Regulatory RNA Networks in Ovarian Follicular Cysts in Dairy Cows: Implications for Human Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Sample Collection
2.4. Follicular Fluid Mature miRNA Profiling Using Real-Time PCR
2.5. Bioinformatics Analyses
2.5.1. Conserved Nucleotide Sequences
2.5.2. Identification of Target Genes
2.5.3. Protein–Protein Interaction (PPI) Network
2.5.4. Network-Based Methodology for Analyzing Regulatory Interplay Among miRNA, circRNA, lncRNA, snRNA, and mRNA
3. Results
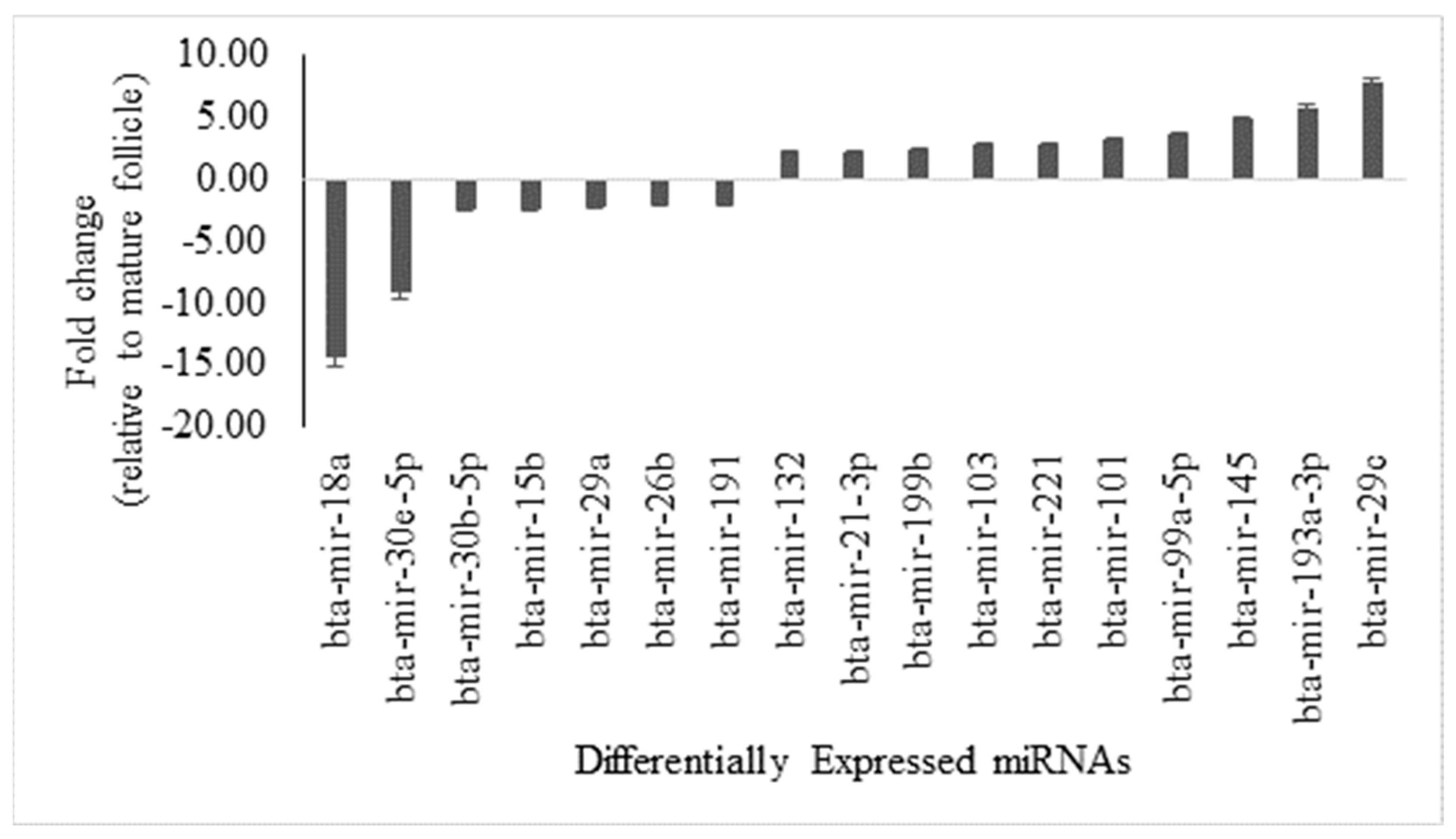
3.1. Comparative Analysis of miRNA Expression in Antral Fluid from Mature Ovarian Follicles Versus Ovarian Follicular Cysts, with Sequence Similarity Insights
3.2. Bovine and Human miRNA Sequence Similarity Insights
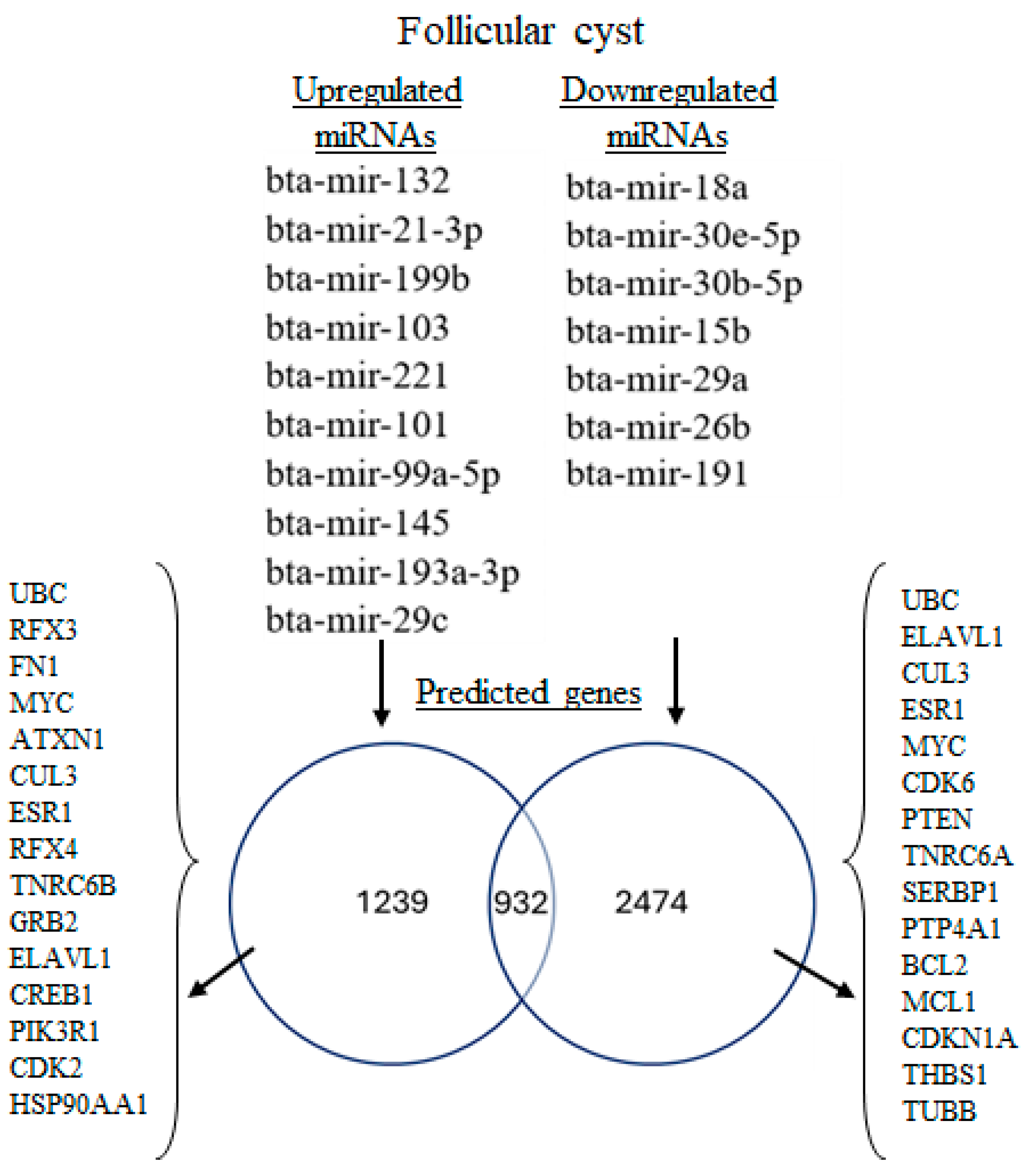
3.3. miRNA-mRNA Interaction Analysis for Upregulated and Downregulated miRNAs
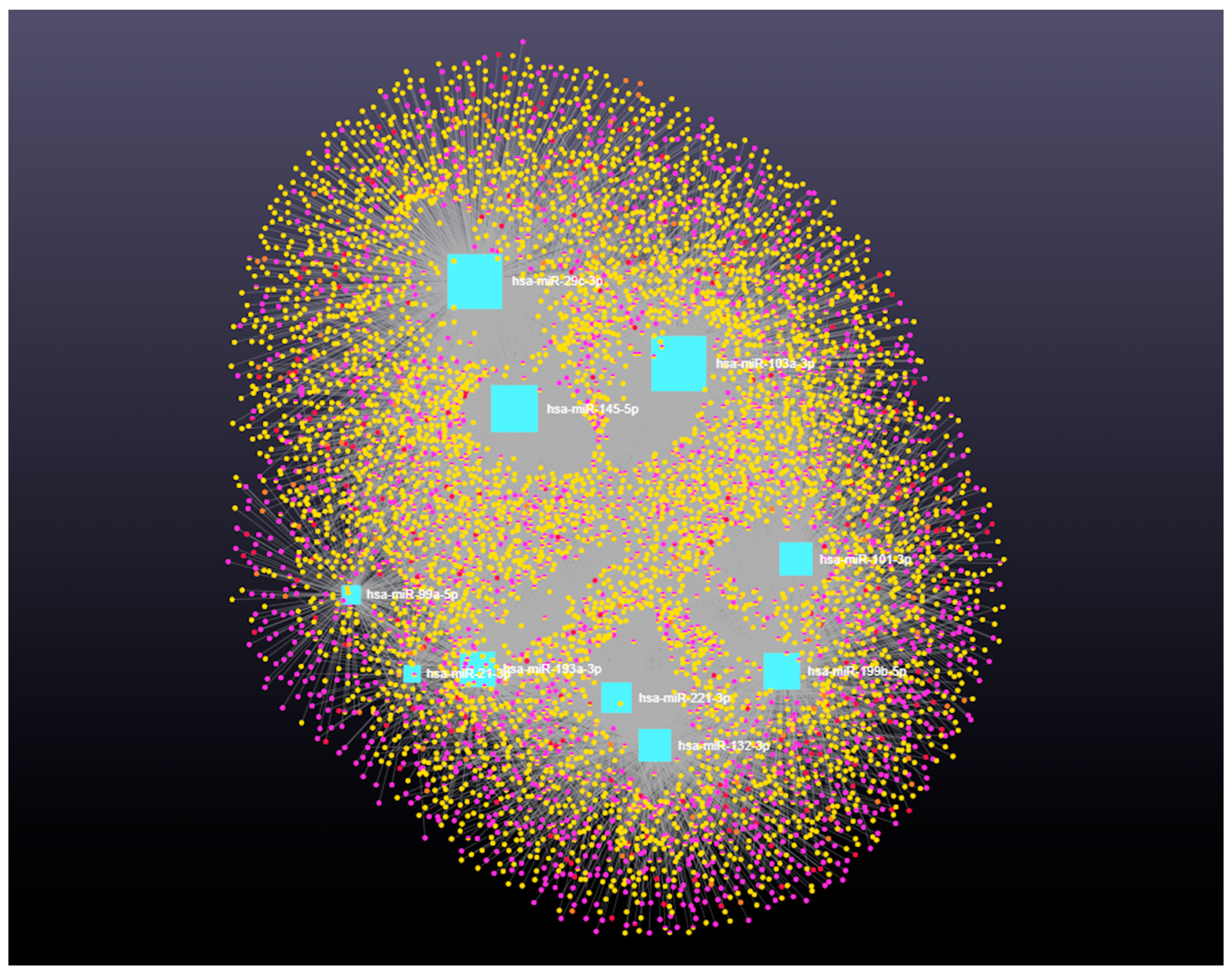
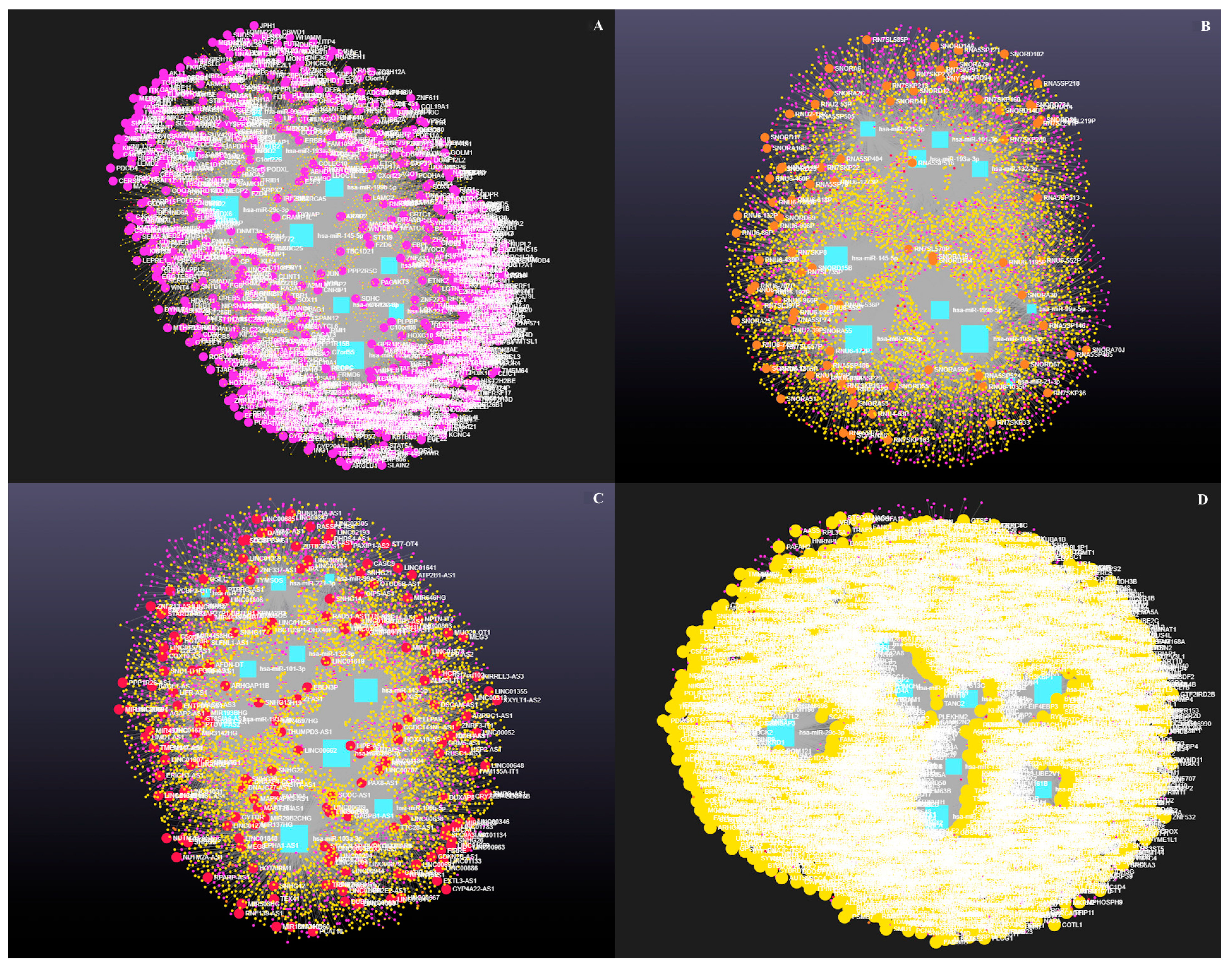
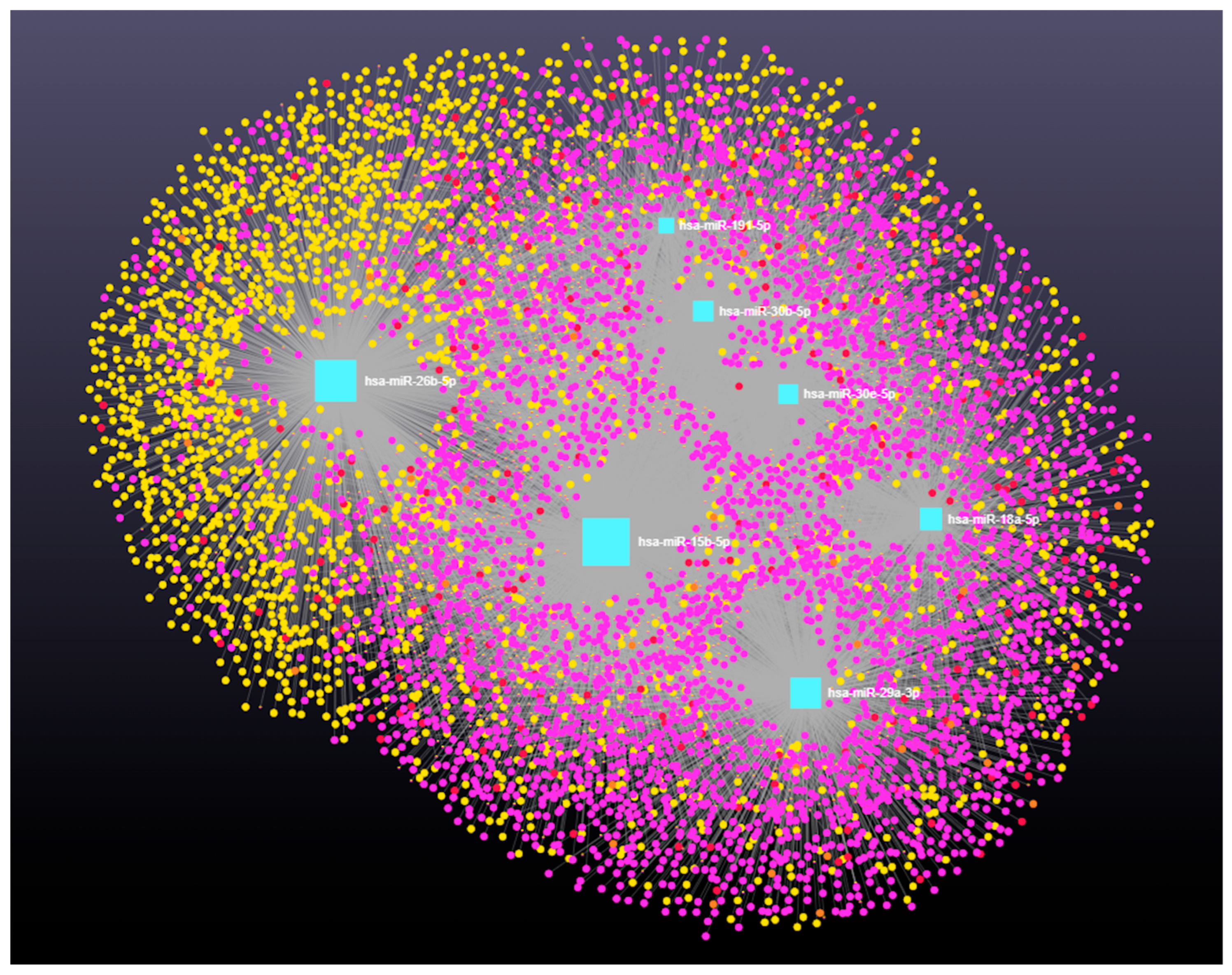
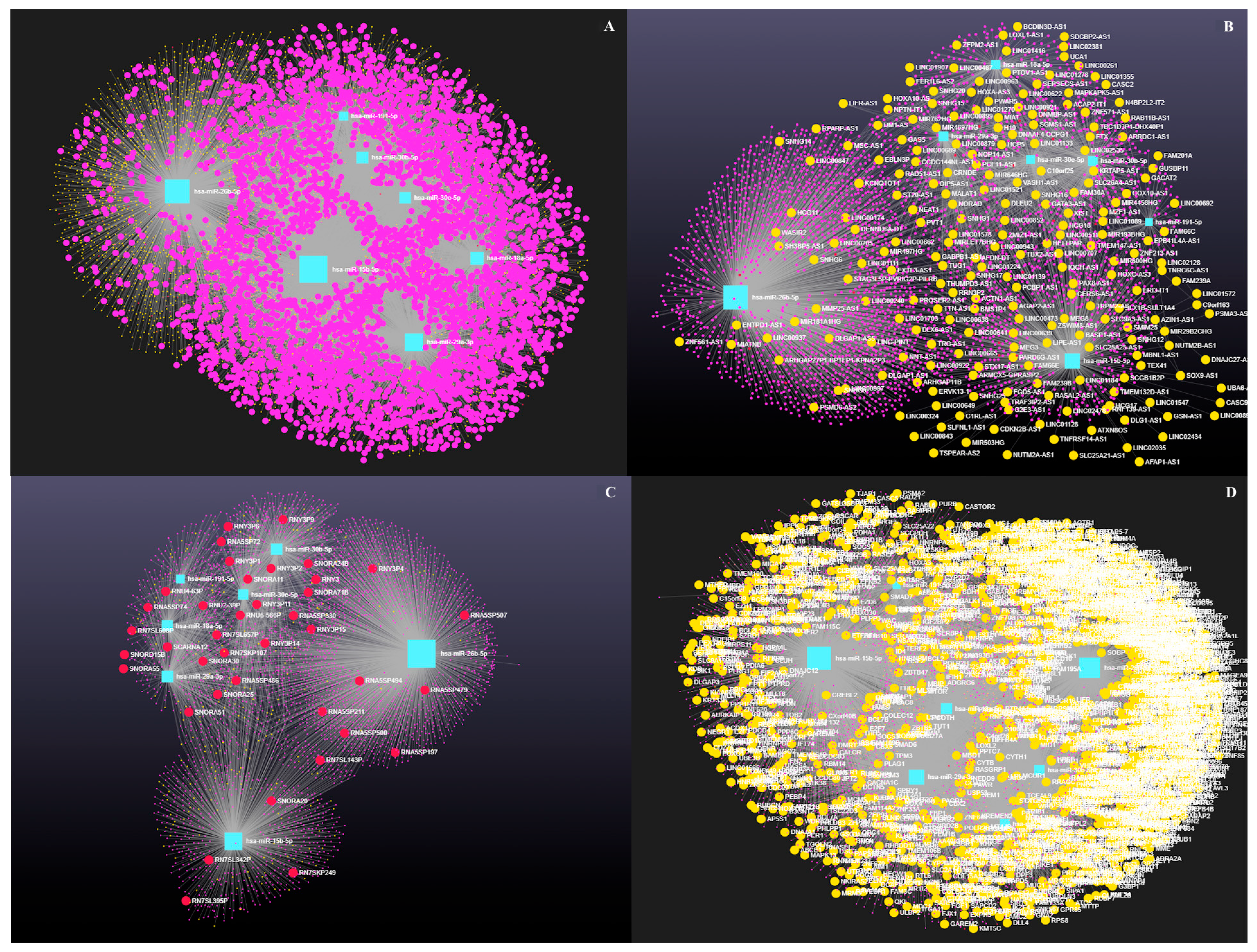
3.4. Decoding the RNA Interactome: A Network Approach to Non-Coding and Coding RNA Interactions
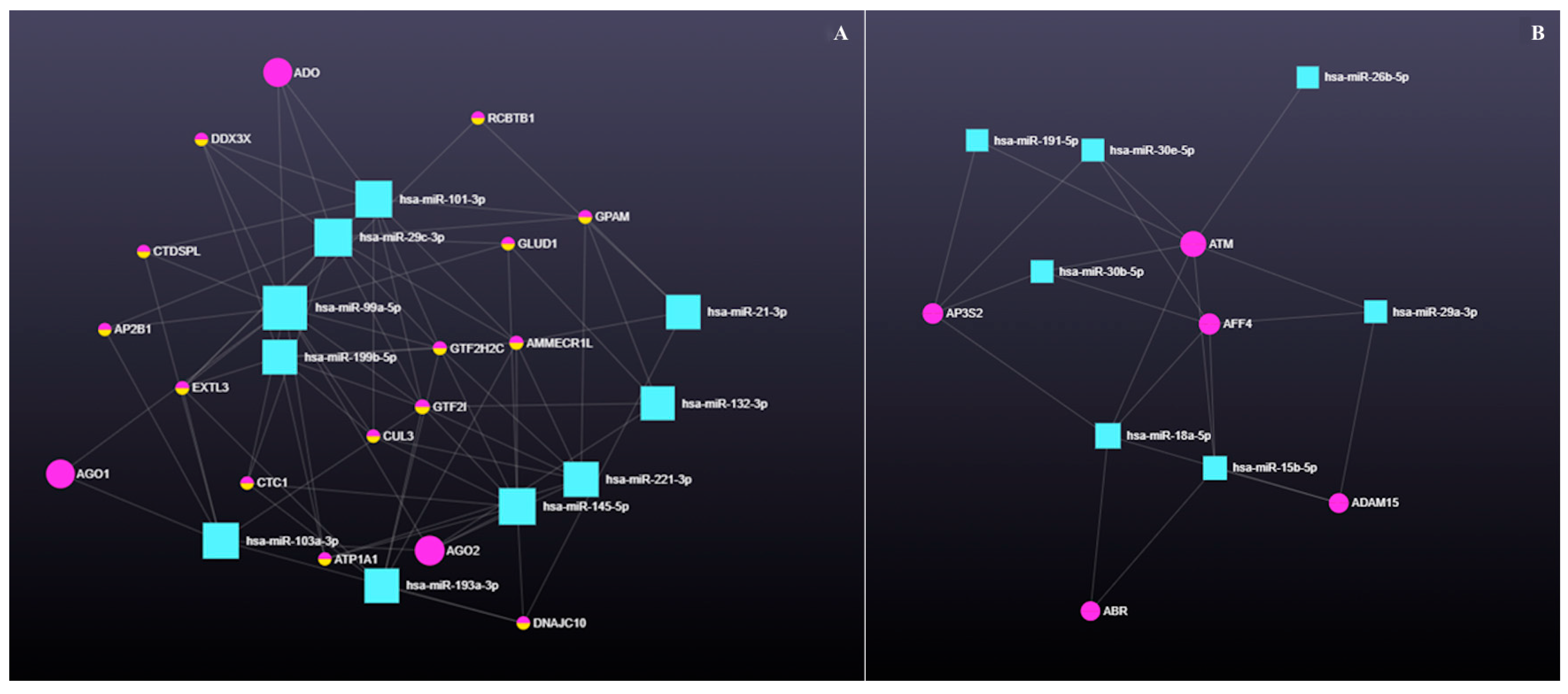
3.4.1. The Role of Circular RNAs (circRNAs) in miRNA-Mediated Gene Regulation
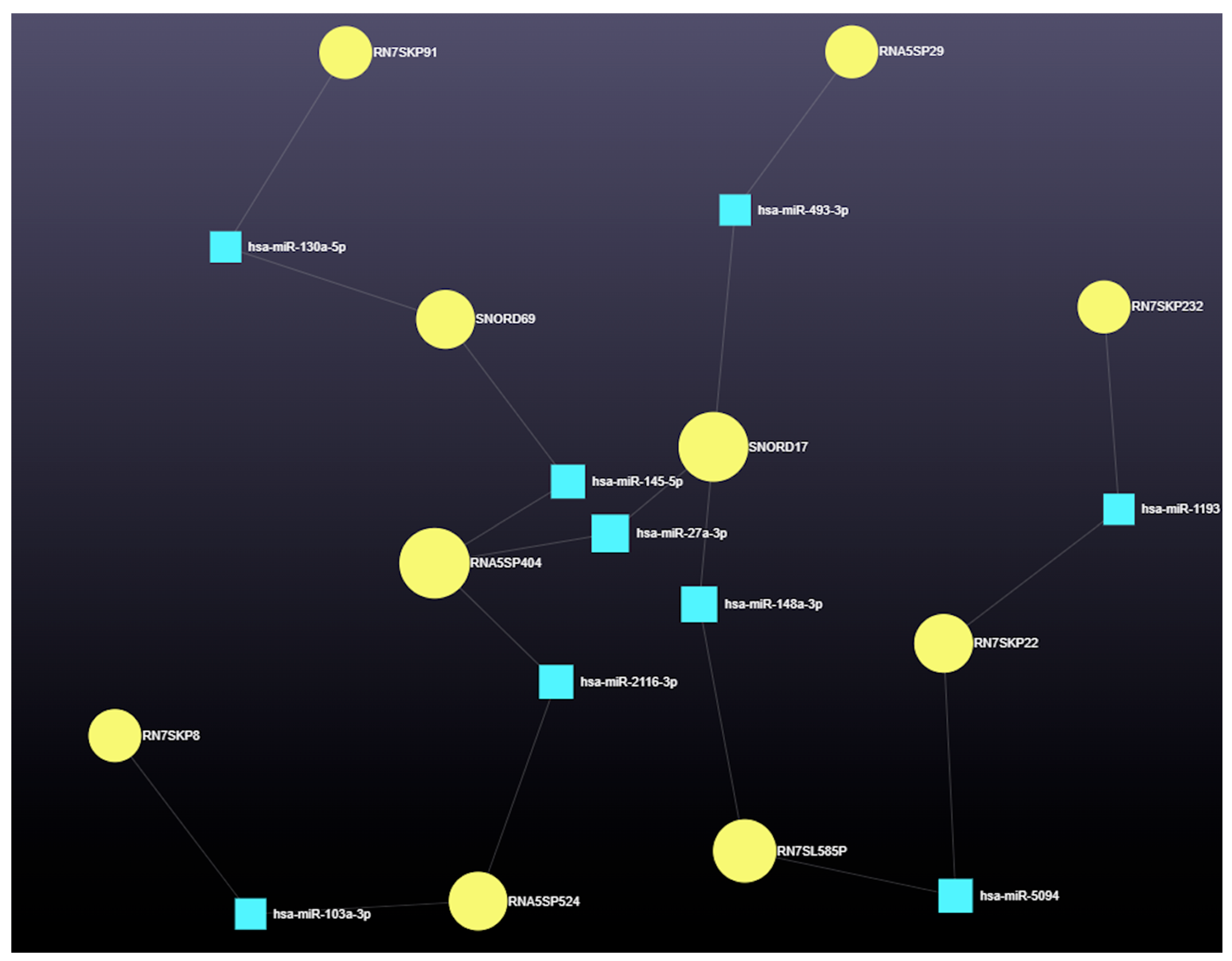
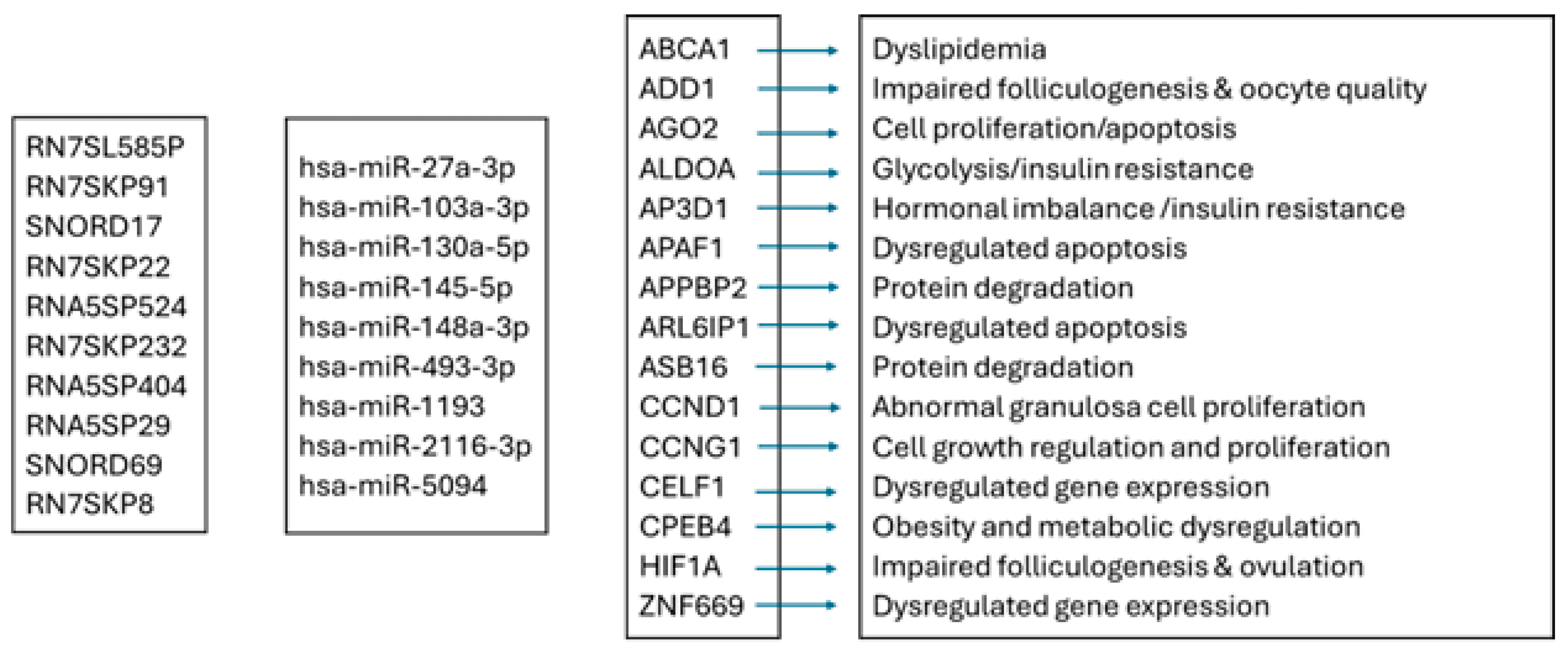
3.4.2. The Role of Small Nuclear RNAs (snRNAs) in miRNA-Mediated Gene Regulation
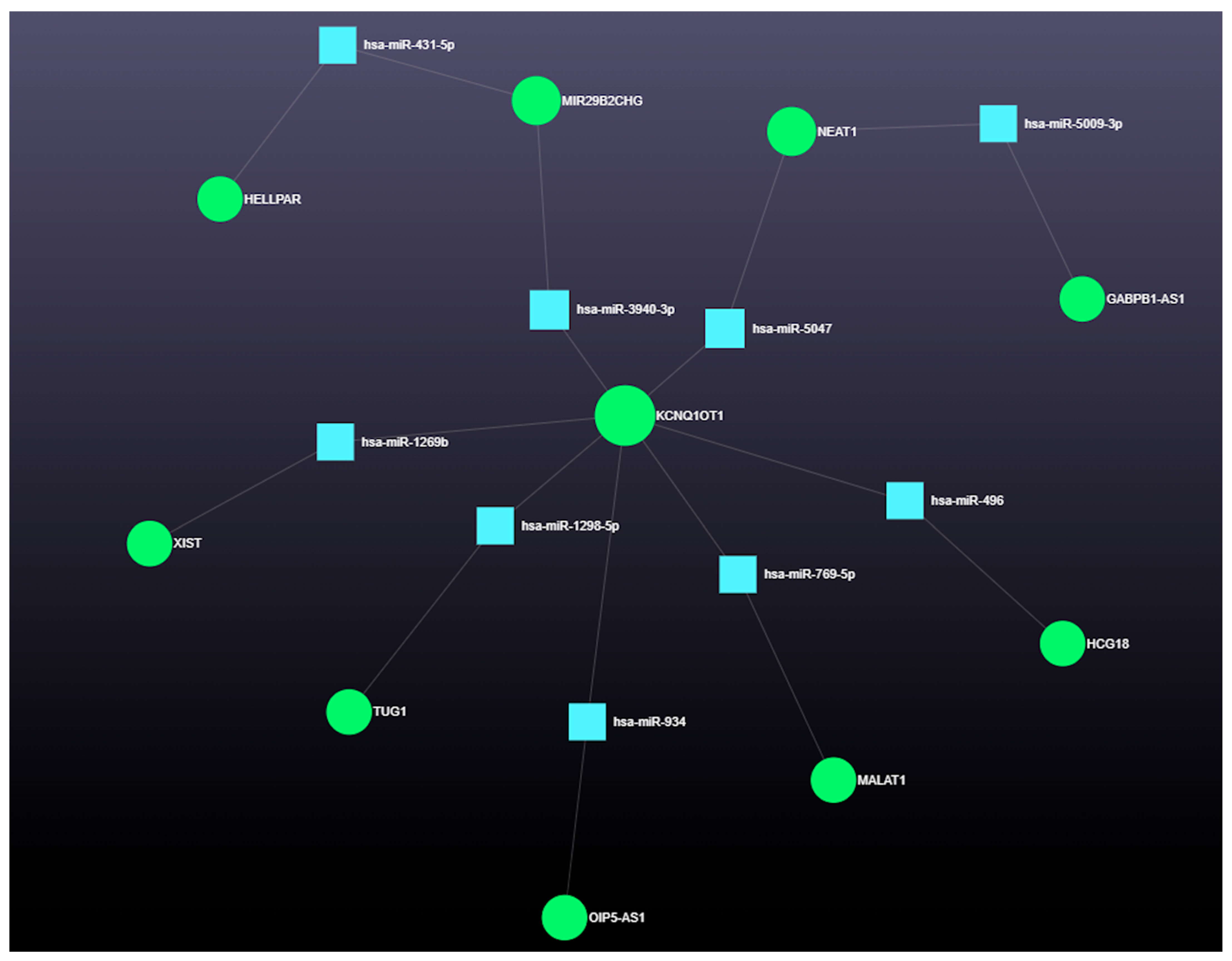
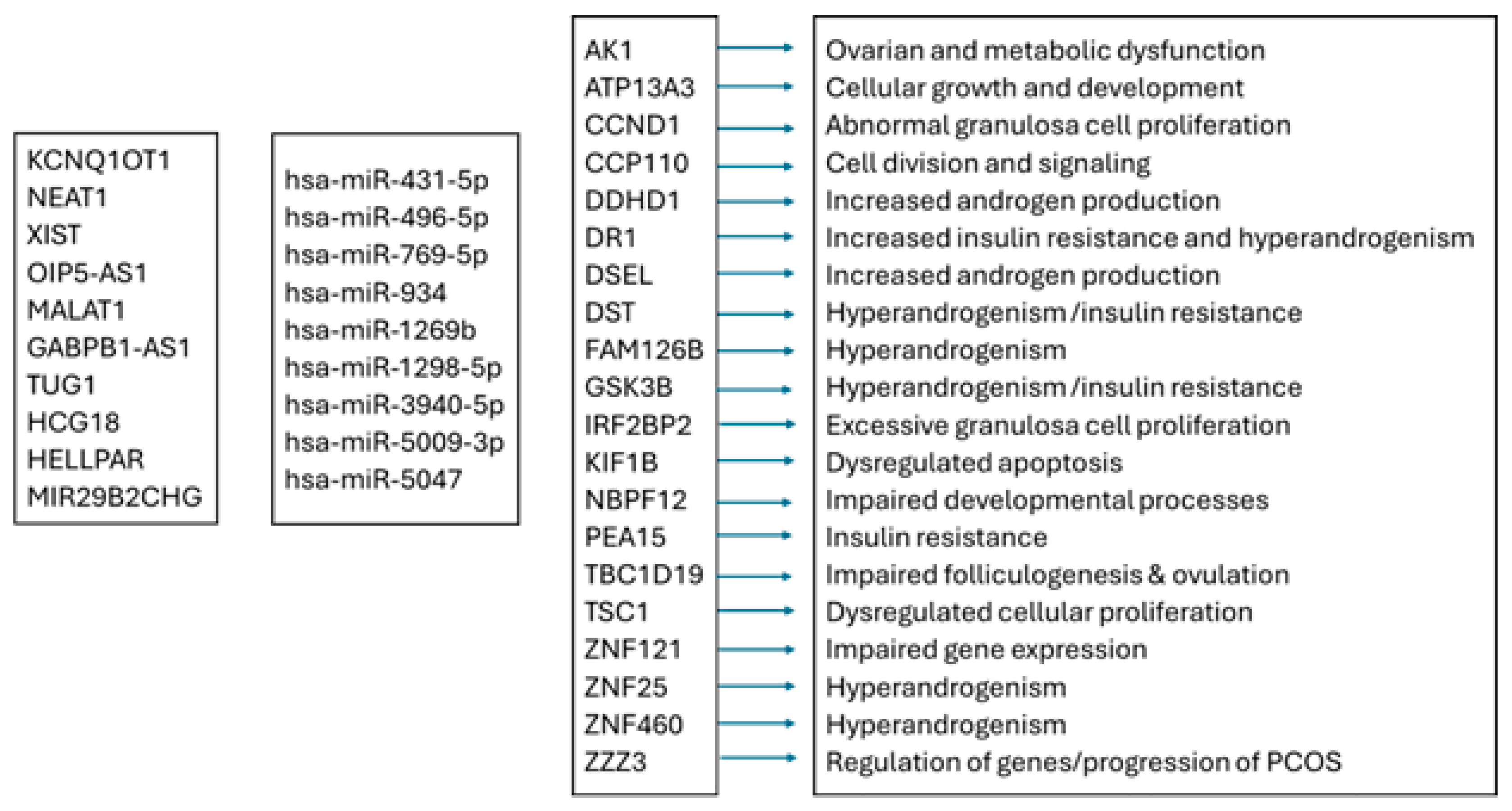
3.4.3. The Role of Long Coding RNAs (lncRNAs) in miRNA-Mediated Gene Regulation
4. Discussion
4.1. Functional Roles of Dysregulated miRNAs in Ovarian Cyst Pathogenesis in Dairy Cows
4.2. Network Analysis Reveals Predicted Gene Expression Shifts in Dairy Cow Ovarian Follicular Cysts
4.3. Integrated Analysis of miRNA-Regulated Coding and Non-Coding RNAs in Ovarian Follicular Cysts
4.4. Emerging Role of Circular RNAs in Post-Transcriptional Control of Bovine Ovarian Cyst Pathways
4.5. Integrated ncRNA and Proteostasis Network Disruption in Bovine Ovarian Cysts
4.6. Shared Molecular Pathways Link Ovarian Follicular Cysts in Dairy Cows and PCOS in Women
4.7. Translational Insights into PCOS from miRNA Profiling of Ovarian Cysts in Cows
4.7.1. MicroRNA-Based Parallels Between Bovine Follicular Cysts and Human PCOS: A Translational Perspective
Comparative Pathophysiology: Similarities and Differences
The Role of microRNAs in Ovarian Dysfunction
Challenges in miRNA-Based Therapeutics
Cross-Species Comparative Research and Its Benefits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kesler, D.J.; Garverick, H.A. Ovarian cysts in dairy cattle: A review. J. Anim. Sci. 1982, 55, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Garverick, H.A. Ovarian follicular cysts in dairy cows. J. Dairy Sci. 1997, 80, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Signorini, M.L.; Bertoli, J.; Bartolomé, J.A.; Gareis, N.C.; Díaz, P.U.; Bó, G.A.; Ortega, H.H. Epidemiological description of cystic ovarian disease in Argentine dairy herds: Risk factors and effects on the reproductive performance of lactating cows. Reprod. Domest. Anim. 2014, 49, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T. An update on cystic ovarian degeneration in cattle. Reprod. Domest. Anim. 2004, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silvia, W.J.; Hatler, T.B.; Nugent, A.M.; Laranja da Fonseca, L.F. Ovarian follicular cysts in dairy cows: An abnormality in folliculogenesis. Domest. Anim. Endocrinol. 