Genetic Diseases of Fucosylation: Insights from Model Organisms
Abstract
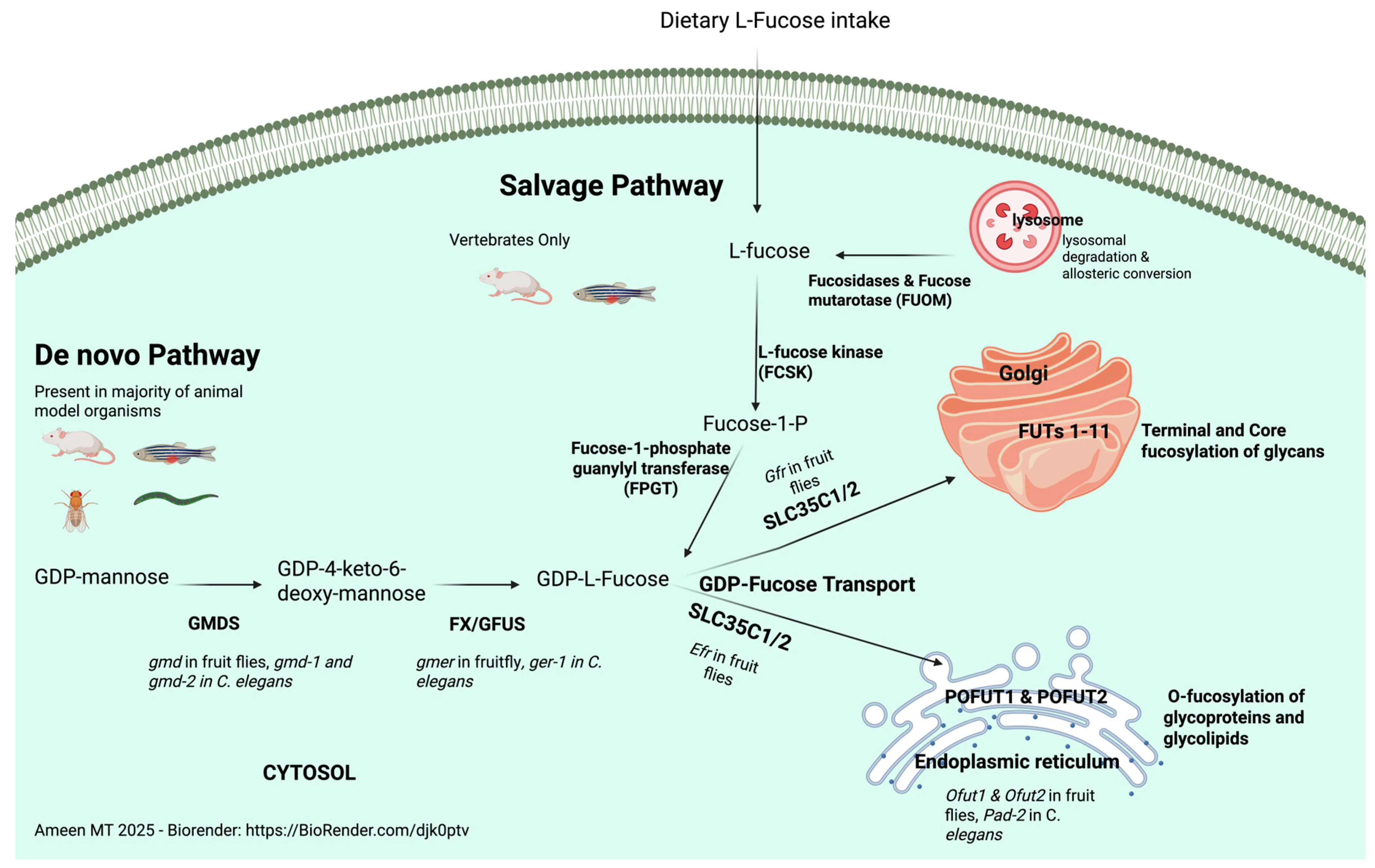
1. Overview of Fucosylation
2. Diseases of Fucosylation
2.1. Congenital Fucosylation Diseases
2.2. Fucosylation Association with Complex Disease
2.3. Abnormal Fucosylation in Cancers
2.4. Fucosylation of Notch Receptors and Disease
3. Animal Models of Fucosylation Disease
3.1. Mouse and Rat Models of Fucosylation-Related Diseases
3.2. Zebrafish as a Model for Fucosylation in Development and Disease
3.3. Invertebrate Models of Fucosylation Disease
4. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDP | Guanosine Diphosphate |
| GMDS | GDP-mannose 4,6-dehydratase |
| EGF | Epidermal Growth Factor |
| TSRs | Thrombospondin type |
| FUT | Fucosyltransferases |
| EMI | Elastin Microfibril Interface |
| HCC | Hepatocellular Carcinoma |
| TGF | Transforming Growth Factor |
| MMP | Matrix metalloproteinases |
| NICD | Notch Intracellular Domain |
| CDG | Congenital Disease of Glycosylation |
| GWAS | Genome Wide Association Studies |
| LADII | Leukocyte adhesion deficiency 2 |
| CDG IIC | Congenital disorder of glycosylation type IIc |
| FPGT | fucose-1-phosphate guanylyl transferase |
| ER | Endoplasmic reticulum |
| POFUT | Protein O-Fucosyltransferase |
| POAG | Primary Open Angle Glaucoma |
| OMIM | Online Mendelian Inheritance in Man |
| FCSK | Fucokinase |
| GFUS | GDP-L-Fucose Synthase |
| DDD | Dowling-Degos disease |
| SNPs | Single nucleotide polymorphisms |
| CADASIL | Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy |
| CSVD | cerebral small vessel diseases |
| CS | congenital scoliosis |
| SCD | spondylocostal dysostoses |
| LNFG | Lunatic Fringe |
| PAR | passive avoidance memorization |
| RGCs | Retinal ganglion cells |
| OCT | optical coherence tomography |
| PTZ | Pentylenetetrazol |
| hpf | hour post fertilization |
| CEFT | C. elegans fucosyltransferases |
References
- Yurchenco, P.D.; Atkinson, P.H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry 1977, 16, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Skurska, E.; Szulc, B.; Kreczko, K.; Olczak, M. Mutations in the SLC35C1 gene, contributing to significant differences in fucosylation patterns, may underlie the diverse phenotypic manifestations observed in leukocyte adhesion deficiency type II patients. Int. J. Biochem. Cell Biol. 2024, 173, 106602. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hou, X.; Shi, S.; Korner, C.; Stanley, P. Slc35c2 promotes Notch1 fucosylation and is required for optimal Notch signaling in mammalian cells. J. Biol. Chem. 2010, 285, 36245–36254. [Google Scholar] [CrossRef]
- Lu, L.; Varshney, S.; Yuan, Y.; Wei, H.X.; Tanwar, A.; Sundaram, S.; Nauman, M.; Haltiwanger, R.S.; Stanley, P. In vivo evidence for GDP-fucose transport in the absence of transporter SLC35C1 and putative transporter SLC35C2. J. Biol. Chem. 2023, 299, 105406. [Google Scholar] [CrossRef]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef]
- Luo, Y.; Haltiwanger, R.S. O-fucosylation of notch occurs in the endoplasmic reticulum. J. Biol. Chem. 2005, 280, 11289–11294. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, K.; Pak, J.E.; Satkunarajah, M.; Zhou, D.; Rini, J.M. Recognition of EGF-like domains by the Notch-modifying O-fucosyltransferase POFUT1. Nat. Chem. Biol. 2017, 13, 757–763. [Google Scholar] [CrossRef]
- Luo, Y.; Nita-Lazar, A.; Haltiwanger, R.S. Two distinct pathways for O-fucosylation of epidermal growth factor-like or thrombospondin type 1 repeats. J. Biol. Chem. 2006, 281, 9385–9392. [Google Scholar] [CrossRef]
