Aquaporin mRNA in Human Saliva
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Cohort
2.2. Baseline Characteristics
- Age;
- Gender (m/f/d);
- Pre-existing conditions: arterial hypertension, [non-]insulin-dependent diabetes mellitus;
- Cardiovascular diseases [including CHD, post-myocardial infarction, PAD, etc.];
- Nicotine abuse;
- Current or previous malignant tumor disease: localization, TNM stage, radiotherapy.
2.3. Collection of Samples
2.4. RNA Quantification
2.5. In Silico Analysis
2.6. Statistical Analysis
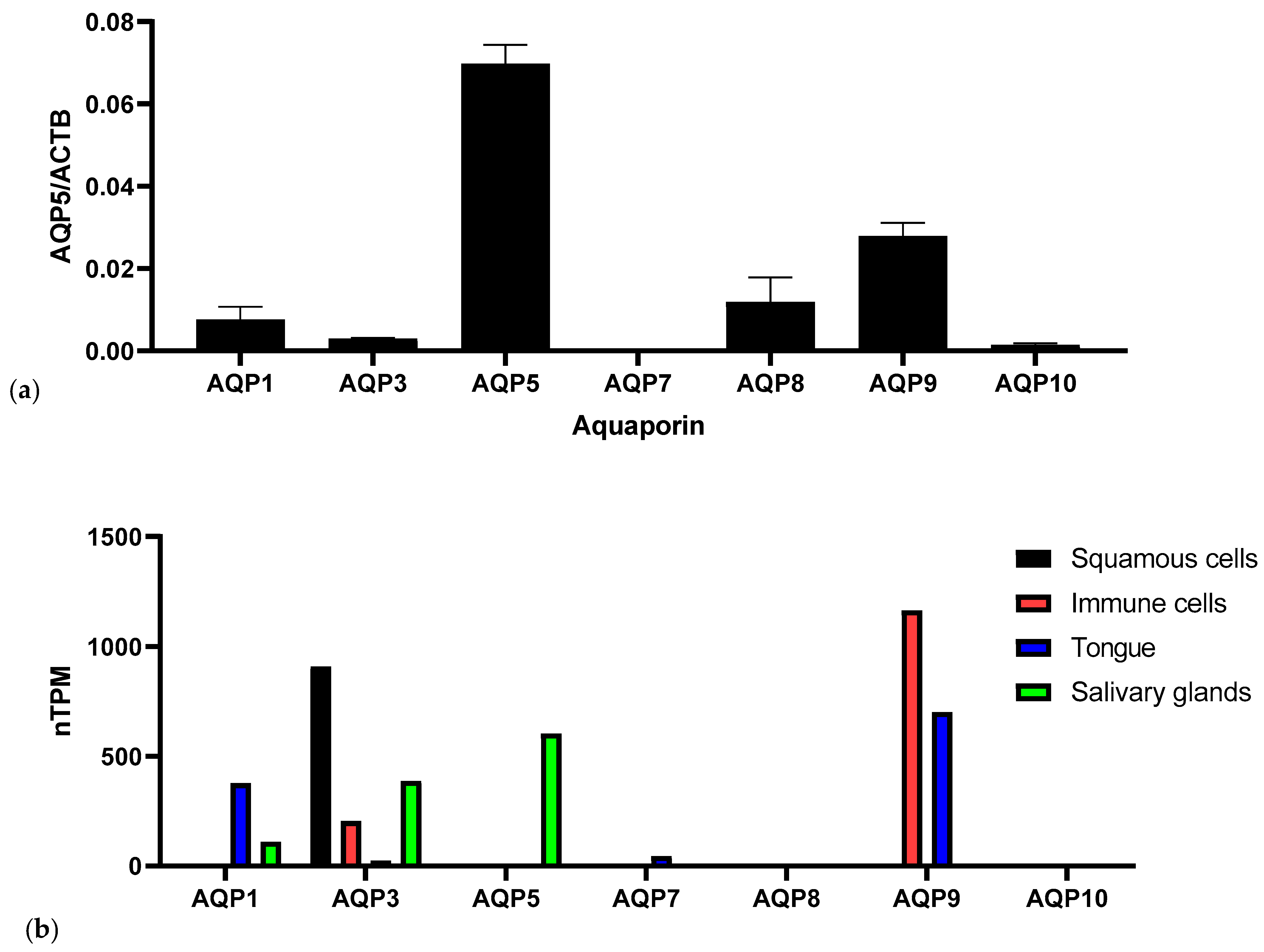
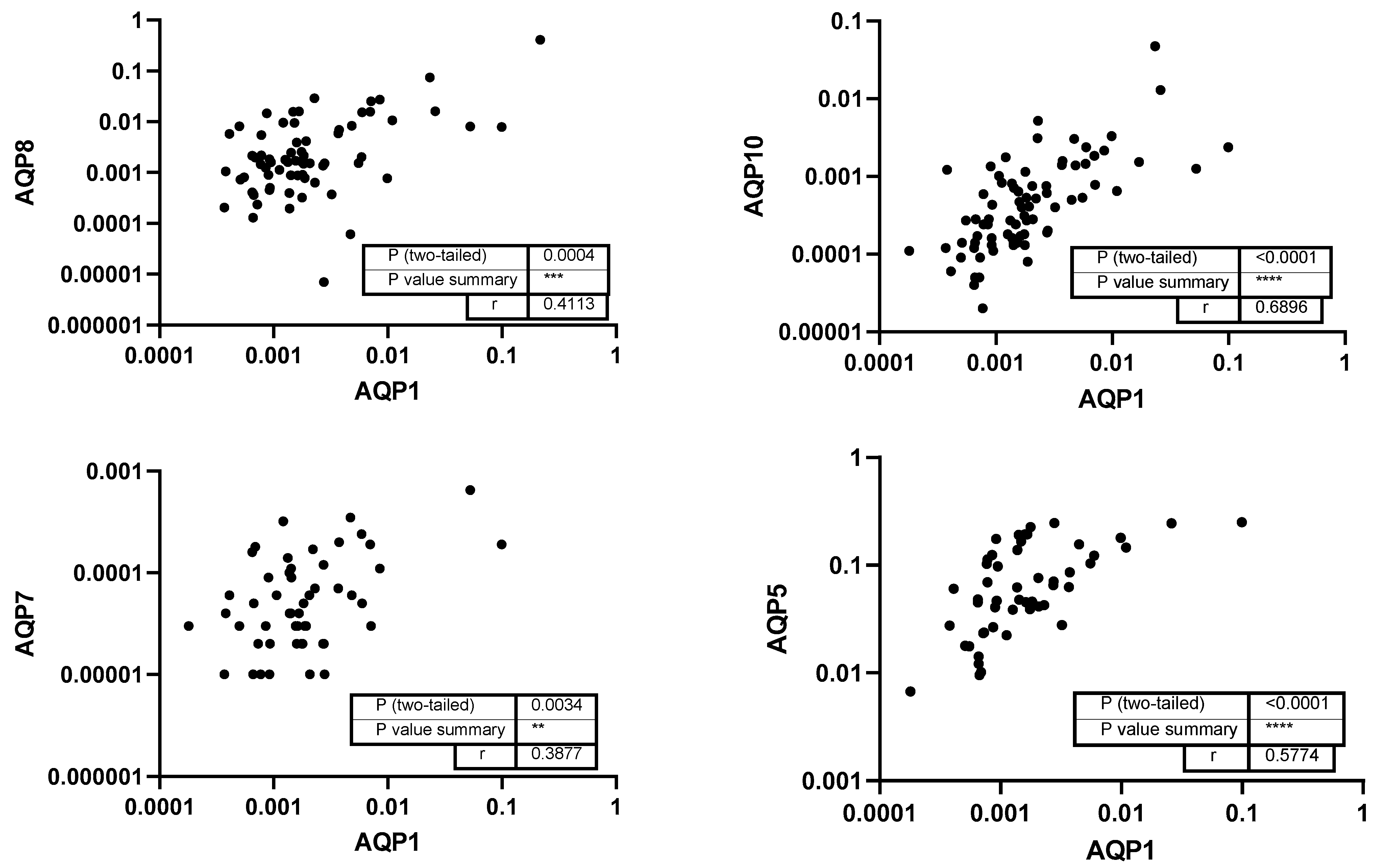
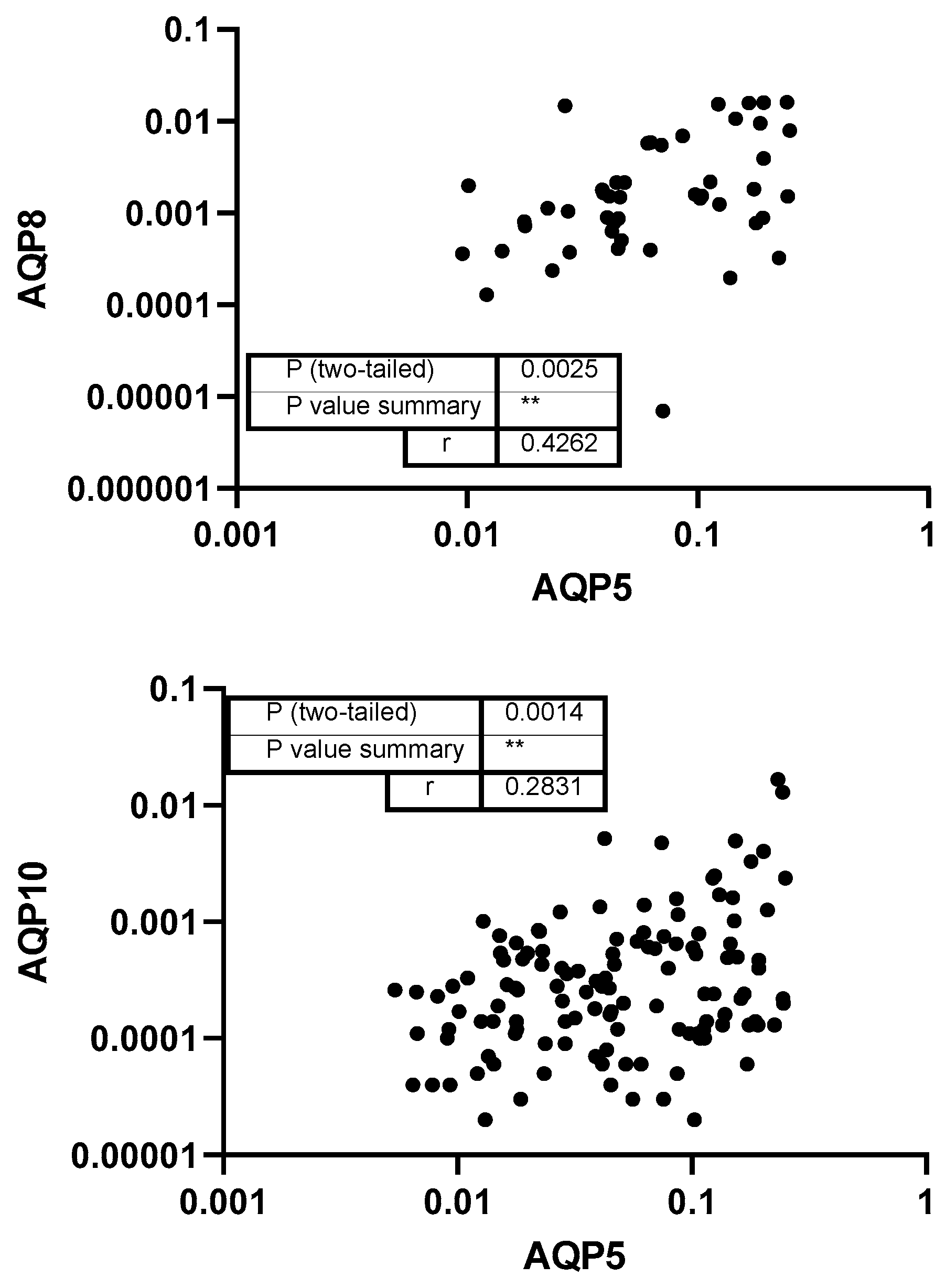
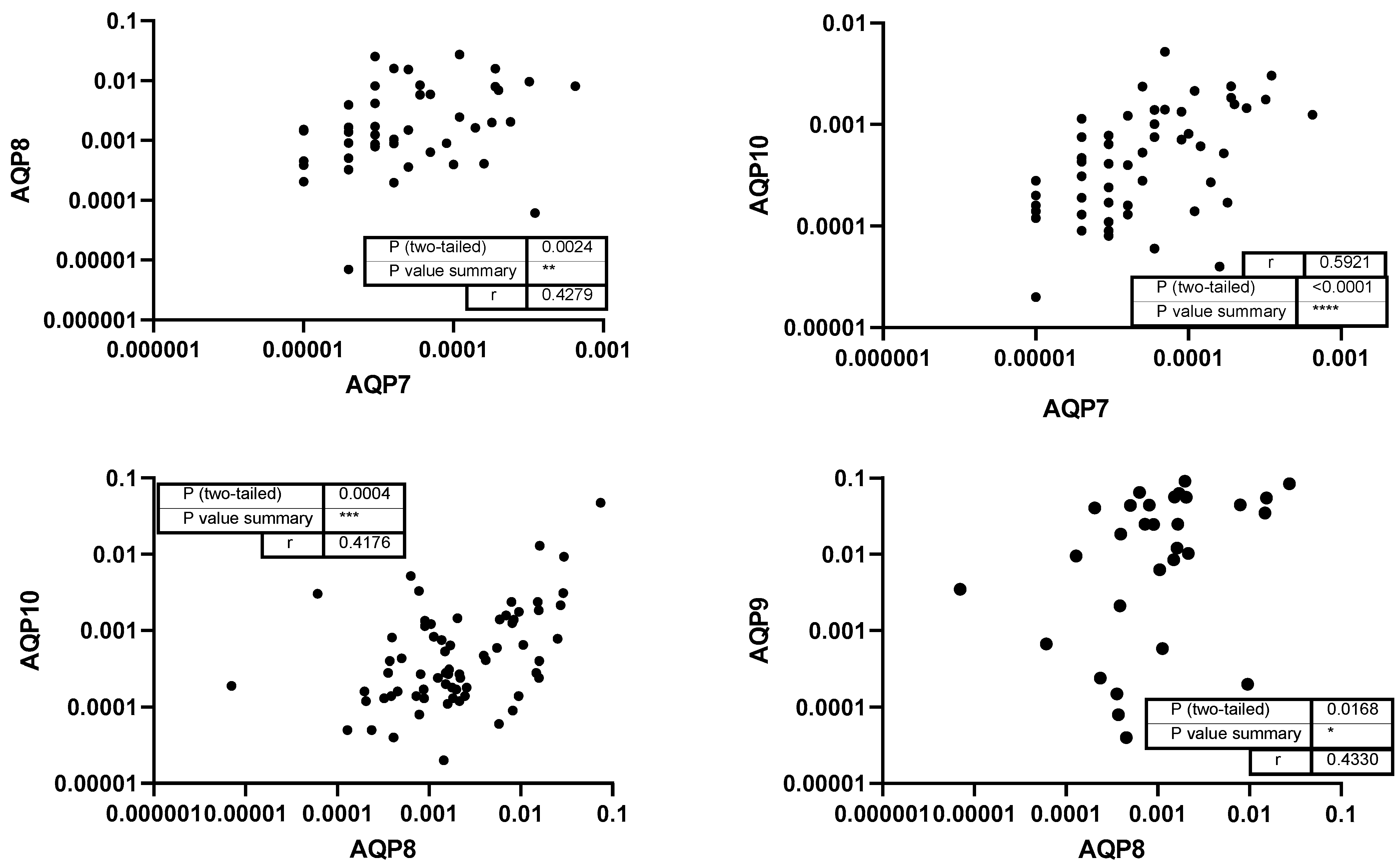
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflug. Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Stookey, G.K. The effect of saliva on dental caries. J. Am. Dent. Assoc. 2008, 139 (Suppl. S2), 11s–17s. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Rodríguez-Montaño, R.; Mosaddad, S.A.; Heboyan, A. Levels of IL-1β, MMP-8, and MMP-9 in the Saliva of Subjects With Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Lab. Anal. 2025, 39, e70040. [Google Scholar] [CrossRef] [PubMed]
- Theda, C.; Hwang, S.H.; Czajko, A.; Loke, Y.J.; Leong, P.; Craig, J.M. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep. 2018, 8, 6944. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; St John, M.A.; Wong, D.T. RNA profiling of cell-free saliva using microarray technology. J. Dent. Res. 2004, 83, 199–203. [Google Scholar] [CrossRef]