2002, 23, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, T.; Opsomer, G.; de Kruif, A. Aetiology and pathogenesis of cystic ovarian follicles in dairy cattle: A review. Reprod. Nutr. Dev. 2006, 46, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.L.; Parfet, J.R.; Smith, C.A.; Moss, G.E.; Youngquist, R.S.; Garverick, H.A. Secretory patterns of LH and FSH during development and hypothalamic and hypophysial characteristics following development of steroid-induced ovarian follicular cysts in dairy cattle. J. Reprod. Fertil. 1991, 91, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Borș, S.I.; Borș, A. Ovarian cysts, an anovulatory condition in dairy cattle. J. Vet. Med. Sci. 2020, 82, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.A.; Garverick, H.A.; Keisler, D.H.; Xu, Z.Z.; Loos, K.; Youngquist, R.S.; Salfen, B.E. Characterization of ovarian follicular cysts and associated endocrine profiles in dairy cows. Biol. Reprod. 1995, 53, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Iwamura, S.; Kamomae, H. Ultrasonic observations on the turnover of ovarian follicular cysts and associated changes of plasma LH, FSH, progesterone and oestradiol-17β in cows. Res. Vet. Sci. 1996, 61, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.W.; Mehta, J.P.; McGettigan, P.A.; Browne, J.A.; Forde, N.; Alibrahim, R.M.; Mulligan, F.J.; Loftus, B.; Crowe, M.A.; Matthews, D.; et al. Effect of the metabolic environment at key stages of follicle development in cattle: Focus on steroid biosynthesis. Physiol. Genom. 2012, 44, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Hatler, T.B.; Hayes, S.H.; Laranja da Fonseca, L.F.; Silvia, W.J. Relationship between endogenous progesterone and follicular dynamics in lactating dairy cows with ovarian follicular cysts. Biol. Reprod. 2003, 69, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Amweg, A.N.; Salvetti, N.R.; Stangaferro, M.L.; Paredes, A.H.; Lara, H.H.; Rodríguez, F.M.; Ortega, H.H. Ovarian localization of 11β-hydroxysteroid dehydrogenase (11βHSD): Effects of ACTH stimulation and its relationship with bovine cystic ovarian disease. Domest. Anim. Endocrinol. 2013, 45, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.E.; Díaz, P.U.; Salvetti, N.R.; Rey, F.; Ortega, H.H. mRNA expression pattern of gonadotropin receptors in bovine follicular cysts. Reprod. Biol. 2014, 14, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Phuong, H.N.; Blavy, P.; Martin, O.; Schmidely, P.; Friggens, N.C. Modelling impacts of performance on the probability of reproducing, and thereby on productive lifespan, allow prediction of lifetime efficiency in dairy cows. Animals 2016, 10, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, C.; Fukihara, S.; Maeda, M.; Kaneko, E.; Montoya, C.A.; Matsui, M.; Shimizu, T.; Matsunaga, N.; Kida, K.; Miyake, Y.; et al. Relationship between metabolic hormones and ovulation of dominant follicle during the first follicular wave post-partum in high-producing dairy cows. Reproduction 2007, 133, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Opsomer, G.; Gröhn, Y.T.; Hertl, J.; Coryn, M.; Deluyker, H.; de Kruif, A. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: A field study. Theriogenology 2000, 53, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Block, S.S.; Butler, W.R.; Ehrhardt, R.A.; Bell, A.W.; Van Amburgh, M.E.; Boisclair, Y.R. Decreased concentration of plasma leptin in periparturient dairy cows is caused by negative energy balance. J. Endocrinol. 2001, 171, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Liefers, S.C.; Veerkamp, R.F.; te Pas, M.F.W.; Delavaud, C.; Chilliard, Y.; van der Lende, T. Leptin concentrations in relation to energy balance, milk yield, intake, live weight, and estrus in dairy cows. J. Dairy Sci. 2003, 86, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Zulu, V.C.; Sawamukai, Y.; Nakada, K.; Kida, K.; Moriyoshi, M. Relationship among insulin-like growth factor-I, blood metabolites and post partum ovarian function in dairy cows. J. Vet. Med. Sci. 2002, 64, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, T.; Leroy, J.L.; Dewulf, J.; Duchateau, L.; Coryn, M.; de Kruif, A.; Opsomer, G. Hormonal and metabolic profiles of high-yielding dairy cows prior to ovarian cyst formation or first ovulation post partum. Reprod. Domest. Anim. 2005, 40, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J. Leptin: A possible metabolic signal affecting reproduction. Domest. Anim. Endocrinol. 2001, 21, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bai, J.; Liu, K.; Xiao, L.; Qin, Y.; Gao, M.; Liu, Y. Association of metabolic and endocrine disorders with bovine ovarian follicular cysts. Animals 2023, 13, 3301. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qin, L.; Yu, H.; Jiang, P.; Xia, L.; Gao, Z.; Yang, R.; Zhao, Y.; Yu, X.; Zhao, Z. Comprehensive analysis of miRNAs and target mRNAs between immature and mature testis tissue in Chinese red steppes cattle. Animals 2021, 11, 3024. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.R.; Kasimanickam, R.K. Differential expression of MicroRNAs in sexually immature and mature canine testes. Theriogenology 2015, 83, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Vendrell-Flotats, M.; García-Martínez, T.; Martínez-Rodero, I.; López-Béjar, M.; LaMarre, J.; Yeste, M.; Mogas, T. In vitro maturation in the presence of Leukemia Inhibitory Factor modulates gene and miRNA expression in bovine oocytes and embryos. Sci. Rep. 2020, 10, 17777. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Leitão, A.L.; Enguita, F.J. MicroRNA profiling in plasma or serum using quantitative RT-PCR. In RNA Mapping; Springer: New York, NY, USA, 2014; Volume 1182, pp. 121–129. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kastelic, J. Circulating cell-free mature microRNAs and their target gene prediction in bovine metritis. Sci. Rep. 2016, 6, 29509. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.C.; Coenen-Stass, A.M.L.; Betts, C.A.; Wood, M.J.A. Detection and quantification of extracellular microRNAs in murine biofluids. Biol. Proced. Online 2014, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Turchinovich, A.; Weiz, L.; Burwinkel, B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem. Sci. 2012, 37, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.; Kasimanickam, R. An efficient approach for RNA extraction from boar sperm and seminal plasma. Bio-Protocol 2019, 9, e3284. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.; Buhr, M.; Kasimanickam, R. Patterns of expression of sperm and seminal plasma microRNAs in boar semen. Theriogenology 2019, 125, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, G.; Soufan, O.; Xia, J. miRNet 2.0: Network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020, 48, W244–W251. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Li, A.; Wu, D.; Yang, Q.; Yi, L.X.; He, G.; Lu, H. The functional role of lncRNAs as ceRNAs in both ovarian processes and associated diseases. Non-Coding RNA Res. 2023, 9, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Gareis, N.C.; Rodríguez, F.M.; Cattaneo Moreyra, M.L.; Stassi, A.F.; Angeli, E.; Etchevers, L.; Salvetti, N.R.; Ortega, H.H.; Hein, G.J.; Rey, F. Contribution of key elements of nutritional metabolism to the development of cystic ovarian disease in dairy cattle. Theriogenology 2023, 197, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Chalmeh, A.; Pourjafar, M.; Nazifi, S.; Momenifar, F.; Mohamadi, M. Insulin resistance in different physiological states of high producing Holstein dairy cows. Acta Sci. Vet. 2015, 43, 1255. [Google Scholar]
- Gareis, N.C.; Stassi, A.F.; Huber, E.; Rodríguez, F.M.; Cattaneo Moreyra, M.L.; Salvetti, N.R.; Ortega, H.H.; Hein, G.J.; Rey, F. Alterations in the insulin signaling pathway in bovine ovaries with experimentally induced follicular persistence. Theriogenology 2020, 158, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Huang, Y.; Chen, J.; Yu, Z.; Chen, C.; Lai, L. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic. Biol. Med. 2019, 141, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Cheung, A.H.; Chan, C.L.; Chan, W.Y. The role of microRNAs in ovarian granulosa cells in health and disease. Front. Endocrinol. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cao, Z.; Zhu, R.; You, L.; Zhang, T. The dual functional role of microRNA-18a (miR-18a) in cancer development. Clin. Transl. Med. 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Zielak-Steciwko, A.E.; Browne, J.A.; McGettigan, P.A.; Gajewska, M.; Dzięcioł, M.; Szulc, T.; Evans, A.C. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol. Genom. 2014, 46, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Ahmad, I.; Gebremedhn, S.; Sahadevan, S.; Hossain, M.D.; Rings, F.; Hoelker, M.; Tholen, E.; Neuhoff, C.; Looft, C.; et al. The expression pattern of microRNAs in granulosa cells of subordinate and dominant follicles during the early luteal phase of the bovine estrous cycle. PLoS ONE 2014, 9, e106795. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.; Insenser, M.; Fernández-Durán, E.; San-Millán, J.L.