- Moloney, D.J.; Panin, V.M.; Johnston, S.H.; Chen, J.; Shao, L.; Wilson, R.; Wang, Y.; Stanley, P.; Irvine, K.D.; Haltiwanger, R.S.; et al. Fringe is a glycosyltransferase that modifies Notch. Nature 2000, 406, 369–375. [Google Scholar] [CrossRef]
- Haltiwanger, R.S.; Stanley, P. Modulation of receptor signaling by glycosylation: Fringe is an O-fucose-beta1,3-N-acetylglucosaminyltransferase. Biochim. Biophys. Acta 2002, 1573, 328–335. [Google Scholar] [CrossRef]
- Larsen, R.D.; Ernst, L.K.; Nair, R.P.; Lowe, J.B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA 1990, 87, 6674–6678. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Rouquier, S.; Giorgi, D.; Lennon, G.G.; Lowe, J.B. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 1995, 270, 4640–4649. [Google Scholar] [CrossRef]
- Hao, H.; Yuan, Y.; Ito, A.; Eberand, B.M.; Tjondro, H.; Cielesh, M.; Norris, N.; Moreno, C.L.; Maxwell, J.W.C.; Neely, G.G.; et al. FUT10 and FUT11 are protein O-fucosyltransferases that modify protein EMI domains. Nat. Chem. Biol. 2025, 21, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Oriol, R.; Mollicone, R.; Cailleau, A.; Balanzino, L.; Breton, C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology 1999, 9, 323–334. [Google Scholar] [CrossRef]
- Costache, M.; Apoil, P.A.; Cailleau, A.; Elmgren, A.; Larson, G.; Henry, S.; Blancher, A.; Iordachescu, D.; Oriol, R.; Mollicone, R. Evolution of fucosyltransferase genes in vertebrates. J. Biol. Chem. 1997, 272, 29721–29728. [Google Scholar] [CrossRef] [PubMed]
- Tomida, S.; Takata, M.; Hirata, T.; Nagae, M.; Nakano, M.; Kizuka, Y. The SH3 domain in the fucosyltransferase FUT8 controls FUT8 activity and localization and is essential for core fucosylation. J. Biol. Chem. 2020, 295, 7992–8004. [Google Scholar] [CrossRef]
- Boruah, B.M.; Kadirvelraj, R.; Liu, L.; Ramiah, A.; Li, C.; Zong, G.; Bosman, G.P.; Yang, J.Y.; Wang, L.X.; Boons, G.J.; et al. Characterizing human alpha-1,6-fucosyltransferase (FUT8) substrate specificity and structural similarities with related fucosyltransferases. J. Biol. Chem. 2020, 295, 17027–17045. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Burdon, K.P.; Fogarty, R.; Sharma, S.; Hewitt, A.W.; Martin, S.; Law, M.H.; Cremin, K.; Bailey, J.N.C.; Loomis, S.J.; et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat. Genet. 2014, 46, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- French, C.R.; Seshadri, S.; Destefano, A.L.; Fornage, M.; Arnold, C.R.; Gage, P.J.; Skarie, J.M.; Dobyns, W.B.; Millen, K.J.; Liu, T.; et al. Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J. Clin. Investig. 2014, 124, 4877–4881. [Google Scholar] [CrossRef]
- Keeley, T.S.; Yang, S.; Lau, E. The Diverse Contributions of Fucose Linkages in Cancer. Cancers 2019, 11, 1241. [Google Scholar] [CrossRef]
- Norman, K.E.; Moore, K.L.; McEver, R.P.; Ley, K. Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood 1995, 86, 4417–4421. [Google Scholar] [CrossRef]