- Quinelato, V.; Mourão, C.F.; Santos, T.A.B.; Cataldo de Felipe Cordeiro, P.; Ladeira Bonato, L.; Gomes Pereira, M.; Calasans-Maia, J.A.; Granjeiro, J.M.; Kawase, T.; Ladeira Casado, P. Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples. Int. J. Mol. Sci. 2025, 26, 2891. [Google Scholar] [CrossRef]
- Atif, S.; Abdul Wahab, N.; Ghafoor, S.; Azlina, A.; Tauseef, A.; Rana, S.; Saeed, M.Q. Salivary aquaporin-3 as a screening biomarker for xerostomia in patients with periodontal disease and the effects of xerostomia on oral health-related quality of life. PLoS ONE 2023, 18, e0283995. [Google Scholar] [CrossRef]
- Ichiyama, T.; Nakatani, E.; Tatsumi, K.; Hideshima, K.; Urano, T.; Nariai, Y.; Sekine, J. Expression of aquaporin 3 and 5 as a potential marker for distinguishing dry mouth from Sjögren’s syndrome. J. Oral Sci. 2018, 60, 212–220. [Google Scholar] [CrossRef]
- Rump, K.; Brendt, P.; Frey, U.H.; Schäfer, S.T.; Siffert, W.; Peters, J.; Adamzik, M. Aquaporin 1 and 5 expression evoked by the β2 adrenoreceptor agonist terbutaline and lipopolysaccharide in mice and in the human monocytic cell line THP-1 is differentially regulated. Shock 2013, 40, 430–436. [Google Scholar] [CrossRef]