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Non-targeted profiling of circulating microRNAs in women with polycystic ovary syndrome (PCOS): Effects of obesity and sex hormones. Metabolism 2018, 86, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.L.; Li, S.D.; Ma, Y.C.; Tang, J.R.; Lv, J.Y.; Zhang, Y.Q.; Miao, Y.L.; Ma, Y.Q.; Li, C.M.; Chu, Y.Y.; et al. MicroRNA-30b regulates insulin sensitivity by targeting SERCA2b in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, X.F.; Dong, M.Z.; Tan, J.; Zhang, J.; Zhuang, L.K.; Liu, S.S.; Xin, Y.N. MiR-30b-5p regulates the lipid metabolism by targeting PPARGC1A in Huh-7 cell line. Lipids Health Dis. 2020, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, M.A.; Fitzpatrick, R.; Kenny, D.A.; Diskin, M.G.; Patton, J.; Murphy, J.J.; Wathes, D.C. Interrelationships between negative energy balance (NEB) and IGF regulation in liver of lactating dairy cows. Domest. Anim. Endocrinol. 2008, 34, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Z.; Zhao, C.; Bai, Y.; Xia, C.; Xu, C. Effect of Negative Energy Balance on Plasma Metabolites, Minerals, Hormones, Cytokines and Ovarian Follicular Growth Rate in Holstein Dairy Cows. J. Vet. Res. 2021, 65, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hailay, T.; Hoelker, M.; Poirier, M.; Gebremedhn, S.; Rings, F.; Saeed-Zidane, M.; Salilew-Wondim, D.; Dauben, C.; Tholen, E.; Neuhoff, C.; et al. Extracellular vesicle-coupled miRNA profiles in follicular fluid of cows with divergent post-calving metabolic status. Sci. Rep. 2019, 9, 12851. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, L.T.; Sørensen, A.E.; Hardikar, A.A.; Joglekar, M.V. The microRNA-29 family: Role in metabolism and metabolic disease. Am. J. Physiol.-Cell Physiol. 2022, 323, C367–C377. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, X.; Qiukai, E.; Shang, Y.; Zhang, X.; Liu, S.; Zhang, X. Long non-coding RNA Xist regulates oocyte loss via suppressing miR-23b-3p/miR-29a-3p maturation and upregulating STX17 in perinatal mouse ovaries. Cell Death Dis. 2021, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, R.; Pan, Z.X.; Zhou, B.; Yu, D.B.; Wang, X.G.; Ma, X.S.; Han, J.; Shen, M.; Liu, H.L. miR-26b promotes granulosa cell apoptosis by targeting ATM during follicular atresia in porcine ovary. PLoS ONE 2012, 7, e38640. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Yoshimura, Y. Deficient proliferation and apoptosis in the granulosa and theca interna cells of the bovine cystic follicle. J. Reprod. Dev. 2007, 53, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, N.R.; Stangaferro, M.L.; Palomar, M.M.; Alfaro, N.S.; Rey, F.; Gimeno, E.J.; Ortega, H.H. Cell proliferation and survival mechanisms underlying the abnormal persistence of follicular cysts in bovines with cystic ovarian disease induced by ACTH. Anim. Reprod. Sci. 2010, 122, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.J.; Irving-Rodgers, H.F.; Russell, D.L. Extracellular matrix of the developing ovarian follicle. Reproduction 2003, 126, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Irving-Rodgers, H.F.; Catanzariti, K.D.; Aspden, W.J.; D’Occhio, M.J.; Rodgers, R.J. Remodeling of extracellular matrix at ovulation of the bovine ovarian follicle. Mol. Reprod. Dev. 2006, 73, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Peralta, M.B.; Baravalle, M.E.; Belotti, E.M.; Stassi, A.F.; Salvetti, N.R.; Ortega, H.H.; Rey, F.; Velázquez, M.M.L. Involvement of matrix metalloproteinases and their inhibitors in bovine cystic ovarian disease. J. Comp. Pathol. 