- Hullen, A.; Falkenstein, K.; Weigel, C.; Huidekoper, H.; Naumann-Bartsch, N.; Spenger, J.; Feichtinger, R.G.; Schaefers, J.; Frenz, S.; Kotlarz, D.; et al. Congenital disorders of glycosylation with defective fucosylation. J. Inherit. Metab. Dis. 2021, 44, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Rosenfeld, J.A.; Emrick, L.; Jain, M.; Burrage, L.C.; Lee, B.; Undiagnosed Diseases, N.; Craigen, W.J.; Bearden, D.R.; Graham, B.H.; et al. Pathogenic Variants in Fucokinase Cause a Congenital Disorder of Glycosylation. Am. J. Hum. Genet. 2018, 103, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Starosta, R.T.; Lee, A.J.; Toolan, E.R.; He, M.; Wongkittichote, P.; Daniel, E.J.P.; Radenkovic, S.; Budhraja, R.; Pandey, A.; Sharma, J.; et al. D-mannose as a new therapy for fucokinase deficiency-related congenital disorder of glycosylation (FCSK-CDG). Mol. Genet. Metab. 2024, 142, 108488. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.G.; Xu, G.; Chandy, N.; Steyermark, J.; Shinde, D.N.; Radtke, K.; Raymond, K.; Lebrilla, C.B.; AlAsmari, A.; Suchy, S.F.; et al. Biallelic Mutations in FUT8 Cause a Congenital Disorder of Glycosylation with Defective Fucosylation. Am. J. Hum. Genet. 2018, 102, 188–195. [Google Scholar] [CrossRef]
- Schweigert, A.; Areaux, R.G., Jr. Childhood glaucoma in association with congenital disorder of glycosylation caused by mutations in fucosyltransferase 8. J. AAPOS 2019, 23, 351–352. [Google Scholar] [CrossRef]
- Feichtinger, R.G.; Hullen, A.; Koller, A.; Kotzot, D.; Grote, V.; Rapp, E.; Hofbauer, P.; Brugger, K.; Thiel, C.; Mayr, J.A.; et al. A spoonful of L-fucose-an efficient therapy for GFUS-CDG, a new glycosylation disorder. EMBO Mol. Med. 2021, 13, e14332. [Google Scholar] [CrossRef]
- Michalewska, B.; Olsson, M.L.; Naremska, G.; Walenciak, J.; Hult, A.K.; Ozog, A.; Guz, K.; Brojer, E.; Storry, J.R. FUT1 mutations responsible for the H-deficient phenotype in the Polish population, including the first example of an abolished start codon. Blood Transfus. 2018, 16, 101–104. [Google Scholar] [CrossRef]
- Soejima, M.; Koda, Y. FUT1 variants responsible for Bombay or para-Bombay phenotypes in a database. Sci. Rep. 2023, 13, 17447. [Google Scholar] [CrossRef]
- Storry, J.R.; Johannesson, J.S.; Poole, J.; Strindberg, J.; Rodrigues, M.J.; Yahalom, V.; Levene, C.; Fujita, C.; Castilho, L.; Hustinx, H.; et al. Identification of six new alleles at the FUT1 and FUT2 loci in ethnically diverse individuals with Bombay and Para-Bombay phenotypes. Transfusion 2006, 46, 2149–2155. [Google Scholar] [CrossRef]
- Koda, Y.; Soejima, M.; Johnson, P.H.; Smart, E.; Kimura, H. Missense mutation of FUT1 and deletion of FUT2 are responsible for Indian Bombay phenotype of ABO blood group system. Biochem. Biophys. Res. Commun. 1997, 238, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Song, D.; Wang, S. Novel deletion of the POFUT1 gene associated with multiple seborrheic keratosis Dowling-Degos disease. J. Dermatol. 2021, 48, e591–e593. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Liu, H.; Fu, X.; Yu, Y.; Yu, G.; Wang, C.; Bao, F.; Liany, H.; Wang, Z.; et al. Analysis of POFUT1 gene mutation in a Chinese family with Dowling-Degos disease. PLoS ONE 2014, 9, e104496. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, H.; Wong, D.; Schneider, M.; Freeze, H.H.; Takeuchi, M.; Berardinelli, S.J.; Ito, A.; Lee, H.; Nelson, S.F.; Haltiwanger, R.S. Variant in human POFUT1 reduces enzymatic activity and likely causes a recessive microcephaly, global developmental delay with cardiac and vascular features. Glycobiology 2018, 28, 276–283. [Google Scholar] [CrossRef]
- Lo-A-Njoe, S.M.; Verberne, E.A.; van der Veken, L.T.; Arends, E.; van Tintelen, J.P.; Postma, A.V.; van Haelst, M.M. Intragenic Deletions Associate with Congenital Heart Disease including Ebstein Anomaly. Cardiogenetics 2023, 13, 106–112. [Google Scholar] [CrossRef]
- Park, J.H.; Reunert, J.; He, M.; Mealer, R.G.; Noel, M.; Wada, Y.; Gruneberg, M.; Horvath, J.; Cummings, R.D.; Schwartz, O.; et al. L-Fucose treatment of FUT8-CDG. Mol. Genet. Metab. Rep. 2020, 25, 100680. [Google Scholar] [CrossRef]
