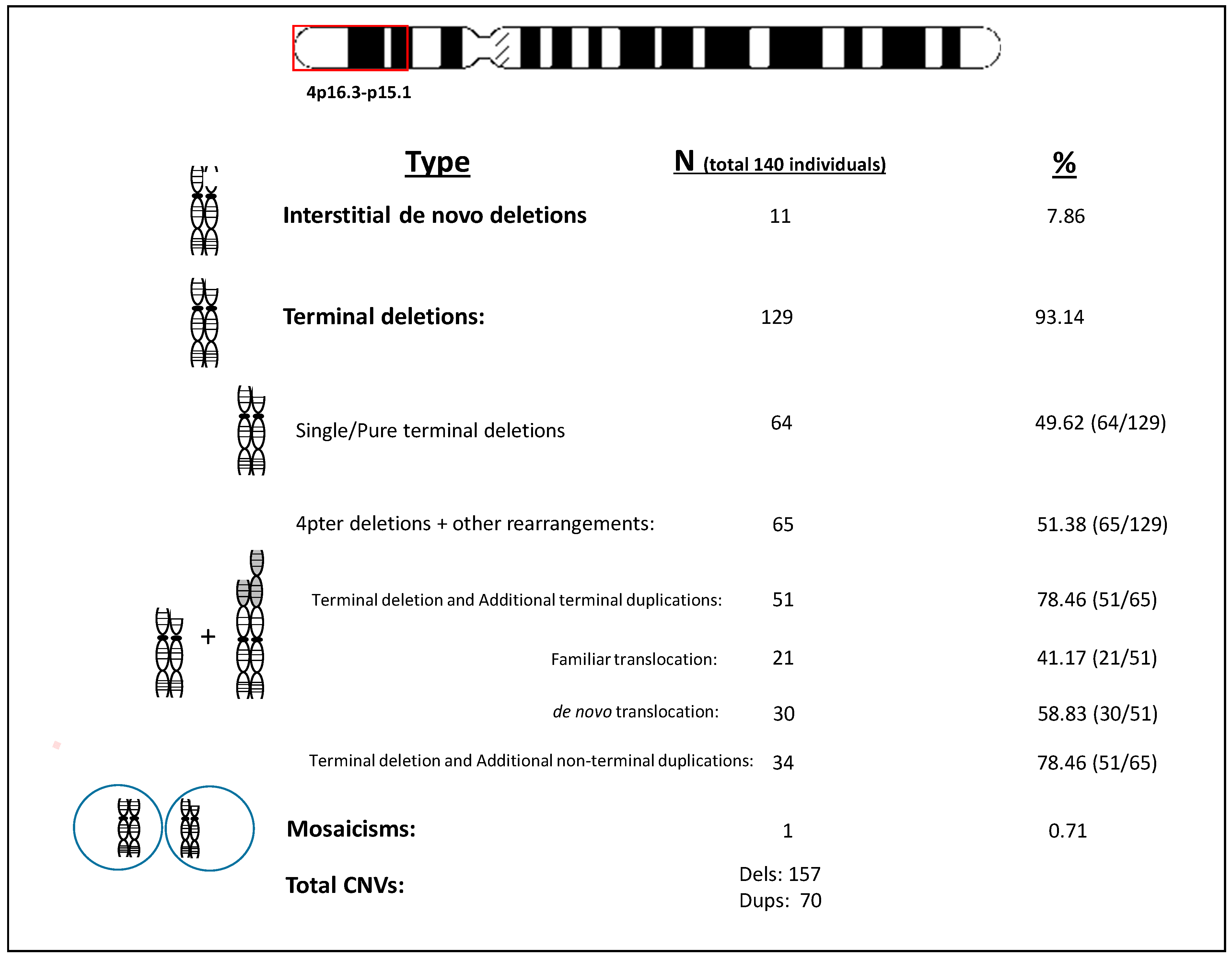
3.2. Genetic Findings
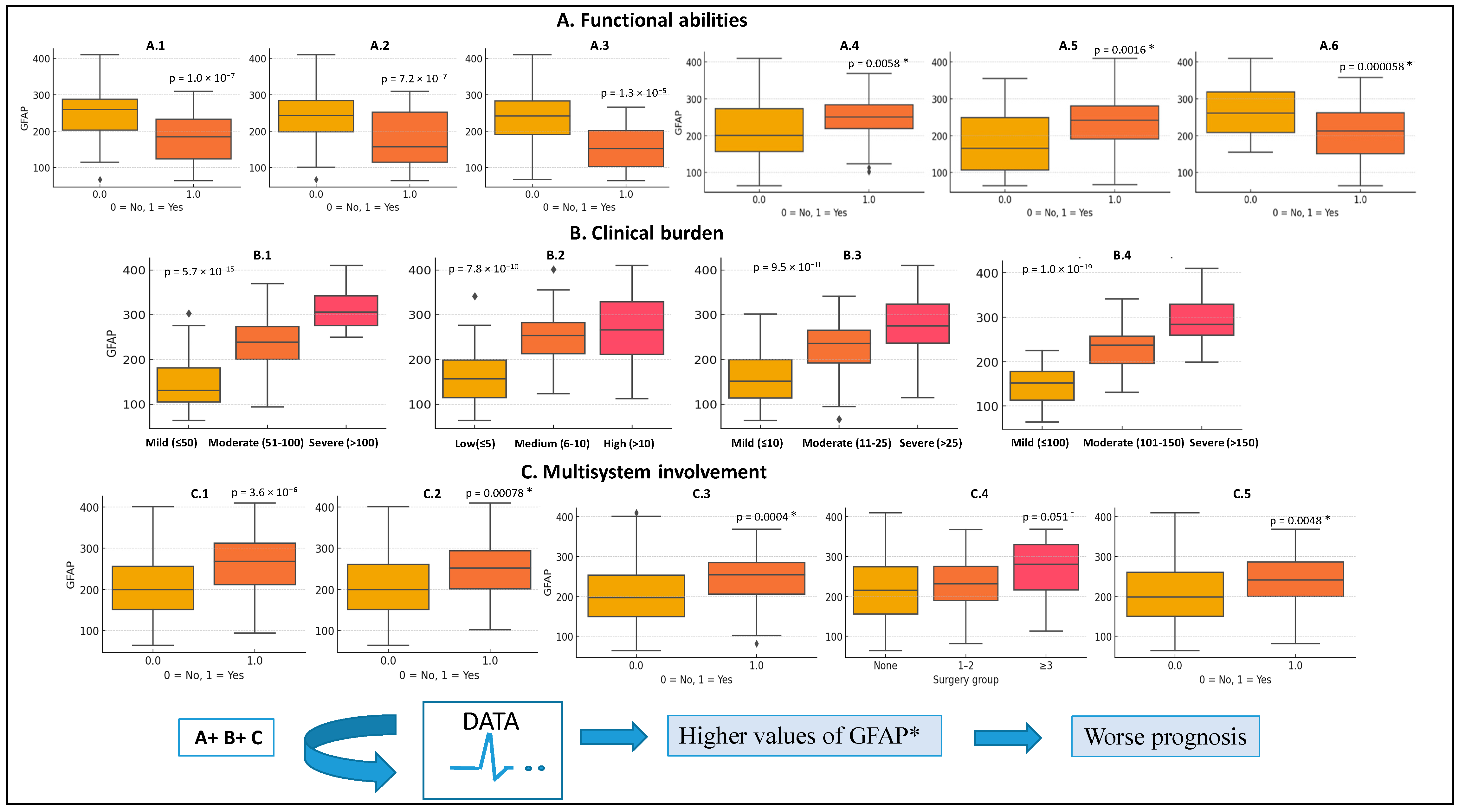
3.3. GFAP Score Distribution and Correlates
3.4. Genotype/Phenotype Analysis
3.5. Ward’s Cluster Analysis Using the Size of the Deletion in the Whole Cohort
3.6. Unsupervised Hierarchical Clusterization and Discriminate Analysis
4. Discussion
5. Future Address
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, T.J.; Ricke, D.O.; Denison, K.; Abmayr, S.; Cotter, P.D.; Hirschhorn, K.; Keinänen, M.; McDonald-McGinn, D.; Somer, M.; Spinner, N.; et al. A transcript map of the newly defined 165 kb Wolf-Hirschhorn syndrome critical region. Hum. Mol. Genet. 1997, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zollino, M.; Lecce, R.; Fischetto, R.; Murdolo, M.; Faravelli, F.; Selicorni, A.; Buttè, C.; Memo, L.; Capovilla, G.; Neri, G. Mapping the Wolf-Hirschhorn syndrome phenotype outside the currently accepted WHS critical region and defining a new critical region, WHSCR-2. Am. J. Hum. Genet. 2003, 72, 590–597. [Google Scholar] [CrossRef]
- Battaglia, A.; Carey, J.C.; South, S.T. Wolf-Hirschhorn syndrome: A review and update. Am. J. Med. Genet. Part C 2015, 169, 216–223. [Google Scholar] [CrossRef]
- Zollino, M.; Doronzio, P.N. Dissecting the Wolf- Hirschhorn syndrome phenotype: WHSC1 is a neurodevelopmental gene contributing to growth delay, intellectual disability, and to the facial dysmorphism. J. Hum. Genet. 2018, 63, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Gandelman, K.Y.; Gibson, L.; Meyn, M.S.; Yang-Feng, T.-L. Molecular definition of the smallest region of deletion overlap in the Wolf-Hirschhorn syndrome. Am. J. Hum. Gene 1992, 51, 571–578. [Google Scholar]
- South, S.T.; Bleyl, S.B.; Carey, J.C. Two unique patients with novel microdeletions in 4p16.3 that exclude the WHS critical regions: Implications for critical region designation. Am. J. Med. Genet. Part A 2007, 143, 2137–2142. [Google Scholar] [CrossRef]
- South, S.T.; Hannes, F.; Fisch, G.S.; Vermeesch, J.R.; Zollino, M. Pathogenic significance of deletions distal to the currently described Wolf-Hirschhorn syndrome critical regions on 4p16.3. Am. J. Med. Genet. Part C 2008, 148, 270–274. [Google Scholar] [CrossRef]
- Corrêa, T.; Mergener, R.; Leite, J.C.L.; Galera, M.F.; Moreira, L.M.D.A.; Vargas, J.E.; Riegel, M. Cytogenomic Integrative Network Analysis of the Critical Region Associated with Wolf-Hirschhorn Syndrome. Biomed. Res. Int. 2018, 2018, 5436187. [Google Scholar] [CrossRef]
- da Silva Mori, X.; Cristina, B.F.; Diéguez, M.; MLÁ, M.Á. Prevalence and geographic distribution of the Wolf-Hirschhorn syndrome in Spain. Rev. Esp. Salud Publica 2022, 96, e202206045. [Google Scholar]