2017, 156, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.S.; Salvetti, N.R.; Velazquez, M.M.; Stangaferro, M.L.; Rey, F.; Ortega, H.H. Steroid receptor mRNA expression in the ovarian follicles of cows with cystic ovarian disease. Res. Vet. Sci. 2012, 92, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.H.; Marelli, B.E.; Rey, F.; Amweg, A.N.; Díaz, P.U.; Stangaferro, M.L.; Salvetti, N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction 2015, 149, R251–R264. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Ovcharenko, D.; Grossmann, R.; Lauková, M.; Mlyncek, M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J. Cell. Physiol. 2009, 219, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Rashid, G.; Khan, N.A.; Elsori, D.; Youness, R.A.; Hassan, H.; Siwan, D.; Seth, N.; Kamal, M.A.; Rizvi, S.; Babker, A.M.; et al. miRNA expression in PCOS: Unveiling a paradigm shift toward biomarker discovery. Arch. Gynecol. Obstet. 2024, 309, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Ramachandran, V.; Hayat, S.; Dargham, S.; Cunningham, T.K.; Benurwar, M.; Sathyapalan, T.; Najafi-Shoushtari, S.H.; Atkin, S.L. Expression of microRNA in follicular fluid in women with and without PCOS. Sci. Rep. 2019, 9, 16306. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wang, H.; Shao, W.; Liu, N. miR-199a-5p stimulates ovarian granulosa cell apoptosis in polycystic ovary syndrome. J. Mol. Endocrinol. 2020, 65, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Chelegahi, A.M.; Ebrahimi, S.O.; Reiisi, S.; Nezamnia, M.A. A glance into the roles of microRNAs (exosomal and non-exosomal) in polycystic ovary syndrome. Obstet. Gynecol. Sci. 2024, 67, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Xiang, S.; Yu, Y.; Song, J.; Zheng, M.; Lian, F. miR-221-3p regulates apoptosis of ovarian granulosa cells via targeting FOXO1 in older women with diminished ovarian reserve (DOR). Mol. Reprod. Dev. 2021, 88, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lingling, G.; Qingxing, Y.; Jianbo, X.; Weijie, W.; Dan, L. MiR-145-5p regulates granulosa cell proliferation by targeting the SET gene in KGN cells. J. Reprod. Dev. 2024, 70, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tang, J.; Jiang, R.; Wang, X.; Yang, Z.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. CircRILPL1 promotes muscle proliferation and differentiation via binding miR-145 to activate IGF1R/PI3K/AKT pathway. Cell Death Dis. 2021, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Acosta, D.A.V.; Egan, T.R.; Skenandore, C.; Sulzberger, S.; French, D.D.; Cardoso, F.C. Steroidogenic, metabolic, and immunological markers in dairy cows diagnosed with cystic ovarian follicles at early and mid-late lactation. Front. Vet. Sci. 2019, 6, 324. [Google Scholar] [CrossRef] [PubMed]