- Etzioni, A.; Tonetti, M. Fucose supplementation in leukocyte adhesion deficiency type II. Blood 2000, 95, 3641–3643. [Google Scholar] [CrossRef]
- Sturla, L.; Puglielli, L.; Tonetti, M.; Berninsone, P.; Hirschberg, C.B.; De Flora, A.; Etzioni, A. Impairment of the Golgi GDP-L-fucose transport and unresponsiveness to fucose replacement therapy in LAD II patients. Pediatr. Res. 2001, 49, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Markus, H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ 2010, 341, c3666. [Google Scholar] [CrossRef]
- Cui, X.J.; Zhao, A.G.; Wang, X.L. Correlations of AFAP1, GMDS and PTGFR gene polymorphisms with intra-ocular pressure response to latanoprost in patients with primary open-angle glaucoma. J. Clin. Pharm. Ther. 2017, 42, 87–92. [Google Scholar] [CrossRef]
- Blanas, A.; Sahasrabudhe, N.M.; Rodriguez, E.; van Kooyk, Y.; van Vliet, S.J. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front. Oncol. 2018, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Wuhrer, M.; Rombouts, Y. Glycosylation characteristics of colorectal cancer. Adv. Cancer Res. 2015, 126, 203–256. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Pucci, M.; Malagolini, N. The Cancer-Associated Antigens Sialyl Lewis(a/x) and Sd(a): Two Opposite Faces of Terminal Glycosylation. Cancers 2021, 13, 5273. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef]
- Liao, C.; An, J.; Yi, S.; Tan, Z.; Wang, H.; Li, H.; Guan, X.; Liu, J.; Wang, Q. FUT8 and Protein Core Fucosylation in Tumours: From Diagnosis to Treatment. J. Cancer 2021, 12, 4109–4120. [Google Scholar] [CrossRef]
- Nakayama, K.; Moriwaki, K.; Imai, T.; Shinzaki, S.; Kamada, Y.; Murata, K.; Miyoshi, E. Mutation of GDP-mannose-4,6-dehydratase in colorectal cancer metastasis. PLoS ONE 2013, 8, e70298. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Suzuki, Y.; Igarashi, K.; Yokota, T.; Mori, S.; Suda, T.; Naitoh, A.; Isemura, M.; Asakura, H. Highly enhanced fucosylation of alpha-fetoprotein in patients with germ cell tumor. Cancer 1993, 72, 615–618. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimizu, T.; Hirokawa, F.; Inoue, Y.; Komeda, K.; Asakuma, M.; Miyamoto, Y.; Takeshita, A.; Shibayama, Y.; Tanigawa, N. Clinicopathological risk factors for recurrence within one year after initial hepatectomy for hepatocellular carcinoma. Am. Surg. 2011, 77, 572–578. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, K.; Qin, H.; Zhu, J.; Qin, Q.; Yu, Y.; Wang, H. GMDS knockdown impairs cell proliferation and survival in human lung adenocarcinoma. BMC Cancer 2018, 18, 600. [Google Scholar] [CrossRef]
- Lai, T.Y.; Chen, I.J.; Lin, R.J.; Liao, G.S.; Yeo, H.L.; Ho, C.L.; Wu, J.C.; Chang, N.C.; Lee, A.C.; Yu, A.L. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells. Cell Death Discov. 2019, 5, 74. [Google Scholar] [CrossRef]
- Taniuchi, F.; Higai, K.; Tanaka, T.; Azuma, Y.; Matsumoto, K. Transcriptional regulation of fucosyltransferase 1 gene expression in colon cancer cells. Sci. World J. 2013, 2013, 105464. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Kobayashi, N.; Ohki, R. FUCA1: An Underexplored p53 Target Gene Linking Glycosylation and Cancer Progression. Cancers 2024, 16, 2753. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, I.; Sawai, Y.; Kawase, T.; Okabe, A.; Tsutsumi, S.; Ichikawa, H.; Kobayashi, Y.; Tashiro, F.; Namiki, H.; Kondo, T.; et al. Novel p53 target gene FUCA1 encodes a fucosidase and regulates growth and survival of cancer cells. Cancer Sci. 2016, 107, 734–745. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, X.; Jankovic, J.; Deng, H. CADASIL: A NOTCH3-associated cerebral small vessel disease. J. Adv. Res. 2024, 66, 223–235. [Google Scholar] [CrossRef]