- Thon, P.; Rahmel, T.; Ziehe, D.; Palmowski, L.; Marko, B.; Nowak, H.; Wolf, A.; Witowski, A.; Orlowski, J.; Ellger, B.; et al. AQP3 and AQP9-Contrary Players in Sepsis? Int. J. Mol. Sci. 2024, 25, 1209. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Marinelli, R.A.; LaRusso, N.F. Water transport by epithelia of the digestive tract. Gastroenterology 2002, 122, 545–562. [Google Scholar] [CrossRef]
- Zhu, S.; Ran, J.; Yang, B.; Mei, Z. Aquaporins in Digestive System. Adv. Exp. Med. Biol. 2017, 969, 123–130. [Google Scholar] [CrossRef] [PubMed]
- de Paula, F.; Tucker, A.S.; Teshima, T.H.N.; de Souza, M.M.; Coutinho-Camillo, C.M.; Nico, M.M.S.; Lourenço, S.V. Characteristics of aquaporin 1, 3, and 5 expression during early murine salivary gland development. J. Anat. 2021, 238, 794–806. [Google Scholar] [CrossRef]
- Hosoi, K.; Yao, C.; Hasegawa, T.; Yoshimura, H.; Akamatsu, T. Dynamics of Salivary Gland AQP5 under Normal and Pathologic Conditions. Int. J. Mol. Sci. 2020, 21, 1182. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Stergiou, C.; Daoussis, D.; Zisimopoulou, P.; Andonopoulos, A.P.; Zolota, V.; Tzartos, S.J. Antibodies to aquaporins are frequent in patients with primary Sjögren’s syndrome. Rheumatology 2017, 56, 2114–2122. [Google Scholar] [CrossRef]
- Hong, X.; Meng, S.; Tang, D.; Wang, T.; Ding, L.; Yu, H.; Li, H.; Liu, D.; Dai, Y.; Yang, M. Single-Cell RNA Sequencing Reveals the Expansion of Cytotoxic CD4+ T Lymphocytes and a Landscape of Immune Cells in Primary Sjögren’s Syndrome. Front. Immunol. 2020, 11, 594658. [Google Scholar] [CrossRef]