- Gareis, N.C.; Angeli, E.; Huber, E.; Salvetti, N.R.; Rodriguez, F.M.; Ortega, H.H.; Hein, G.J.; Rey, F. Alterations in key metabolic sensors involved in bovine cystic ovarian disease. Theriogenology 2018, 120, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Lazzeroni, P.; Sartori, C.; Nicoli, A.; Amarri, S.; La Sala, G.B.; Street, M.E. MiRNAs regulating insulin sensitivity are dysregulated in Polycystic Ovary Syndrome (PCOS) ovaries and are associated with markers of inflammation and insulin Ssnsitivity. Front. Endocrinol. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.E.; Udesen, P.B.; Maciag, G.; Geiger, J.; Saliani, N.; Januszewski, A.S.; Jiang, G.; Ma, R.C.; Hardikar, A.A.; Wissing, M.L.M.; et al. Hyperandrogenism and metabolic syndrome are associated with changes in serum-derived microRNAs in women with polycystic ovary syndrome. Front. Med. 2019, 6, 242. [Google Scholar] [CrossRef] [PubMed]
- Hailay, T.S. Post-Calving Metabolic Status Dependent Changes on Expression of Extracellular Vesicles Coupled microRNAs in Bovine Follicular Fluid and Blood Serum. Ph.D. Dissertation, University of Ghent, Gent, Belgium, 2019. Available online: https://nbn-resolving.org/urn:nbn:de:hbz:5-56655 (accessed on 10 February 2025).
- Krishna, M.B.; Johnson, B.S.; Vasudevan, M.; Pillai, S.M.; Laloraya, M. miRNA-mRNA network in PBMCs of PCOS women identifies overactivated stress-activated kinases. Cell. Physiol. Biochem. 2023, 57, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, Y.; Guo, Z.; Zhou, X. MicroRNA-29c-3p participates in insulin function to modulate polycystic ovary syndrome via targeting Forkhead box O 3. Bioengineered 2022, 13, 4361–4371. [Google Scholar] [CrossRef] [PubMed]
- Stassi, A.F.; Díaz, P.U.; Gasser, F.B.; Velázquez, M.M.L.; Gareis, N.C.; Salvetti, N.R.; Ortega, H.H.; Baravalle, M.E. A review on inflammation and angiogenesis as key mechanisms involved in the pathogenesis of bovine cystic ovarian disease. Theriogenology 2022, 186, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, X.; Zhou, J.; Pan, Z.; Liu, H.; Li, Q. MicroRNA-26b functions as a proapoptotic factor in porcine follicular granulosa cells by targeting Sma-and Mad-related protein 4. Biol. Reprod. 2014, 91, 146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, F.; Yao, W.; Li, X.; Xie, Z.; Liu, H.; Li, Q.; Pan, Z. Conserved miR-26b enhances ovarian granulosa cell apoptosis through HAS2-HA-CD44-Caspase-3 pathway by targeting HAS2. Sci. Rep. 2016, 6, 21197. [Google Scholar] [CrossRef] [PubMed]
- Carletti, M.Z.; Fiedler, S.D.; Christenson, L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010, 83, 28695. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA expression profile in bovine granulosa cells of preovulatory dominant and subordinate follicles during the late follicular phase of the estrous cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.T. miRiad roles for the miR-17-92 cluster in development and disease. Cell 2008, 133, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Seifi Noferesti, S. Bovine Ovarian Hyperstimulation-Induced Changes in the Expression Profile of Circulatory miRNA in Follicular Fluid and Blood Plasma. Ph.D. Dissertation, University of Bonn, Bonn, Germany, 2014. Available online: https://nbn-resolving.org/urn:nbn:de:hbz:5n-38273 (accessed on 27 June 2025).