- Taruscio, D.; Vittozi, L.; Rocchetti, A.; Torreri, P.; Ferrari, L. The Occurrence of 275 Rare Diseases and 47 Rare Disease Groups in Italy. Results from the National Registry of Rare Diseases. Int. J. Environ. Res. Public Health 2018, 15, 1470. [Google Scholar] [CrossRef]
- Martínez-Martín, P.; Jiménez-Jiménez, F.J.; García, E.C.; Alonso-Navarro, H.; Rubio, L.; Calleja, P.; Díaz-Sánchez, M.; Benito-León, J. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J. Clin. Epidemiol. 2010, 63, 767–773. [Google Scholar] [CrossRef]
- Martinez-Martin, P. Composite rating scales. J. Neurol. Sci. 2010, 289, 7–11. [Google Scholar] [CrossRef]
- Weldring, T.; Smith, S.M. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv. Insights 2013, 6, 61–68. [Google Scholar] [PubMed]
- Relja, M. Clinical rating scales. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S229–S232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Powers, J.H.; Patrick, D.L.; Walton, M.K.; Marquis, P.; Cano, S.; Hobart, J.; Isaac, M.; Vamvakas, S.; Slagle, A.; Molsen, E.; et al. Clinician-Reported Outcome Assessments of Treatment Benefit: Report of the ISPOR Clinical Outcome Assessment Emerging Good Practices Task Force John. Value Health 2017, 20, 2–14. [Google Scholar] [CrossRef]
- Austin, C.P.; Cutillo, C.M.; Lau, L.P.; Jonker, A.H.; Rath, A.; Julkowska, D.; Thomson, D.; Terry, S.F.; de Montleau, B.; Ardigò, D.; et al. Future of Rare Diseases Research 2017–2027: An IRDiRC Perspective. Clin. Transl. Sci. 2018, 11, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorder Society UPDRS Revision Task Force. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 5662. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Shannon, N.L.; Maltby, E.L.; Rigby, A.S.; Quarrell, O.W.J. An epidemiological study of Wolf-Hirschhorn syndrome: Life expectancy and cause of mortality. J. Med. Genet. 2001, 38, 674–679. [Google Scholar] [CrossRef]
- Battaglia, A.; Carey, J.C.; Wright, T.J. Wolf-Hirschhorn (4P-) syndrome in adults. Genet. Couns. 2001, 12, 35–48. [Google Scholar]
- Battaglia, A.; Carey, J.C. Health supervision and anticipatory guidance of individuals with Wolf-Hirschhorn syndrome. Am. J. Med. Genet. Part A 1999, 89, 111–115. [Google Scholar] [CrossRef]
- Carey, J.C.; Lortz, A.; Mendel, A.; Battaglia, A. Natural history study of adults with Wolf-Hirschhorn syndrome 2: Patient-reported outcomes study. Am. J. Med. Genet. Part A 2021, 185, 2065–2069. [Google Scholar] [CrossRef]
- Battaglia, A.; Carey, J.C. The delineation of the Wolf-Hirschhorn syndrome over six decades: Illustration of the ongoing advances in phenotype analysis and cytogenomic technology. Am. J. Med. Genet. Part A 2021, 185, 2748–2755. [Google Scholar] [CrossRef]
