Cascade Genetic Testing for Hereditary Cancer Predisposition: Characterization of Patients in a Catchment Area of Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Probands and Relatives
2.3. Data Analysis
3. Results
3.1. Characterization of Patients
3.2. Evaluation of the Perception of Cancer Risk and the Gender of Patients
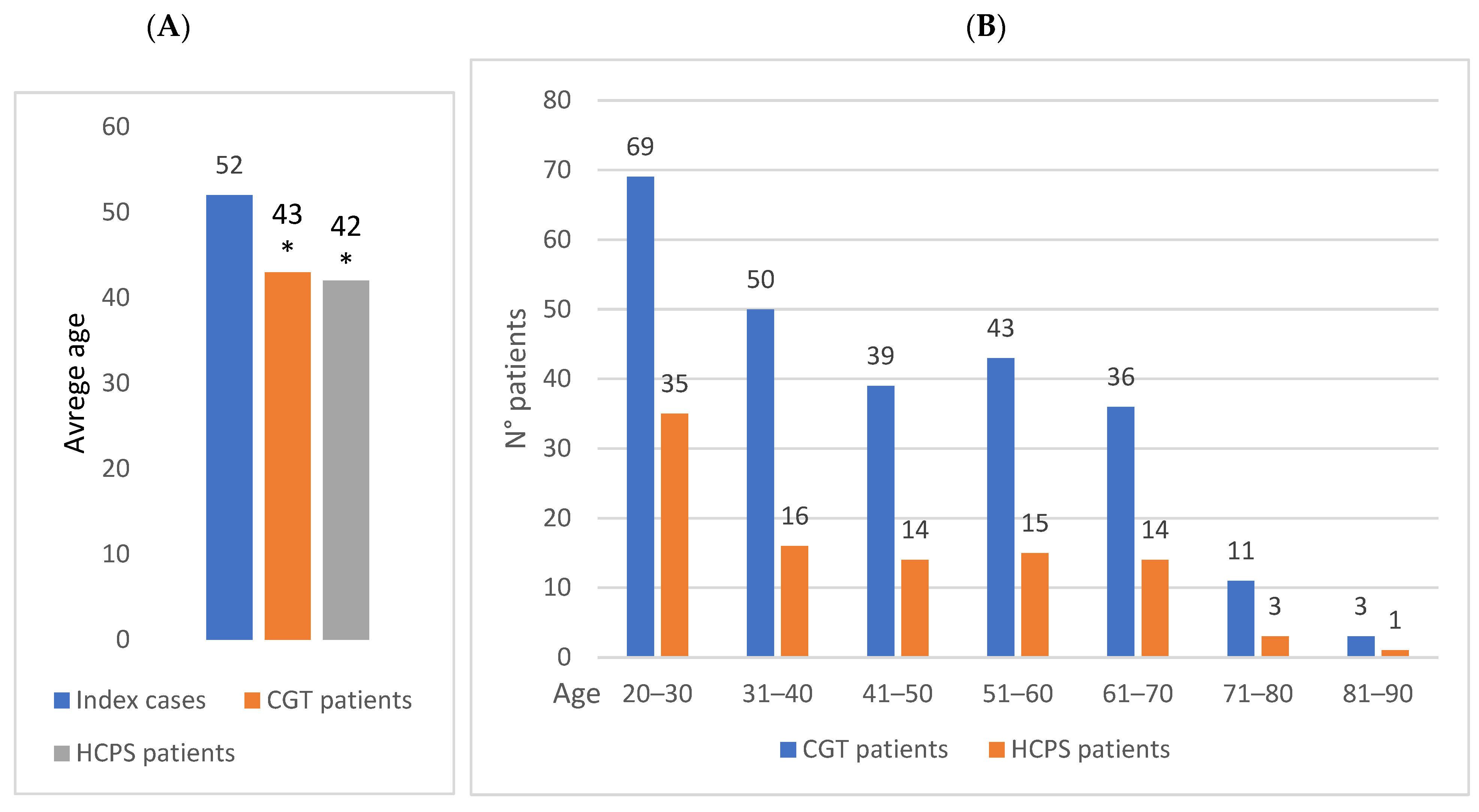
3.3. Evaluation of the Age of Patients
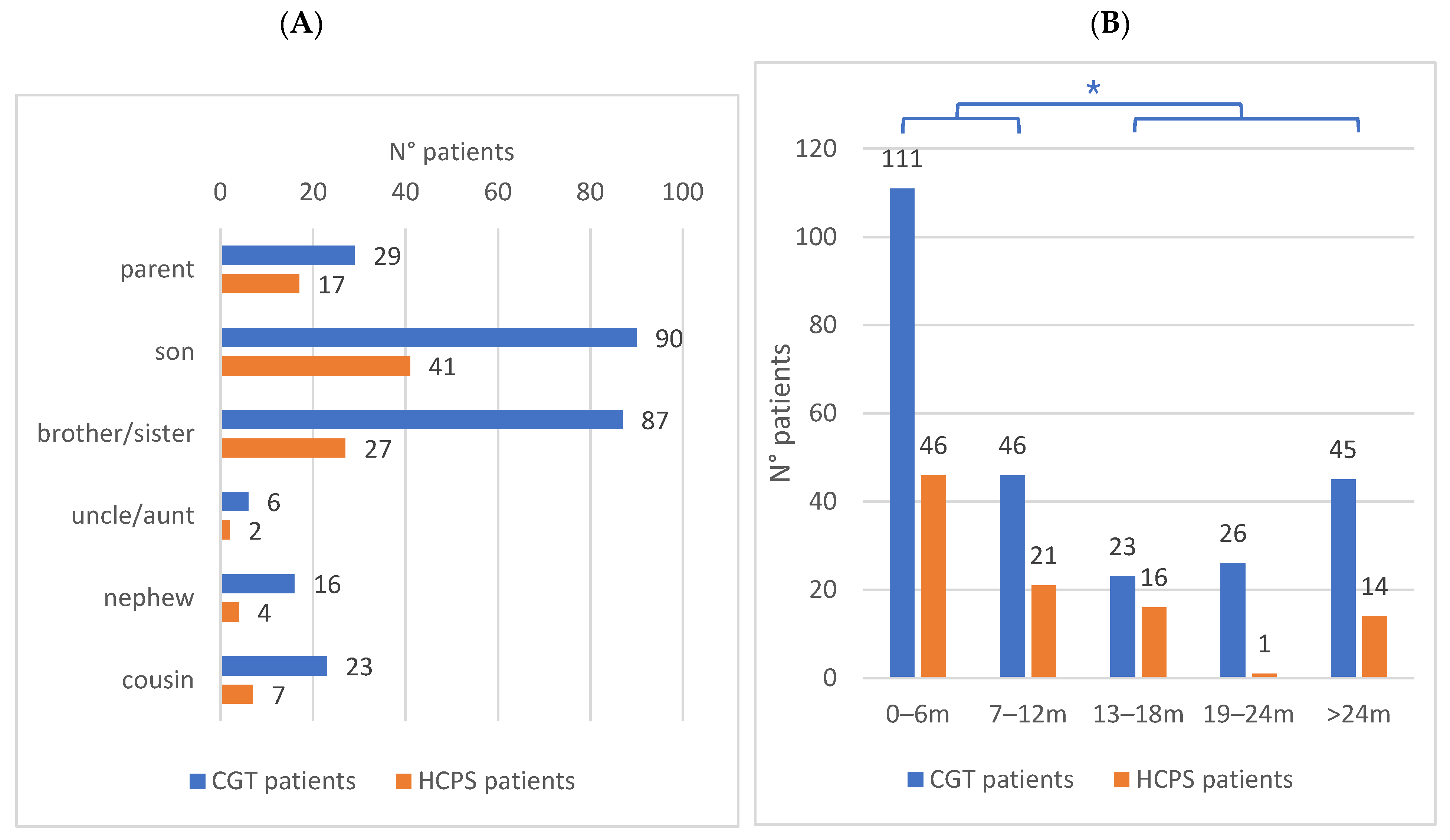
3.4. Evaluation of Family Relationship Between Patients
3.5. Evaluation of Time Interval of Information
3.6. Evaluation of the Uptake of CGTs
3.7. Evaluation of PVs Identified and Relative Frequencies
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandelker, D.; Zhang, L.; Kemel, Y.; Stadler, Z.K.; Joseph, V.; Zehir, A.; Pradhan, N.; Arnold, A.; Walsh, M.F.; Li, Y.; et al. Mutation Detection in Patients With Advanced Cancer by Universal Sequencing of Cancer-Related Genes in Tumor and Normal DNA vs Guideline-Based Germline Testing. J. Am. Med. Assoc. 2017, 318, 825. [Google Scholar] [CrossRef] [PubMed]
- Tsaousis, G.N.; Papadopoulou, E.; Apessos, A.; Agiannitopoulos, K.; Pepe, G.; Kampouri, S.; Diamantopoulos, N.; Floros, T.; Iosifidou, R.; Katopodi, O.; et al. Analysis of Hereditary Cancer Syndromes by Using a Panel of Genes: Novel and Multiple Pathogenic Mutations. BMC Cancer 2019, 19, 535. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Sweet, K.; Eng, C. Highly Penetrant Hereditary Cancer Syndromes. Oncogene 2004, 23, 6445–6470. [Google Scholar] [CrossRef] [PubMed]
- McGee, R.B.; Nichols, K.E. Introduction to Cancer Genetic Susceptibility Syndromes. Hematology 2016, 2016, 293–301. [Google Scholar] [CrossRef]
- Akras, Z.; Bungo, B.; Leach, B.H.; Marquard, J.; Ahluwalia, M.; Carraway, H.; Grivas, P.; Sohal, D.P.S.; Funchain, P. Primer on Hereditary Cancer Predisposition Genes Included Within Somatic Next-Generation Sequencing Panels. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Sokolova, A.; Johnstone, K.J.; McCart Reed, A.E.; Simpson, P.T.; Lakhani, S.R. Hereditary Breast Cancer: Syndromes, Tumour Pathology and Molecular Testing. Histopathology 2023, 82, 70–82. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 380–391. [Google Scholar] [CrossRef]
- Tomlinson-Hansen, S.; Beaston, M. Hereditary Cancer Genes and Related Risks. Rhode Isl. Med. J. 2023, 106, 12–17. [Google Scholar]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Stasiewicz, M. Carcinogenic Microbiota and Its Role in Colorectal Cancer Development. Semin. Cancer Biol. 2022, 86, 420–430. [Google Scholar] [CrossRef]