- Arboleda-Velasquez, J.F.; Rampal, R.; Fung, E.; Darland, D.C.; Liu, M.; Martinez, M.C.; Donahue, C.P.; Navarro-Gonzalez, M.F.; Libby, P.; D’Amore, P.A.; et al. CADASIL mutations impair Notch3 glycosylation by Fringe. Hum. Mol. Genet. 2005, 14, 1631–1639. [Google Scholar] [CrossRef]
- Takeda, K.; Kou, I.; Mizumoto, S.; Yamada, S.; Kawakami, N.; Nakajima, M.; Otomo, N.; Ogura, Y.; Miyake, N.; Matsumoto, N.; et al. Screening of known disease genes in congenital scoliosis. Mol. Genet. Genom. Med. 2018, 6, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, D.B.; Chapman, G.; Wouters, M.A.; Whittock, N.V.; Ellard, S.; Fatkin, D.; Turnpenny, P.D.; Kusumi, K.; Sillence, D.; Dunwoodie, S.L. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am. J. Hum. Genet. 2006, 78, 28–37. [Google Scholar] [CrossRef]
- Bulman, M.P.; Kusumi, K.; Frayling, T.M.; McKeown, C.; Garrett, C.; Lander, E.S.; Krumlauf, R.; Hattersley, A.T.; Ellard, S.; Turnpenny, P.D. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat. Genet. 2000, 24, 438–441. [Google Scholar] [CrossRef]
- Hellbusch, C.C.; Sperandio, M.; Frommhold, D.; Yakubenia, S.; Wild, M.K.; Popovici, D.; Vestweber, D.; Grone, H.J.; von Figura, K.; Lubke, T.; et al. Golgi GDP-fucose transporter-deficient mice mimic congenital disorder of glycosylation IIc/leukocyte adhesion deficiency II. J. Biol. Chem. 2007, 282, 10762–10772. [Google Scholar] [CrossRef]
- Yakubenia, S.; Frommhold, D.; Scholch, D.; Hellbusch, C.C.; Korner, C.; Petri, B.; Jones, C.; Ipe, U.; Bixel, M.G.; Krempien, R.; et al. Leukocyte trafficking in a mouse model for leukocyte adhesion deficiency II/congenital disorder of glycosylation IIc. Blood 2008, 112, 1472–1481. [Google Scholar] [CrossRef]
- Ishikawa, H.O.; Higashi, S.; Ayukawa, T.; Sasamura, T.; Kitagawa, M.; Harigaya, K.; Aoki, K.; Ishida, N.; Sanai, Y.; Matsuno, K. Notch deficiency implicated in the pathogenesis of congenital disorder of glycosylation IIc. Proc. Natl. Acad. Sci. USA 2005, 102, 18532–18537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Zou, T.T.; Liu, H.H.; Jia, H.B.; Zhang, X.Q. Knockout of the fcsk gene in zebrafish causes neurodevelopmental defects. Zool. Res. 2025, 46, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hayashiji, N.; Kawahara, G.; Xu, X.; Fukuda, T.; Kerever, A.; Gu, J.; Hayashi, Y.K.; Arikawa-Hirasawa, E. alpha-1,6-Fucosyltransferase Is Essential for Myogenesis in Zebrafish. Cells 2022, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, J.; Miyoshi, E.; Honke, K.; Taniguchi, N. Phenotype changes of Fut8 knockout mouse: Core fucosylation is crucial for the function of growth factor receptor(s). Methods Enzymol. 2006, 417, 11–22. [Google Scholar] [CrossRef]
- Smith, P.L.; Myers, J.T.; Rogers, C.E.; Zhou, L.; Petryniak, B.; Becker, D.J.; Homeister, J.W.; Lowe, J.B. Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J. Cell Biol. 2002, 158, 801–815. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Chen, K.Y.; Cui, M.; Wang, W.; Huang, X.; Awadellah, A.; Li, Q.; Friedman, A.; Xin, W.W.; et al. Fucosylation Deficiency in Mice Leads to Colitis and Adenocarcinoma. Gastroenterology 2017, 152, 193–205.e10. [Google Scholar] [CrossRef]