- Battaglia, A. Deletion 4p: Wolf-Hirschhorn syndrome. In Cassidy and Allanson’s Management of Genetic Syndromes, 4th ed.; Carey, J.C., Battaglia, A., Viskochil, D., Cassidy, S.B., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2021; pp. 265–280. 4p. [Google Scholar]
- Martínez-Quintana, E.; Rodríguez-González, F. Clinical features in adult patient with Wolf-Hirschhorn syndrome. Morphologie 2014, 98, 86–89. [Google Scholar] [CrossRef]
- Blanco-Lago, R.; Malaga-Dieguez, I.; Granizo-Martinez, J.J.; Carrera-Garcia, L.; Barruz-Galian, P.; Lapunzina, P.; Nevado-Blanco, J. Wolf-Hirschhorn syndrome. Description of a Spanish cohort of 51 cases and a literature review. Rev. Neurol. 2017, 64, 393–400. [Google Scholar] [CrossRef]
- Nevado, J.; Ho, K.S.; Zollino, M.; Blanco, R.; Cobaleda, C.; Golzio, C.; Beaudry-Bellefeuille, I.; Berrocoso, S.; Limeres, J.; Barrúz, P.; et al. International meeting on Wolf-Hirschhorn syndrome: Update on the nosology and new insights on the pathogenic mechanisms for seizures and growth delay. Am. J. Med. Genet. Part A 2020, 182, 257–267. [Google Scholar] [CrossRef]
- Cammarata-Scalisi, F.; Araque, D.; Da Silva, G.; Lacruz-Rengel, M.A.; Avendaño, A. Wolf-Hirschhorn syndrome. Description of five cases characterized by means of single nucleotide polymorphism microarrays. Arch. Argent. Pediatr. 2019, 117, e406–e412. [Google Scholar] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Hierarchical Clustering. Stat. Sci. 2001, 16, 222–248. [Google Scholar]
- Wieczorek, D.; Krause, M.; Majewski, F.; Albrecht, B.; Horn, D.; Riess, O.; Gillessen-Kaesbach, G. Effect of the size of the deletion and clinical manifestation in Wolf–Hirschhorn syndrome: Analysis of 13 patients with a de novo deletion. Eur. J. Hum. Genet. 2000, 8, 519–526. [Google Scholar] [CrossRef]
- Zollino, M.; Di Stefano, C.; Zampino, G.; Mastroiacovo, P.; Wright, T.J.; Sorge, G.; Selicorni, A.; Tenconi, R.; Zappalà, A.; Battaglia, A.; et al. Genotype-phenotype correlations and clinical diagnostic criteria in Wolf-Hirschhorn syndrome. Am. J. Med. Genet. Part A 2000, 94, 254–261. [Google Scholar] [CrossRef]
- Zollino, M.; Murdolo, M.; Marangi, G.; Pecile, V.; Galasso, C.; Mazzanti, L.; Neri, G. On the nosology and pathogenesis of Wolf-Hirschhorn syndrome: Genotype-phenotype correlation analysis of 80 patients and literature review. Am. J. Med. Genet C 2008, 148, 257–269. [Google Scholar] [CrossRef]