- Bhai, P.; Levy, M.A.; Rooney, K.; Carere, D.A.; Reilly, J.; Kerkhof, J.; Volodarsky, M.; Stuart, A.; Kadour, M.; Panabaker, K.; et al. Analysis of Sequence and Copy Number Variants in Canadian Patient Cohort With Familial Cancer Syndromes Using a Unique Next Generation Sequencing Based Approach. Front. Genet. 2021, 12, 698595. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.J.; Arcila, M.E.; Corless, C.; Kamel-Reid, S.; Lubin, I.M.; Pfeifer, J.; Temple-Smolkin, R.L.; Voelkerding, K.V.; Nikiforova, M.N. Guidelines for Validation of Next-Generation Sequencing–Based Oncology Panels. J. Mol. Diagn. 2017, 19, 341–365. [Google Scholar] [CrossRef] [PubMed]
- Tuffaha, H.W.; Mitchell, A.; Ward, R.L.; Connelly, L.; Butler, J.R.G.; Norris, S.; Scuffham, P.A. Cost-Effectiveness Analysis of Germ-Line BRCA Testing in Women with Breast Cancer and Cascade Testing in Family Members of Mutation Carriers. Genet. Med. 2018, 20, 985–994. [Google Scholar] [CrossRef]
- AIOM. Raccomandazioni per l’implementazione Del Test BRCA Predittivo e Preventivo Nei Tumori Della Mammella, Dell’ovaio, Del Pancreas e Della Prostata; AIOM (Associazione Italiana Oncologia Medica): Milano, Italy, 2021. [Google Scholar]
- AIOM. Raccomandazioni 2019 per l’implementazione Del Test BRCA Nelle Pazienti Con Carcinoma Mammario e Nei Familiari a Rischio Elevato di Neoplasia; AIOM (Associazione Italiana Oncologia Medica): Milano, Italy, 2019. [Google Scholar]
- Whitaker, K.D.; Obeid, E.; Daly, M.B.; Hall, M.J. Cascade Genetic Testing for Hereditary Cancer Risk: An Underutilized Tool for Cancer Prevention. JCO Precis. Oncol. 2021, 5, 1387–1396. [Google Scholar] [CrossRef]
- Griffin, N.E.; Buchanan, T.R.; Smith, S.H.; Leon, A.A.; Meyer, M.F.; Liu, J.; Tabak, R.G.; Fuh, K.C.; Thaker, P.H.; Powell, M.A.; et al. Low Rates of Cascade Genetic Testing among Families with Hereditary Gynecologic Cancer: An Opportunity to Improve Cancer Prevention. Gynecol. Oncol. 2020, 156, 140–146. [Google Scholar] [CrossRef]
- AIOM. Raccomandazioni AIOM-I Tumori Ereditari Dello Stomaco e Del Colon-Retto; AIOM (Associazione Italiana Oncologia Medica): Milano, Italy, 2022. [Google Scholar]