- Waterhouse, C.C.; Johnson, S.; Phillipson, M.; Zbytnuik, L.; Petri, B.; Kelly, M.; Lowe, J.B.; Kubes, P. Secretory cell hyperplasia and defects in Notch activity in a mouse model of leukocyte adhesion deficiency type II. Gastroenterology 2010, 138, 1079–1090.e5. [Google Scholar] [CrossRef]
- Sasamura, T.; Sasaki, N.; Miyashita, F.; Nakao, S.; Ishikawa, H.O.; Ito, M.; Kitagawa, M.; Harigaya, K.; Spana, E.; Bilder, D.; et al. neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 2003, 130, 4785–4795. [Google Scholar] [CrossRef]
- Fowler, G.; French, D.V.; Rose, A.; Squires, P.; Aniceto da Silva, C.; Ohata, S.; Okamoto, H.; French, C.R. Protein fucosylation is required for Notch dependent vascular integrity in zebrafish. Dev. Biol. 2021, 480, 62–68. [Google Scholar] [CrossRef]
- Ameen, M.T.; Alloway, H.; Longjohn, M.N.; Gendron, R.L.; Paradis, H.; Benoukraf, T.; French, C.R. Genomic Analysis of Glaucoma Pathogenesis Due to gmds Mutation in Zebrafish. Exp. Eye Res. 2025, 258, 110497. [Google Scholar] [CrossRef]
- Song, Y.; Willer, J.R.; Scherer, P.C.; Panzer, J.A.; Kugath, A.; Skordalakes, E.; Gregg, R.G.; Willer, G.B.; Balice-Gordon, R.J. Neural and synaptic defects in slytherin, a zebrafish model for human congenital disorders of glycosylation. PLoS ONE 2010, 5, e13743. [Google Scholar] [CrossRef] [PubMed]
- Ohata, S.; Kinoshita, S.; Aoki, R.; Tanaka, H.; Wada, H.; Tsuruoka-Kinoshita, S.; Tsuboi, T.; Watabe, S.; Okamoto, H. Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development 2009, 136, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Justice, M.J.; Dhillon, P. Using the mouse to model human disease: Increasing validity and reproducibility. Dis. Model. Mech. 2016, 9, 101–103. [Google Scholar] [CrossRef]
- Mouse Genome Sequencing, C.; Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Wang, W.; Tang, X.; Duan, C.; Tian, S.; Han, C.; Qian, W.; Jiang, X.; Hou, X.; Lin, R. Intestinal epithelium-specific Fut2 deficiency promotes colorectal cancer through down-regulating fucosylation of MCAM. J. Transl. Med. 2023, 21, 82. [Google Scholar] [CrossRef]
- Wang, X.; Inoue, S.; Gu, J.; Miyoshi, E.; Noda, K.; Li, W.; Mizuno-Horikawa, Y.; Nakano, M.; Asahi, M.; Takahashi, M.; et al. Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. USA 2005, 102, 15791–15796. [Google Scholar] [CrossRef]
- He, S.; Luo, Y.; Ma, W.; Wang, X.; Yan, C.; Hao, W.; Fang, Y.; Su, H.; Lai, B.; Liu, J.; et al. Endothelial POFUT1 controls injury-induced liver fibrosis by repressing fibrinogen synthesis. J. Hepatol. 2024, 81, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Ramirez, M.; Vazquez, E.; Barranco, A.; Gruart, A.; Delgado-Garcia, J.M.; Buck, R.; Rueda, R.; Martin, M.J. Oral supplementation of 2′-fucosyllactose during lactation improves memory and learning in rats. J. Nutr. Biochem. 2016, 31, 20–27. [Google Scholar] [CrossRef]
- Lorenzini, C.G.; Baldi, E.; Bucherelli, C.; Sacchetti, B.; Tassoni, G. 2-Deoxy-D-galactose effects on passive avoidance memorization in the rat. Neurobiol. Learn. Mem. 1997, 68, 317–324. [Google Scholar] [CrossRef]
- Shen, N.; Lin, H.; Wu, T.; Wang, D.; Wang, W.; Xie, H.; Zhang, J.; Feng, Z. Inhibition of TGF-beta1-receptor posttranslational core fucosylation attenuates rat renal interstitial fibrosis. Kidney Int. 2013, 84, 64–77. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Dehnert, K.W.; Beahm, B.J.; Huynh, T.T.; Baskin, J.M.; Laughlin, S.T.; Wang, W.; Wu, P.; Amacher, S.L.; Bertozzi, C.R. Metabolic labeling of fucosylated glycans in developing zebrafish. ACS Chem. Biol. 2011, 6, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bell, K.; Herrmann, A.; Arnhold, S.; Mercieca, K.; Anders, F.; Nagel-Wolfrum, K.; Thanos, S.; Prokosch, V. Crystallins Play a Crucial Role in Glaucoma and Promote Neuronal Cell Survival in an In Vitro Model Through Modulating Muller Cell Secretion. Invest. Ophthalmol. Vis. Sci. 2022, 63, 3. [Google Scholar] [CrossRef]