- Shimizu, K.; Wakui, K.; Kosho, T.; Okamoto, N.; Mizuno, S.; Itomi, K.; Hattori, S.; Nishio, K.; Samura, O.; Kobayashi, Y.; et al. Microarray and FISH-based genotype-phenotype analysis of 22 Japanese patients with Wolf-Hirschhorn syndrome. Am. J. Med. Genet. A 2014, 164, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Maas, N.M.C.; Van Buggenhout, G.; Hannes, F.; Thienpont, B.; Sanlaville, D.; Kok, K.; Midro, A.; Andrieux, J.; Anderlid, B.M.; Schoumans, J.; et al. Genotype-phenotype correlation in 21 patients with Wolf-Hirschhorn syndrome using high resolution array comparative genome hybridization (CGH). J. Med. Genet. 2008, 45, 71–80. [Google Scholar] [CrossRef]
- Mekkawy, M.K.; Kamel, A.K.; Thomas, M.M.; Ashaat, E.A.; Zaki, M.S.; Eid, O.M.; Ismail, S.; Hammad, S.A.; Megahed, H.; ElAwady, H.; et al. Clinical and genetic characterization of ten Egyptian patients with Wolf-Hirschhorn syndrome and review of literature. Mol. Genet. Genomic Med. 2021, 9, e1546. [Google Scholar] [CrossRef]
- Chaudhry, C.; Kaur, A.; Panigrahi, I.; Kaur, A. Wolf-Hirschhorn syndrome: A case series from India. Am. J. Med. Genet. A 2020, 182, 3048–3051. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, A.; Filippi, T.; Carey, J.C. Update on the clinical features and natural history of Wolf-Hirschhorn (4p-) syndrome: Experience with 87 patients and recommendations for routine health supervision. Am. J. Med. Genet. Part C 2008, 148, 246–251. [Google Scholar] [CrossRef]
- Wieczorek, D.; Krause, M.; Majewski, F.; Albrecht, B.; Meinecke, P.; Riess, O.; Gillessen-Kaesbach, O. Unexpected high frequency of the novo unbalanced translocation in patients with Wolf –Hirschhorn syndrome (WHS). J. Med. Genet. 2000, 37, 798–804. [Google Scholar] [CrossRef][Green Version]
- South, S.T.; Whitby, H.; Battaglia, A.; Carey, J.C.; Brothman, A.R. Comprehensive analysis of Wolf-Hirschhorn syndrome using array CGH indicates a high prevalence of translocations. Eur. J. Hum. Genet. 2008, 16, 45–52. [Google Scholar] [CrossRef]
- Antonius, T.; Draaisma, J.; Levichenko, E.; Knoers, N.; Renier, W.; Van Ravensawaaii, C. Growth charts for Wolf-Hirschhorn (4p-) syndrome (0-4 years of age). Eur. J. Pediatr. 2008, 167, 807–810. [Google Scholar] [CrossRef]
- Calhoun, A.R.U.L.; Lortz, T.; Casper, C.; Carey, J. Extended Growth Curves for the Wolf-Hirschhorn Syndrome (4p-). Am. J. Med. Genet. A 2025, 197, e64075. [Google Scholar] [CrossRef] [PubMed]
- Kerzendorfer, C.; Hannes, F.; Colnaghi, R.; Abramowicz, I.; Carpenter, G.; Vermeesch, J.R.; O’Driscoll, M. Characterizing the functional consequences of haploinsufficiency of NELF-A (WHSC2) and SLBP identifies novel cellular phenotypes in Wolf-Hirschhorn syndrome. Hum. Mol. Genet. 2012, 21, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Nevado, J.; Bel-Fenellós, C.; Sandoval-Talamantes, A.K.; Hernández, A.; Biencinto-López, C.; Martínez-Fernández, M.L.; Barrúz, P.; Santos-Simarro, F.; Mori-Álvarez, M.Á.; Mansilla, E.; et al. Deep Phenotyping and Genetic Characterization of a Cohort of 70 Individuals With 5p Minus Syndrome. Front Genet 2021, 12, 645595. [Google Scholar] [CrossRef] [PubMed]