- Karlsson, T.; Glogauer, M.; Ellen, R.P.; Loitto, V.M.; Magnusson, K.E.; Magalhães, M.A. Aquaporin 9 phosphorylation mediates membrane localization and neutrophil polarization. J. Leukoc. Biol. 2011, 90, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Delporte, C.; Steinfeld, S. Distribution and roles of aquaporins in salivary glands. Biochim. Biophys. Acta 2006, 1758, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Clarke-Bland, C.E.; Bill, R.M.; Devitt, A. Emerging roles for AQP in mammalian extracellular vesicles. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183826. [Google Scholar] [CrossRef]
- Gresz, V.; Burghardt, B.; Ferguson, C.J.; Hurley, P.T.; Takács, M.; Nielsen, S.; Varga, G.; Zelles, T.; Case, R.M.; Steward, M.C. Expression of aquaporin 1 (AQP1) water channels in human labial salivary glands. Arch. Oral. Biol. 1999, 44 (Suppl. S1), S53–S57. [Google Scholar] [CrossRef]
- Delporte, C.; O’Connell, B.C.; He, X.; Lancaster, H.E.; O’Connell, A.C.; Agre, P.; Baum, B.J. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc. Natl. Acad. Sci. USA 1997, 94, 3268–3273. [Google Scholar] [CrossRef]
- Rump, K.; Rahmel, T.; Rustige, A.M.; Unterberg, M.; Nowak, H.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The Aquaporin3 Promoter Polymorphism −1431 A/G is Associated with Acute Graft Rejection and Cytomegalovirus Infection in Kidney Recipients Due to Altered Immune Cell Migration. Cells 