- Hodan, R.; Gupta, S.; Weiss, J.M.; Axell, L.; Burke, C.A.; Chen, L.-M.; Chung, D.C.; Clayback, K.M.; Felder, S.; Foda, Z.; et al. Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric, Version 3.2024, NCCN Clinical Practice Guidelines In Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 695–711. [Google Scholar] [CrossRef]
- Sessa, C.; Balmaña, J.; Bober, S.L.; Cardoso, M.J.; Colombo, N.; Curigliano, G.; Domchek, S.M.; Evans, D.G.; Fischerova, D.; Harbeck, N.; et al. Risk Reduction and Screening of Cancer in Hereditary Breast-Ovarian Cancer Syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 33–47. [Google Scholar] [CrossRef]
- Derbez, B.; de Pauw, A.; Stoppa-Lyonnet, D.; de Montgolfier, S. Supporting Disclosure of Genetic Information to Family Members: Professional Practice and Timelines in Cancer Genetics. Fam. Cancer 2017, 16, 447–457. [Google Scholar] [CrossRef]
- Di Pietro, M.L.; Zaçe, D.; Orfino, A.; Di Raimo, F.R.; Poscia, A.; de Matteis, E.; Turchetti, D.; Godino, L.; Bertonazzi, B.; Franiuk, M.; et al. Intrafamilial Communication of Hereditary Breast and Ovarian Cancer Genetic Information in Italian Women: Towards a Personalised Approach. Eur. J. Hum. Genet. 2021, 29, 250–261. [Google Scholar] [CrossRef]
- Pensabene, M.; Calabrese, A.; von Arx, C.; Caputo, R.; De Laurentiis, M. Cancer Genetic Counselling for Hereditary Breast Cancer in the Era of Precision Oncology. Cancer Treat. Rev. 2024, 125, 102702. [Google Scholar] [CrossRef]
- Menko, F.H.; Jeanson, K.N.; Bleiker, E.M.A.; van Tiggelen, C.W.M.; Hogervorst, F.B.L.; ter Stege, J.A.; Ait Moha, D.; van der Kolk, L.E. The Uptake of Predictive DNA Testing in 40 Families with a Pathogenic BRCA1/BRCA2 Variant. An Evaluation of the Proband-Mediated Procedure. Eur. J. Hum. Genet. 2020, 28, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Agiannitopoulos, K.; Potska, K.; Katseli, A.; Ntogka, C.; Tsaousis, G.N.; Pepe, G.; Bouzarelou, D.; Tsoulos, N.; Papathanasiou, A.; Ziogas, D.; et al. Only 32.3% of Breast Cancer Families with Pathogenic Variants in Cancer Genes Utilized Cascade Genetic Testing. Cancers 2023, 15, 5218. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; ter Stege, J.A.; van der Kolk, L.E.; Jeanson, K.N.; Schats, W.; Moha, D.A.; Bleiker, E.M.A. The Uptake of Presymptomatic Genetic Testing in Hereditary Breast-Ovarian Cancer and Lynch Syndrome: A Systematic Review of the Literature and Implications for Clinical Practice. Fam. Cancer 2019, 18, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Schmidlen, T.J.; Bristow, S.L.; Hatchell, K.E.; Esplin, E.D.; Nussbaum, R.L.; Haverfield, E.V. The Impact of Proband Indication for Genetic Testing on the Uptake of Cascade Testing Among Relatives. Front. Genet. 2022, 13, 867226. [Google Scholar] [CrossRef]