- Nevado, J.; García-Miñaúr, S.; Palomares-Bralo, M.; Vallespín, E.; Guillén-Navarro, E.; Rosell, J.; Bel-Fenellós, C.; Mori, M.Á.; Milá, M.; Del Campo, M.; et al. Variability in Phelan-McDermid Syndrome in a Cohort of 210 Individuals. Front. Genet. 2022, 13, 652454. [Google Scholar] [CrossRef]
- Nevado, J.; Escalada, B.; Muñoz-GªPorrero, Y.; Adan, C.; Tenorio-Castaño, J.; Lapunzina, P.D. Genotype-Phenotype Associations in Phelan-McDermid Syndrome: Insights into Novel Genes beyond SHANK3. Int. J. Mol. Sci. 2025, 26, 4653. [Google Scholar] [CrossRef]
| Mean | Median | Range | |
|---|---|---|---|
| GFAP | 227.23 ± 75.64 | 235.00 | 64–410 |
| i.- Developmental delay milestones corrected by age | 19.78 ± 11.97 | 16.00 | 2–45 |
| ii.- Comorbidities | 8.15 ± 5.26 | 7.00 | 0–45 |
| iii.- Several items affecting developmental aspects | 130.40 ± 43.38 | 131.50 | 30–230 |
| iv.- Global epilepsy | 68.45 ± 31.89 | 67.00 | 0–170 |
| Gender | Males (n = 44) | Females (n = 95) | ||||
|---|---|---|---|---|---|---|
| Range | Median | Mean | Range | Median | Mean | |
| GFAP | 64–410 | 254 | 248.09 ± 88.09 * | 82–359 | 219 | 217.25 ± 67.20 |
| i.- Developmental delay items corrected by age | 2–40 | 27.50 | 22.50 ± 11.87 t | 2–45 | 15 | 18.48 ± 11.86 |
| ii.- Comorbidities | 1–21 | 9.0 | 9.32 ± 3.99 t | 0–45 | 8.0 | 7.59 ± 5.7 |
| iii.- Different items affecting developmental aspects | 30–230 | 150 | 141.16 ± 47.73 * | 35–210 | 128.0 | 125.25 ± 40.39 |
| iv.- Global epilepsy | 0–170 | 77 | 75.95 ± 39.21 | 0–135 | 65 | 64.86 ± 27.24 |
| Motor Milestones (motor delay) (up to 6) | 0–6 | 3 | 2.73 ± 1.89 * | 0–6 | 4 | 3.72 ± 2.18 |
| Cognitive milestones (cognitive delay) (up to 5) | 0–5 | 2 | 2.30 ± 1.27 * | 0–5 | 3 | 2.75 ± 1.29 |
| Genetics | 4p Minus Single Deletions (n = 63) | 4p Minus Deletions + Additional Rearrangement (n = 69) | ||||
|---|---|---|---|---|---|---|
| Range | Median | Mean | Range | Median | Mean | |
| GFAP | 102–410 | 256.50 | 246.07 ± 71.36 | 82–369 | 221 | 218.74 ± 71.35 * |
| i.- Developmental delay items corrected by age | 2–45 | 21.50 | 22.27 ± 12.08 | 2–21 | 15 | 17.78 ± 11.39 * |
| ii.- Comorbidities | 2–45 | 8.0 | 9.52 ± 6.31 | 0–45 | 7.0 | 7.33 ± 4.01 |
| iii.- Several items affecting developmental aspects | 30–230 | 150.50 | 141.02 ± 40.23 | 30–230 | 128.0 | 124.28 ± 43.11 * |