2020, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Gresz, V.; Kwon, T.H.; Hurley, P.T.; Varga, G.; Zelles, T.; Nielsen, S.; Case, R.M.; Steward, M.C. Identification and localization of aquaporin water channels in human salivary glands. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G247–G254. [Google Scholar] [CrossRef]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Iena, F.M.; Jul, J.B.; Vegger, J.B.; Lodberg, A.; Thomsen, J.S.; Brüel, A.; Lebeck, J. Sex-Specific Effect of High-Fat Diet on Glycerol Metabolism in Murine Adipose Tissue and Liver. Front. Endocrinol. 2020, 11, 577650. [Google Scholar] [CrossRef]
- Xing, L.; Jin, B.; Fu, X.; Zhu, J.; Guo, X.; Xu, W.; Mou, X.; Wang, Z.; Jiang, F.; Zhou, Y.; et al. Identification of functional estrogen response elements in glycerol channel Aquaporin-7 gene. Climacteric 2019, 22, 466–471. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Soveral, G. Aquaporins in Obesity. Adv. Exp. Med. Biol. 2023, 1398, 289–302. [Google Scholar] [CrossRef]
- Lopes, P.A.; Fonseca, E.; da Silva, I.V.; Vigia, E.; Paulino, J.; Soveral, G. Aquaporins Transcripts with Potential Prognostic Value in Pancreatic Cancer. Genes 2023, 14, 1694. [Google Scholar] [CrossRef]
- Nielsen, S.; Frøkiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: From molecules to medicine. Physiol. Rev. 2002, 82, 205–244. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Anjum, I.; Shabbir, A.; Khurram Syed, S.; Mobeen, I.; Hassaan Shahid, M.; Sarwar, K. Amelioration of Ovalbumin-Induced Allergic Asthma by Juglans regia via Downregulation of Inflammatory Cytokines and Upregulation of Aquaporin-1 and Aquaporin-5 in Mice. J. Trop. Med. 2022, 2022, 6530095. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, H.; Wang, C.; Hu, Y.; Wang, S.; Shen, L. Aquaporin 4 deficiency alleviates experimental colitis in mice. FASEB J. 2019, 33, 8935–8944. [Google Scholar] [CrossRef]
- Squillacioti, C.; Mirabella, N.; Liguori, G.; Germano, G.; Pelagalli, A. Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis. Animals 2021, 11, 1539. [Google Scholar] [CrossRef]
- Pimpão, C.; da Silva, I.V.; Soveral, G. The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. Int. J. Mol. Sci. 2025, 26, 1330. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, B.; Li, C.; Wang, W. Expression Regulation and Trafficking of Aquaporins. Adv. Exp. Med. Biol. 2023, 1398, 39–51. [Google Scholar] [CrossRef]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Ren. Physiol. 2020, 318, F193–F203. [Google Scholar] [CrossRef]
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int. J. Mol. Sci. 2019, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, N.; Hus, A.; Yadav, J.S. Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications. Respir. Med. 2020, 174, 106193. [Google Scholar] [CrossRef] [PubMed]