- Mesa-Chavez, F.; Chavarri-Guerra, Y.; Aguilar-y-Mendez, D.; Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Carrillo-Bedoya, A.; Santiesteban-González, S.; Aranda-Gutierrez, A.; Rodríguez-Faure, A.; Obregon-Leal, D.; et al. Uptake of Risk-Reducing Measures, Cascade Testing, and Related Challenges Among Carriers of Breast Cancer–Associated Germline Pathogenic Variants in Mexico. JCO Glob. Oncol. 2024, 10, e2300417. [Google Scholar] [CrossRef]
- Nycum, G.; Avard, D.; Knoppers, B.M. Factors Influencing Intrafamilial Communication of Hereditary Breast and Ovarian Cancer Genetic Information. Eur. J. Hum. Genet. 2009, 17, 872–880. [Google Scholar] [CrossRef]
- Elrick, A.; Ashida, S.; Ivanovich, J.; Lyons, S.; Biesecker, B.B.; Goodman, M.S.; Kaphingst, K.A. Psychosocial and Clinical Factors Associated with Family Communication of Cancer Genetic Test Results among Women Diagnosed with Breast Cancer at a Young Age. J. Genet. Couns. 2017, 26, 173–181. [Google Scholar] [CrossRef]
- Paduano, F.; Colao, E.; Fabiani, F.; Rocca, V.; Dinatolo, F.; Dattola, A.; D’Antona, L.; Amato, R.; Trapasso, F.; Baudi, F.; et al. Germline Testing in a Cohort of Patients at High Risk of Hereditary Cancer Predisposition Syndromes: First Two-Year Results from South Italy. Genes 2022, 13, 1286. [Google Scholar] [CrossRef]
- Rocca, V.; Lo Feudo, E.; Dinatolo, F.; Lavano, S.M.; Bilotta, A.; Amato, R.; D’Antona, L.; Trapasso, F.; Baudi, F.; Colao, E.; et al. Germline Variant Spectrum in Southern Italian High-Risk Hereditary Breast Cancer Patients: Insights from Multi-Gene Panel Testing. Curr. Issues Mol. Biol. 2024, 46, 13003–13020. [Google Scholar] [CrossRef]
- Baudi, F.; Quaresima, B.; Grandinetti, C.; Cuda, G.; Faniello, C.; Tassone, P.; Barbieri, V.; Bisegna, R.; Ricevuto, E.; Conforti, S.; et al. Evidence of a Founder Mutation of BRCA1 in a Highly Homogeneous Population from Southern Italy with Breast/Ovarian Cancer. Hum. Mutat. 2001, 18, 163–164. [Google Scholar] [CrossRef]
- Paduano, F.; Fabiani, F.; Colao, E.; Trapasso, F.; Perrotti, N.; Barbieri, V.; Baudi, F.; Iuliano, R. Case Report: Identification of a Novel Pathogenic Germline TP53 Variant in a Family With Li–Fraumeni Syndrome. Front. Genet. 2021, 12, 734809. [Google Scholar] [CrossRef] [PubMed]