| iv.- Global epilepsy | 0–170 | 68 | 73.58 ± 31.90 | 20–135 | 65 | 67.64 ± 28.51 |
| Motor Milestones (up to 6) | 0–6 | 3 | 2.92 ± 2.03 | 0–6 | 4 | 3.74 ± 1.95 * |
| Cognitive milestones (up to 5) | 0–5 | 2 | 2.50 ± 1.28 | 0–5 | 2 | 2.62 ± 1.26 |
| Variable | Cluster A | Cluster B |
|---|---|---|
| Gender (Female/Male) | 18F/12M (1.50:1) | 77F/33M (2.33:1) |
| size of deletion (Mb) | 18.98 ± 6.75 (15.98) range 12.00–41.50 | 6.37 ± 3.45 (6.26) range 0.01–15.46 |
| Subpop Sph/Lat | 17/13 (1.31:1) | 57/53 (1.07:1) |
| Age at evaluation (years) | 5.11 ± 5.76 (3.12) range 0.01–34.04 | 8.53 ± 8.44 (5.60) range 0.01–39.04 |
| Age at diagnosis (months) | 12.97 ± 27.87 (3) range 0.01–144 | 31.11 ± 56.92 (12) range 0.1–384 |
| Additional duplications | Not, 23/Yes, 7 (30.43%) | Not, 55/Yes, 55 (50%) |
| GFAP score (AU) | 267.96 ± 53.12 (261) range 164–369 | 215. 87 ± 77.07 (220) range 64–346 |
| Weighted Psychomotor delay milestones corrected by age | 28.19 ± 10.32 (30) range 2–45 | 18.83 ± 11.56 (17) range 2–38 |
| Comorbidities | 9.70 ± 5.26 (9) range 2–23 | 7.58 ± 4.13 (7) range 0–21 |
| DD affecting items | 148.67 ± 37.96 (151) range 80–150 | 125.87 ± 43.46 (126) range 30–190 |
| Global Epilepsy items | 81.41 ± 26.23 (92) range 32–98 | 64.96 ± 37.72 (65) range 0–150 |
| Prenatal/Neonatal | ||
| IUGR | 29/29 (100%) | 101/109 (92.70%) |
| Medro faillure | 23/27 (85.20%) | 101/109 (92.70%) |
| Gestational week | 35.65 ± 4.37 (37) range 17–42 | 36.87 ± 2.44 (37) range 28–41 |
| Weight at birth (gr) | 1830.19 ± 414.36 (1800) range 440–2700 | 2049.14 ± 498.13 (2010) range 840–3700 |
| height at birth (cm) | 42.48 ± 3.33 (43) range 29.5–46 | 44.09 ± 3.52 (44) range 33–52 |
| OFC at birth (cm) | 30.76 ± 3.15 (31) range 19.5–39 | 31.46 ± 0.26 (31) range 21–39 |
| EPILEPSY | ||
| Seizures | 27/28 (96.50%) | 99/109 (90.80%) |
| Age of seizures (months) | 7.72 ± 4.49 (8) range 0–18 | 10.26 ± 7.03 (9) range 0.01–36 |
| Seizures w fever | 16/27 (59.30%) | 79/107 (73.80%) |
| Seizures w/o fever | 20/27 (74.10%) | 61/107 (57.0%) |
| Status | 18/27 (66.70%) | 62/107 (57.90%) |
| Number status | 3.56 ± 6.07 (2) range 0–30 | 2.93 ± 6.56 (1) range 0–55 |
| Status to ICU | 12/27 (44.40%) | 46/107 (43.0%) |
| AEDs | 25/27 (92.600%) | 90/107 (84.10%) |
| Number of AEDs | 2.15 ± 1.13 (2) range 0–5 | 2.16 ± 1.52 (2) range 0–6 |
| Monotherapy | 12/27 (48.10%) | 50/108 (46.29%) |
| Max number of AEDs used simultaneously. | 1.81 ± 1.0 (2) range 0–5 | 1.54 ± 0.90 (1) range 0–4 |
| Took drug for epilepsy not now | 3/27 (11.10%) | 17/108 (15.74%) |
| Crisis control (1 to 6) | 2.52 ± 1.31 (2) range 0–5 | 3.91 ± 1.58 (5) range 1–6 |
| MOTOR | ||