- Andley, U.P. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Dulle, J.E.; Rubsam, A.; Garnai, S.J.; Pawar, H.S.; Fort, P.E. BetaB2-crystallin mutations associated with cataract and glaucoma leads to mitochondrial alterations in lens epithelial cells and retinal neurons. Exp. Eye Res. 2017, 155, 85–90. [Google Scholar] [CrossRef]
- Piri, N.; Kwong, J.M.; Caprioli, J. Crystallins in retinal ganglion cell survival and regeneration. Mol. Neurobiol. 2013, 48, 819–828. [Google Scholar] [CrossRef]
- Prokosch, V.; Schallenberg, M.; Thanos, S. Crystallins are regulated biomarkers for monitoring topical therapy of glaucomatous optic neuropathy. PLoS ONE 2013, 8, e49730. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Li, M.; Cheng, R.; Liang, J.; Yan, H.; Zhang, H.; Yang, L.; Li, C.; Jiao, Q.; Lu, Z.; He, J.; et al. Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am. J. Hum. Genet. 2013, 92, 895–903. [Google Scholar] [CrossRef]
- Barrows, B.D.; Haslam, S.M.; Bischof, L.J.; Morris, H.R.; Dell, A.; Aroian, R.V. Resistance to Bacillus thuringiensis toxin in Caenorhabditis elegans from loss of fucose. J. Biol. Chem. 2007, 282, 3302–3311. [Google Scholar] [CrossRef]
- Roos, C.; Kolmer, M.; Mattila, P.; Renkonen, R. Composition of Drosophila melanogaster proteome involved in fucosylated glycan metabolism. J. Biol. Chem. 2002, 277, 3168–3175. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Koles, K.; Vorndam, W.; Haltiwanger, R.S.; Panin, V.M. Protein O-fucosyltransferase 2 adds O-fucose to thrombospondin type 1 repeats. J. Biol. Chem. 2006, 281, 9393–9399. [Google Scholar] [CrossRef] [PubMed]
- Okajima, T.; Irvine, K.D. Regulation of notch signaling by o-linked fucose. Cell 2002, 111, 893–904. [Google Scholar] [CrossRef]
- Rendic, D.; Linder, A.; Paschinger, K.; Borth, N.; Wilson, I.B.; Fabini, G. Modulation of neural carbohydrate epitope expression in Drosophila melanogaster cells. J. Biol. Chem. 2006, 281, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Paschinger, K.; Staudacher, E.; Stemmer, U.; Fabini, G.; Wilson, I.B. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology 2005, 15, 463–474. [Google Scholar] [CrossRef]
- Staudacher, E.; Altmann, F.; Wilson, I.B.; Marz, L. Fucose in N-glycans: From plant to man. Biochim. Biophys. Acta 1999, 1473, 216–236. [Google Scholar] [CrossRef]
- Geisler, C.; Kotu, V.; Sharrow, M.; Rendic, D.; Poltl, G.; Tiemeyer, M.; Wilson, I.B.; Jarvis, D.L. The Drosophila neurally altered carbohydrate mutant has a defective Golgi GDP-fucose transporter. J. Biol. Chem. 2012, 287, 29599–29609. [Google Scholar] [CrossRef]
- Perdigoto, C.N.; Schweisguth, F.; Bardin, A.J. Distinct levels of Notch activity for commitment and terminal differentiation of stem cells in the adult fly intestine. Development 2011, 138, 4585–4595. [Google Scholar] [CrossRef]
- Glittenberg, M.T.; Kounatidis, I.; Atilano, M.; Ligoxygakis, P. A genetic screen in Drosophila reveals the role of fucosylation in host susceptibility to Candida infection. Dis. Model. Mech. 2022, 15, dmm049218. [Google Scholar] [CrossRef]
- Nguyen, K.; van Die, I.; Grundahl, K.M.; Kawar, Z.S.; Cummings, R.D. Molecular cloning and characterization of the Caenorhabditis elegans alpha1,3-fucosyltransferase family. Glycobiology 2007, 17, 586–599. [Google Scholar] [CrossRef]