- Tomečková, V.; Krajčíková, K.; Dolinská, S.; Komanický, V.; Samuely, T. The effect of different diet on saliva. Spectr. Anal. Rev. 2017, 5, 37–48. [Google Scholar] [CrossRef]
- Nelson, N.; Malhan, D.; Hesse, J.; Aboumanify, O.; Yalçin, M.; Lüers, G.; Relógio, A. Comprehensive integrative analysis of circadian rhythms in human saliva. npj Biol. Timing Sleep 2025, 2, 17. [Google Scholar] [CrossRef]
- Lozano, C.; García-Manríquez, N.; Gambetta-Tessini, K.; Echeverría, C.; Fernández, C.E.; Giacaman, R.A. Circadian Influence on Saliva Biochemical Composition: A Pilot Clinical Investigation. Res. Sq. 2024. preprint. [Google Scholar]
- Gomes, A.; da Silva, I.V.; Rodrigues, C.M.P.; Castro, R.E.; Soveral, G. The Emerging Role of microRNAs in Aquaporin Regulation. Front. Chem. 2018, 6, 238. [Google Scholar] [CrossRef]
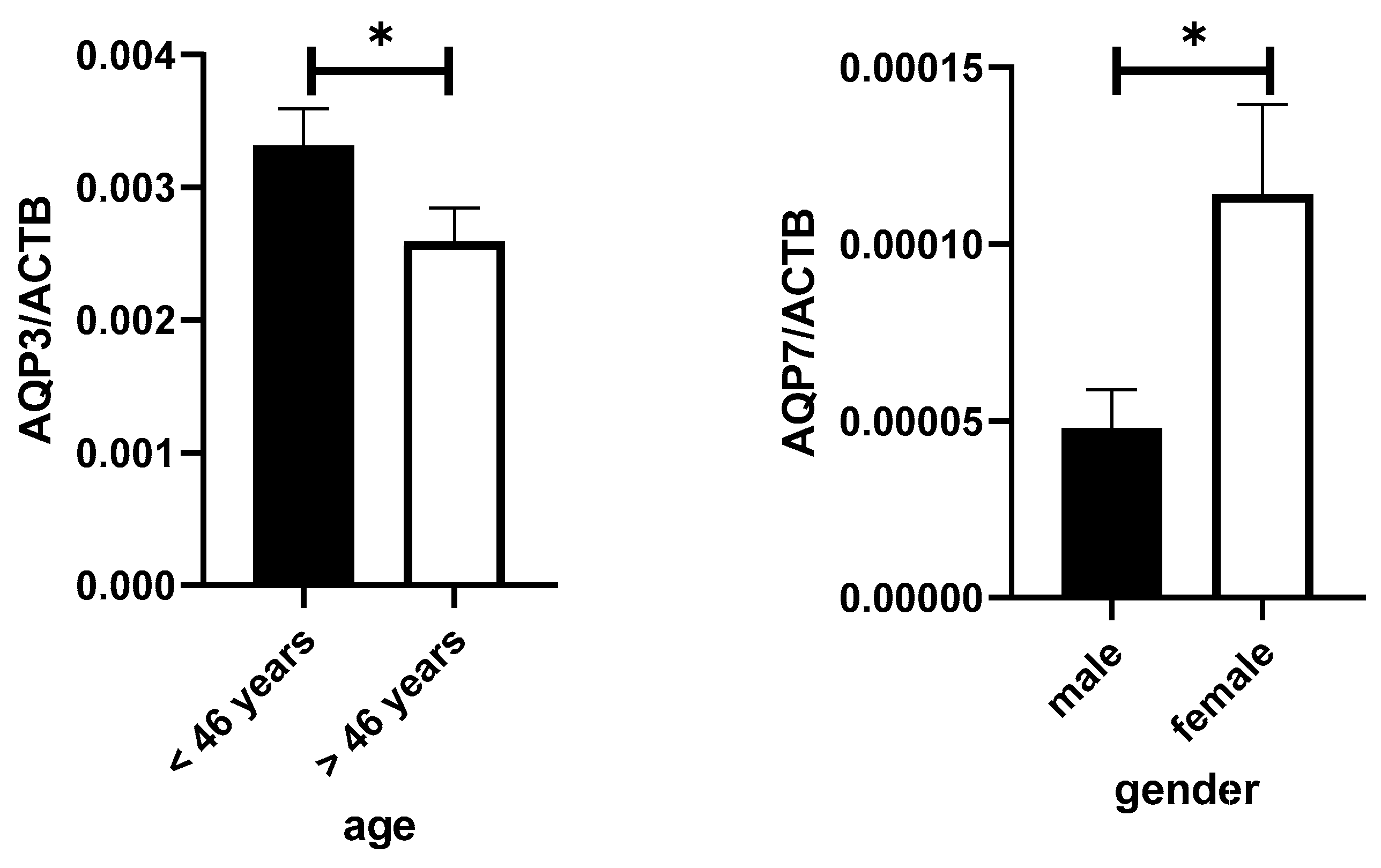
| n = 204 | |
|---|---|
| Gender male | 82 (40.2%) |
| Age median (IQR) | 46.5 (35–56.75) |
| Arterial hypertonia (yes) | 30 (14.75) |
| Heart disease | 11 (5.4%) |
| Smoking (yes) | 46 (22.5%) |
| Smoking in years | 4.85 ± 11.3 |
| Diabetes | 5 (2.5%) |
| Cancer | 3 (1.5%) |
| Primer Name | Sequence |
|---|---|
| AQP1_Rt_Se | GCCATCCTCTCAGGCATCAC |
| AQP1_RT_Human_AS | GTAGCCAGCACGCATAGCAC |
| AQP3_Rt2_Se | GGAATAGTTTTTGGGCTGTA |
| AQP3_Rt2_AS | GGCTGTGCCTATGAACTGGT |
| AQP5_RT_2_SE | TCGGTTCAGCCCCGCTCACT |
| AQP5_RT_2_AS | GCCACACGCTCACTCAGGCT |
| AQP7_Rt2_Se | GGACTGGGGACACAGGGATA |
| AQP7_Rt2_As | GCTGAAAGTGCAATCCACGG |