| Able to support head | 18/27 (66.70%) | 104/110 (94.54%) |
| Able to seat | 14/27 (51.90%) | 89/110 (80.90%) |
| Able to seat unaided | 9/27 (33.30%) | 83/110 (75.45%) |
| Able to walk with help | 7/27 (25.90%) | 71/110 (64.54%) |
| Able to walk unaided | 13/27 (48.10%) | 48/110 (43.64%) |
| Able to eat unaided | 2/27 (7.40%) | 36/110 (32.72%) |
| COGNITIVE | ||
| Non-sphincter control | 24/27 (88.90%) | 82/110 (74.54%) |
| Able to communicate with environment | 24/27 (88.90%) | 100/110 (90.90%) |
| Communication with alternative tools | 14/27 (51.90%) | 81/110 (73.66%) |
| Able to say some words | 3/27 (11.10%) | 44/110 (40.0%) |
| Able to make short sentences | 1/27 (3.70%) | 18/110 (16.36%) |
| Motor milestones total | 1.75 ± 1.58 (2) range 0–6 | 3.84 ± 1.91 (4) range 0–6 |
| Cognitive milestones total | 2.11 ± 1.22 (2) range 0–5 | 2.74 ± 1.29 (3) range 0–5 |
| COMORBIDITY | ||
| C-gastrostomy | 3/27 (11.10%) | 12/110 (10.90%) |
| Cardiovascular problems | 13/27 (48.10%) | 48/110 (43.63%) |
| Nephro-urogenital anomalies | 19/27 (70.40%) | 53/110 (48.18%) |
| Ophthalmological problems | 20/27 (74.10%) | 58/110 (52.72%) |
| Auditive problems | 12/27 (44.40%) | 44/110 (40.0%) |
| Recurrent air tract infections | 17/27 (63.0%) | 62/110 (56.36%) |
| Brain anomalies by MRI | 15/21 (71.42%) | 58/110 (52.72%) |
| Number of Surgeries | 1.22 (1) range 0–6 | 0.93 (1) range 0–7 |
| SOCIAL | ||
| A family member quit job to care | 15/27 (55.60%) | 8/22 (36.36%) |
| Variable | Group-1 (Mean) | Group-2 (Mean) | p-Value | More Affected Group |
|---|---|---|---|---|
| Gender | Male, 17.20%; female, 82.80% | Male, 48.50%; female, 52.50% | 0.0031 | |
| Deletion size (Mb) | 5.09 ± 3.68 | 10.85 ± 5.65 ▲ | 0.00001 | group-2 |
| GFAP score (AU) | 206.24 | 342.14 ▲ | <0.00001 | group-2 |
| Develomental delay corrected by age | 17.63 | 31.52 ▲ | <0.00001 | group-2 |
| Global epilepsy | 61.97 | 103.90 ▲ | <0.00001 | group-2 |
| Comorbidities | 7.30 | 12.81 ▲ | <0.00001 | group-2 |
| Alterations affecting development | 119.82 | 188.33 ▲ | <0.00001 | group-2 |
| Motor delay | 3.67 ▲ | 1.90 | 0.0002 | group-2 |
| Cognitive delay | 2.76 ▲ | 1.76 | 0.0011 | group-2 |
| Number of AEDS | 1.70 ± 1.44 | 2.46 ± 1.37 ▲ | 0.003 | group-2 |
| Cardiovascular anomalies | 0.37 | 0.86 ▲ | 0.0001 | group-2 |
| Nephro-urological anomalies | 0.50 | 0.71 ▲ | 0.0014; Odds ratio = 0.3 | group-2 |
| Ophthalmological anomalies | 0.54 | 0.71 ▲ | 0.05; Odds ratio = 0.47 | group-2 |
| Recurrent respiratory infections | 0.55 | 0.71 ▲ | 0.05; Odds ratio = 0.47 | group-2 |
| Non-sphincter control | 0.67 | 0.87 ▲ | <0.001; Odds ratio = 0.074 | group-2 |
| MRI anomalies | 0.53 | 0.70 ▲ | 0.012 | group-2 |