- Giorgi Rossi, P.; Carrozzi, G.; Federici, A.; Mancuso, P.; Sampaolo, L.; Zappa, M. Invitation Coverage and Participation in Italian Cervical, Breast and Colorectal Cancer Screening Programmes. J. Med. Screen. 2018, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zappa, M.; Bevere, F.; Braga, M.; Broccoli, S.; Cosentino, M.; Galeone, D.; Federici, A.; Marvulli, M.; Vasselli, S.; Venturelli, F.; et al. Development of a Conceptual Model for Interpretation of Monitoring Indicators of Cancer Screenings from the Italian National Prevention Plan. Epidemiol. Prev. 2019, 43, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, L.; Godino, L.; Battistuzzi, L.; Innella, G.; Luppi, E.; Buzzatti, G.; Gismondi, V.; Blondeaux, E.; Bonelli, L.A.; Turchetti, D.; et al. Cascade Testing in Italian Hereditary Breast Ovarian Cancer Families: A Missed Opportunity for Cancer Prevention? Fam. Cancer 2024, 23, 197–207. [Google Scholar] [CrossRef]
- Frey, M.K.; Ahsan, M.D.; Bergeron, H.; Lin, J.; Li, X.; Fowlkes, R.K.; Narayan, P.; Nitecki, R.; Rauh-Hain, J.A.; Moss, H.A.; et al. Cascade Testing for Hereditary Cancer Syndromes: Should We Move Toward Direct Relative Contact? A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2022, 40, 4129–4143. [Google Scholar] [CrossRef]
- Kilbride, M.K. Genetic Privacy, Disease Prevention, and the Principle of Rescue. Hastings Cent. Rep. 2018, 48, 10–17. [Google Scholar] [CrossRef]
- Battistuzzi, L. The Challenges of Cascade Genetic Testing in Hereditary Cancer Syndromes: A Few Ethical Considerations. Tumori J. 2024. [Google Scholar] [CrossRef]
- Varesco, L.; Di Tano, F.; Monducci, J.; Sciallero, S.; Turchetti, D.; Bighin, C.; Buzzatti, G.; Giannubilo, I.; Trevisan, L.; Battistuzzi, L. Cascade Genetic Testing in Hereditary Cancer: Exploring the Boundaries of the Italian Legal Framework. Fam. Cancer 2024, 24, 9. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hampel, H.; Leeman, J.; Patel, A.; Kulchak Rahm, A.; Reuland, D.S.; Roberts, M.C. Stakeholder Perspectives on Overcoming Barriers to Cascade Testing in Lynch Syndrome: A Qualitative Study. Cancer Prev. Res. 2020, 13, 1037–1046. [Google Scholar] [CrossRef]
- Dheensa, S.; Lucassen, A.; Fenwick, A. Limitations and Pitfalls of Using Family Letters to Communicate Genetic Risk: A Qualitative Study with Patients and Healthcare Professionals. J. Genet. Couns. 2018, 27, 689–701. [Google Scholar] [CrossRef]