- Zheng, Q.; Van Die, I.; Cummings, R.D. Molecular cloning and characterization of a novel alpha 1,2-fucosyltransferase (CE2FT-1) from Caenorhabditis elegans. J. Biol. Chem. 2002, 277, 39823–39832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Van Die, I.; Cummings, R.D. A novel alpha1,2-fucosyltransferase (CE2FT-2) in Caenorhabditis elegans generates H-type 3 glycan structures. Glycobiology 2008, 18, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Rhomberg, S.; Fuchsluger, C.; Rendic, D.; Paschinger, K.; Jantsch, V.; Kosma, P.; Wilson, I.B. Reconstitution in vitro of the GDP-fucose biosynthetic pathways of Caenorhabditis elegans and Drosophila melanogaster. FEBS J. 2006, 273, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jin, C.; Wilson, I.B.; Paschinger, K. Comparisons of Caenorhabditis Fucosyltransferase Mutants Reveal a Multiplicity of Isomeric N-Glycan Structures. J. Proteome Res. 2015, 14, 5291–5305. [Google Scholar] [CrossRef]
| Human Gene | Gene Function | Human Phenotype | Animal/Cell Mutant Allele | Animal/Cell Based Phenotype | References |
|---|---|---|---|---|---|
| SLC35C1 | GDP-fucose transporter | LADII, leukocytosis, recurrent infections, growth retardation | Slc35c1-/- (mouse) nac-/- (Drosophila) | Growth retardation, reduced neutrophil rolling, hypocellular lymph nodes, dilated alveoles | [2,22,59,60,61] |
| FCSK | L-fucose kinase in salvage pathway | Severe infantile-onset epilepsy, neurodevelopmental delay, optical abnormalities | fcsk-/- (zebrafish), fcsk morpholino | Seizures, cerebral hemorrhage, growth retardation, social behaviors, brain atrophy | [22,23,62] |
| FUT8 | Core alpha 1,6-fucosyltransferase | Epilepsy, microcephaly, emphysema, myogenesis defect, congenital glaucoma | Fut8-/- (mouse), fut8 morpholino (zebrafish) | Growth retardation, reduced survival, abnormal lung development | [22,25,26,63,64] |
| GFUS (FX) | GDP-keto-6-deoxy mannose epimerase/reductase | Developmental delay, leukocytosis, colitis, adenocarcinoma, brain abnormalities, feeding aversion | Gfus-/- (mouse) | Reduced survival, leukocytosis, colitis, adenocarcinoma | [22,27,65,66,67] |
| POFUT1 | Protein O-fucosyltransferase | Dowling-Degos disease, liver fibrosis, hypopigmentation | Pofut1 conditional KO (mouse), pofut1 morpholino (zebrafish), nti-/- (Drosophila) | Injury induced liver fibrosis, hypopigmentation | [32,33,34,68] |
| GMDS | GDP-mannose 4,6-dehydratase | congenital heart defect with Ebstein Anomaly, Glaucoma, cancer biomarker, cerebral small vessel diseases (CSVD) | gmds-/- (zebrafish), GMDS-shRNA (cell lines), gmd-/- mutant (drosophila) | Altered neural migration, synaptogenesis, hemorrhage, curly tail, reduced retinal ganglion cell number | [19,35,49,69,70,71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, M.T.; French, C.R. Genetic Diseases of Fucosylation: Insights from Model Organisms. Genes 2025, 16, 800. https://doi.org/10.3390/genes16070800
Ameen MT, French CR. Genetic Diseases of Fucosylation: Insights from Model Organisms. Genes. 2025; 16(7):800. https://doi.org/10.3390/genes16070800
Chicago/Turabian StyleAmeen, Muhammad T., and Curtis R. French. 2025. "Genetic Diseases of Fucosylation: Insights from Model Organisms" Genes 16, no. 7: 800. https://doi.org/10.3390/genes16070800
APA StyleAmeen, M. T., & French, C. R. (2025). Genetic Diseases of Fucosylation: Insights from Model Organisms. Genes, 16(7), 800. https://doi.org/10.3390/genes16070800