| IUGR | 0.93 | 1.00 ▲ | 0.06t; Odds ratio = 0.15 | group-2 |
| Seizures | 0.91 | 0.95 ▲ | 0.016; Odds ratio = 0.11 | group-2 |
| Status | 0.57 | 0.71 ▲ | 0.0003; Odds ratio = 0.249 | group-2 |
| Number of Status | 2.97 | 3.48 ▲ | 0.019 | group 2 |
| Able to support head | 0.92 ▲ | 0.71 | 0.00028; Odds ratio = infinite | group-2 |
| Able to seat unaided | 0.73 ▲ | 0.33 | 0.0010 | group-2 |
| Able to communicate to the environment | 0.75 ▲ | 0.38 | 0.0009 | group-2 |
| Able to walk unaided | 0.43 ▲ | 0.05 | 0.0022 | group-2 |
| To be able to say words | 0.38 ▲ | 0.10 | 0.011 | group-2 |
| To be able to make sentences | 0.17 ▲ | 0.001 | 0.0012 | group-2 |
| Weight at birth | 2,120 ± 536 ▲ | 1,930 ± 44.2 | 0.026 | group-2 |
| OFC at birth | 32.17 ± 2.71 ▲ | 30.78 ± 2.73 | 0.006 | group-2 |
| Age at diagnosis (months) | 41.04 ± 61.27 ▲ | 18.91 ± 44.83 | 0.006 | group-2 |
| Age at evaluation (years) | 11.32 ± 9.62 ▲ | 5.69 ± 5.92 | 0.00001 | group-2 |
| Familiar has to quit her/his job to care | 0.54 | 0.76 ▲ | 0.064 t; Odds ratio = 0.5 | group-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevado, J.; Blanco-Lago, R.; Bel-Fenellós, C.; Hernández, A.; Mori-Álvarez, M.A.; Biencinto-López, C.; Málaga, I.; Pachajoa, H.; Mansilla, E.; García-Santiago, F.A.; et al. Clinician-Based Functional Scoring and Genomic Insights for Prognostic Stratification in Wolf–Hirschhorn Syndrome. Genes 2025, 16, 820. https://doi.org/10.3390/genes16070820
Nevado J, Blanco-Lago R, Bel-Fenellós C, Hernández A, Mori-Álvarez MA, Biencinto-López C, Málaga I, Pachajoa H, Mansilla E, García-Santiago FA, et al. Clinician-Based Functional Scoring and Genomic Insights for Prognostic Stratification in Wolf–Hirschhorn Syndrome. Genes. 2025; 16(7):820. https://doi.org/10.3390/genes16070820
Chicago/Turabian StyleNevado, Julián, Raquel Blanco-Lago, Cristina Bel-Fenellós, Adolfo Hernández, María A. Mori-Álvarez, Chantal Biencinto-López, Ignacio Málaga, Harry Pachajoa, Elena Mansilla, Fe A. García-Santiago, and et al. 2025. "Clinician-Based Functional Scoring and Genomic Insights for Prognostic Stratification in Wolf–Hirschhorn Syndrome" Genes 16, no. 7: 820. https://doi.org/10.3390/genes16070820
APA StyleNevado, J., Blanco-Lago, R., Bel-Fenellós, C., Hernández, A., Mori-Álvarez, M. A., Biencinto-López, C., Málaga, I., Pachajoa, H., Mansilla, E., García-Santiago, F. A., Barrúz, P., Tenorio-Castaño, J. A., Muñoz-GªPorrero, Y., Vallcorba, I., & Lapunzina, P. (2025). Clinician-Based Functional Scoring and Genomic Insights for Prognostic Stratification in Wolf–Hirschhorn Syndrome. Genes, 16(7), 820. https://doi.org/10.3390/genes16070820