| AQP8_RT_SE | GAGATCATCCTGACGACGCT |
| AQP8_RT_AS | TTCATGCAGCCTCCAGACAC |
| AQP9_RT_SE_neu | GCGAACGCATTTGCAGATCA |
| AQP9_RT_AS_neu | CAACCAAAGGGCCCACTACA |
| AQP10_RT_SE | CTACGTGGGTGGTAACGTCTC |
| AQP10_RT_AS | TAGGTGGCAAAAATGGAGGCT |
| AQP8 | AQP10 | AQP1 | AQP5 | AQP7 | AQP9 | AQP3 | Color Scheme | |
|---|---|---|---|---|---|---|---|---|
| AQP8 | 0.7 | |||||||
| AQP10 | 0.6 | |||||||
| AQP1 | 0.45 | |||||||
| AQP5 | 0.3 | |||||||
| AQP7 | 0.15 | |||||||
| AQP9 | 0 | |||||||
| AQP3 | −0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rump, K.; Pakosch-Nowak, D.; Witowski, A.; Koos, B.; Ziehe, D.; Orlowski, J.; Adamzik, M.; Kunkel, M.; Baumann, M. Aquaporin mRNA in Human Saliva. Genes 2025, 16, 804. https://doi.org/10.3390/genes16070804
Rump K, Pakosch-Nowak D, Witowski A, Koos B, Ziehe D, Orlowski J, Adamzik M, Kunkel M, Baumann M. Aquaporin mRNA in Human Saliva. Genes. 2025; 16(7):804. https://doi.org/10.3390/genes16070804
Chicago/Turabian StyleRump, Katharina, Daria Pakosch-Nowak, Andrea Witowski, Bjoern Koos, Dominik Ziehe, Jennifer Orlowski, Michael Adamzik, Martin Kunkel, and Markus Baumann. 2025. "Aquaporin mRNA in Human Saliva" Genes 16, no. 7: 804. https://doi.org/10.3390/genes16070804
APA StyleRump, K., Pakosch-Nowak, D., Witowski, A., Koos, B., Ziehe, D., Orlowski, J., Adamzik, M., Kunkel, M., & Baumann, M. (2025). Aquaporin mRNA in Human Saliva. Genes, 16(7), 804. https://doi.org/10.3390/genes16070804