- Evans, D.G.; Green, K.; Burghel, G.J.; Forde, C.; Lalloo, F.; Schlecht, H.; Woodward, E.R. Cascade Screening in HBOC and Lynch Syndrome: Guidelines and Procedures in a UK Centre. Fam. Cancer 2024, 23, 187–195. [Google Scholar] [CrossRef]
- Tommasi, S.; Maurmo, L.; Rizzo, A.; Carella, C.; Ranieri, G.; De Summa, S.; Mannavola, F.; Chiurì, V.E.; Guida, M.; Nisi, C.; et al. The Molecular Tumor Board as a Step in Cancer Patient Management: A Southern Italian Experience. Front. Med. 2024, 11, 1432628. [Google Scholar] [CrossRef]

| Index Cases | CGT Patients | HCPS Patients | |
|---|---|---|---|
| Total Patients | 116 | 251 (206 first-degree) | 98 (89 first-degree) |
| NGSp/o “R. Dulbecco” | 25 | 72 (65 first-grade) | 24 (23 first-grade) |
| NGS p/o other facilities | 54 | 106 (85 first-grade) | 45 (40 first-grade) |
| Sanger report | 37 | 73 (56 first-grade) | 29 (26 first-grade) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilotta, A.; Lo Feudo, E.; Rocca, V.; Colao, E.; Dinatolo, F.; Lavano, S.M.; Malatesta, P.; D’Antona, L.; Amato, R.; Trapasso, F.; et al. Cascade Genetic Testing for Hereditary Cancer Predisposition: Characterization of Patients in a Catchment Area of Southern Italy. Genes 2025, 16, 795. https://doi.org/10.3390/genes16070795
Bilotta A, Lo Feudo E, Rocca V, Colao E, Dinatolo F, Lavano SM, Malatesta P, D’Antona L, Amato R, Trapasso F, et al. Cascade Genetic Testing for Hereditary Cancer Predisposition: Characterization of Patients in a Catchment Area of Southern Italy. Genes. 2025; 16(7):795. https://doi.org/10.3390/genes16070795
Chicago/Turabian StyleBilotta, Anna, Elisa Lo Feudo, Valentina Rocca, Emma Colao, Francesca Dinatolo, Serena Marianna Lavano, Paola Malatesta, Lucia D’Antona, Rosario Amato, Francesco Trapasso, and et al. 2025. "Cascade Genetic Testing for Hereditary Cancer Predisposition: Characterization of Patients in a Catchment Area of Southern Italy" Genes 16, no. 7: 795. https://doi.org/10.3390/genes16070795
APA StyleBilotta, A., Lo Feudo, E., Rocca, V., Colao, E., Dinatolo, F., Lavano, S. M., Malatesta, P., D’Antona, L., Amato, R., Trapasso, F., Perrotti, N., Viglietto, G., Baudi, F., & Iuliano, R. (2025). Cascade Genetic Testing for Hereditary Cancer Predisposition: Characterization of Patients in a Catchment Area of Southern Italy. Genes, 16(7), 795. https://doi.org/10.3390/genes16070795