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef] [PubMed]
- Strum, J.C.; Johnson, J.H.; Ward, J.; Xie, H.; Feild, J.; Hester, A.; Alford, A.; Waters, K.M. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol. 2009, 23, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dong, J.; Jiang, T.; Shi, Z.; Yu, B.; Zhu, Y.; Chen, D.; Xu, J.; Huo, R.; Dai, J.; et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE 2011, 6, e23925. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Tranguch, S.; Daikoku, T.; Jensen, K.; Furneaux, H.; Dey, S.K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc. Natl. Acad. Sci. USA 2007, 104, 15144–15149. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.M.M.; Markert, U.R. MicroRNAs in pregnancy. J. Reprod. Immunol. 2011, 88, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Piontkewitz, Y.; Sundfeldt, K.; Hedin, L. The expression of c-myc during follicular growth and luteal formation in the rat ovary in vivo. J. Endocrinol. 1997, 152, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ataei-Nazari, S.; Amoushahi, M.; Madsen, J.F.; Jensen, J.; Heuck, A.; Mohammadi-Sangcheshmeh, A.; Lykke-Hartmann, K. Cyclin-dependent kinase 6 (CDK6) as a potent regulator of the ovarian primordial-to-primary follicle transition. Front. Cell Dev. Biol. 2022, 10, 1036917. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mo, H.; Chen, W.; Li, L.; Xiao, Y.; Zhang, J.; Li, X.; Lu, Y. Role of the PI3K-Akt signaling pathway in the pathogenesis of polycystic ovary syndrome. Reprod. Sci. 2017, 24, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, N.R.; Acosta, J.C.; Gimeno, E.J.; Müller, L.A.; Mazzini, R.A.; Taboada, A.F.; Ortega, H.H. Estrogen receptors alpha and beta and progesterone receptors in normal bovine ovarian follicles and cystic ovarian disease. Vet. Pathol. 2007, 44, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sugiaman-Trapman, D.; Vitezic, M.; Jouhilahti, E.M.; Mathelier, A.; Lauter, G.; Misra, S.; Daub, C.O.; Kere, J.; Swoboda, P. Characterization of the human RFX transcription factor family by regulatory and target gene analysis. BMC Genom. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Q.; Ding, J.; Yin, T.; Ye, P.; Zhang, Y. The conceivable functions of protein ubiquitination and deubiquitination in reproduction. Front. Physiol. 2022, 13, 886261. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Hsueh, A.J. Tissue-specific Bcl-2 protein partners in apoptosis: An ovarian paradigm. Physiol. Rev. 2000, 80, 593–614. [Google Scholar] [CrossRef] [PubMed]
- ElMonier, A.A.; El-Boghdady, N.A.; Fahim, S.A.; Sabry, D.; Elsetohy, K.A.; Shaheen, A.A.; ElMonier, A.A.; El-Boghdady, N.A.; Fahim, S.A.; Sabry, D.; et al. LncRNA NEAT1 and MALAT1 are involved in polycystic ovary syndrome pathogenesis by functioning as competing endogenous RNAs to control the expression of PCOS-related target genes. Non-Coding RNA Res. 2023, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Khoueiry, R.; Ibala-Rhomdane, S.; Méry, L.; Blachère, T.; Guérin, J.F.; Lornage, J.; Lefèvre, A. Dynamic CpG methylation of the KCNQ1OT1 gene during maturation of human oocytes. J. Med. Genet. 2008, 45, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Agbu, P.; Carthew, R.W. MicroRNA-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Seo, J.; Murakami, K.; Salem, E.S.B.; Bernhard, E.; Borra, V.J.; Choi, K.; Yuan, C.L.; Chan, C.C.; Chen, X.; et al. Hepatic Ago2-mediated RNA silencing controls energy metabolism linked to AMPK activation and obesity-associated pathophysiology. Nat. Commun. 2018, 9, 3658. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Rao, L.V.M. The role of microRNAs in inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor suppressor and metabolic regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- De Felici, M.; Klinger, F.G. PI3K/PTEN/AKT signaling pathways in germ cell development and their involvement in germ cell tumors and ovarian dysfunctions. Int. J. Mol. Sci. 2012, 22, 9838. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Liu, Y.D.; Zhou, X.Y.; Chen, X.; Zhe, J.; Zhang, Q.Y.; Zhang, X.F.; Chen, Y.X.; Wang, Z.; et al. Long non-coding RNA TUG1 and its molecular mechanisms in polycystic ovary syndrome. RNA Biol. 2020, 17, 1798–1810. [Google Scholar] [CrossRef] [PubMed]
- Stassi, A.F.; Gasser, F.; Velazquez, M.M.L.; Belotti, E.M.; Gareis, N.C.; Rey, F. Contribution of the VEGF system to the follicular persistence associated with bovine cystic ovaries. Theriogenology 2019, 138, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Bahramrezaie, M.; Amidi, F.; Aleyasin, A.; Saremi, A.; Aghahoseini, M.; Brenjian, S.; Khodarahmian, M.; Pooladi, A. Effects of resveratrol on VEGF & HIF1 genes expression in granulosa cells in the angiogenesis pathway and laboratory parameters of polycystic ovary syndrome: A triple-blind randomized clinical trial. J. Assist. Reprod. Genet. 2019, 36, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Imbar, T.; Eisenberg, I. Regulatory role of microRNAs in ovarian function. Fertil. Steril. 2014, 101, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Winchester, L.; van Bijsterveldt, L.; Dhawan, A.; Wigfield, S.; Triantafyllidis, C.; Haider, S.; McIntyre, A.; Humphrey, T.C.; Harris, A.L.; Buffa, F.M. Dicer-to-Argonaute switch controls biogenesis of oncogenic miRNA. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lee, S.S.; Min, H.; Ha, J.Y.; Kim, B.H.; Choi, M.S.; Kim, S. Dysregulation of the miRNA biogenesis components DICER1, DROSHA, DGCR8 and AGO2 in clear cell renal cell carcinoma in both a Korean cohort and the cancer genome atlas kidney clear cell carcinoma cohort. Oncol. Lett. 2019, 18, 4337–4345. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, S.; Haber, D.A. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 2007, 131, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, A.; Yang, Y.; Plath, K. The role of Xist in X-Chromosome dosage compensation. Trends Cell Biol. 2018, 28, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, H.; Chen, Z.; Hu, X.; Wu, T.; Liu, W. Long noncoding RNA X-Inactive-Specific Transcript promotes the secretion of inflammatory cytokines in LPS stimulated astrocyte cell via sponging miR-29c-3p and regulating nuclear factor of activated T cell 5 expression. Front. Endocrinol. 2021, 12, 573143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Liu, Z.; Lin, B.; Deng, X.; Xiao, Q.; Chen, Z.; Ye, H.; Chen, D.; Su, Y.; et al. Downregulation of Kcnq1ot1 attenuates β-cell proliferation and insulin secretion via the miR-15b-5p/Ccnd1 and Ccnd2 axis. Acta Diabetol. 2022, 59, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, R.; Kang, F.; Lai, H.; Wang, Y. KCNQ1OT1 promotes ovarian cancer progression via modulating miR-142-5p/CAPN10 axis. Mol. Genet. Genom. Med. 2020, 8, e1077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, Y.; Zhou, X.; Zheng, L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod. Biol. Endocrinol. 2019, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Thys-Jacobs, S.; Donovan, D.; Papadopoulos, A.; Sarrel, P.; Bilezikian, J.P. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999, 64, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.; Haghollahi, F.; Shariat, M.; Zayerii, F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: A pilot study. Taiwan. J. Obstet. Gynecol. 2009, 48, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Han, B.; Li, Q.; Yuan, Y.; Li, J.; Sun, D. Using RNA sequencing to identify putative competing endogenous RNAs (ceRNAs) potentially regulating fat metabolism in bovine liver. Sci. Rep. 2017, 7, 6396. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cheng, Y.; Guo, T.; Guo, X.; Zhang, H.; Ma, X.; Pan, Y.; Kebreab, E.; Wang, D.; Lyu, L. Analyzing the interactions of mRNAs, miRNAs and lncRNAs to predict ceRNA networks in bovine cystic follicular granulosa cells. Front. Vet. Sci. 2022, 9, 1028867. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Nasser, J.S.; Altahoo, N.; Almosawi, S.; Alhermi, A.; Butler, A.E. The Role of microRNA, long non-coding RNA and circular RNA in the pathogenesis of polycystic ovary syndrome: A literature review. Int. J. Mol. Sci. 2024, 25, 903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Lai, M.; Li, J.; Zhan, J.; Wen, Q.; Ma, H. Circular RNA expression profiling of granulosa cells in women of reproductive age with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2019, 300, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. Oxidative stress and the multifaceted roles of ATM in maintaining cellular redox homeostasis. Redox Biol. 2024, 75, 103269. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. Role of circular RNAs in DNA repair. RNA Biol. 2024, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Saaoud, F.; Drummer, I.V.C.; Shao, Y.; Sun, Y.; Lu, Y.; Xu, K.; Ni, D.; Jiang, X.; Wang, H.; Yang, X. Circular RNAs are a novel type of non-coding RNAs in ROS regulation, cardiovascular metabolic inflammations, and cancers. Pharmacol. Ther. 2021, 220, 107715. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, X.; Shi, Y.; Wang, Z. The signaling pathways involved in ovarian follicle development. Front. Physiol. 2021, 12, 730196. [Google Scholar] [CrossRef] [PubMed]
- Carbonari, A.; Martino, N.A.; Burgio, M.; Cicirelli, V.; Frattina, L.; Dell’Aquila, M.E.; Rizzo, A. New insights in bovine follicular cysts. Reprod. Domest. Anim. 2025, 60, e70048. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, J.I.; Rabaglino, M.B.; Denicol, A.C. Ovarian preantral follicles are responsive to FSH as early as the primary stage of development. J. Endocrinol. 2020, 247, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.J.; Candelaria, J.I.; McDonnell, S.P.; Zgodzay, D.P.; Denicol, A.C. Review: Roles of follicle-stimulating hormone in preantral folliculogenesis of domestic animals: What can we learn from model species and where do we go from here? Animal 2023, 17 (Suppl. S1), 100743. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Goldberg, A.L. Ubiquitinated proteins promote the association of proteasomes with the deubiquitinating enzyme Usp14 and the ubiquitin ligase Ube3c. Proc. Natl. Acad. Sci. USA 2017, 114, E3404–E3413. [Google Scholar] [CrossRef] [PubMed]
- Noferesti, S.S.; Sohel, M.M.; Hoelker, M.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Neuhoff, C.; Schellander, K.; Tesfaye, D. Controlled ovarian hyperstimulation induced changes in the expression of circulatory miRNA in bovine follicular fluid and blood plasma. J. Ovarian Res. 2015, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Otávio, K.S.; Passos, J.R.S.; Silva, R.F.; Lima, L.F.; Cadenas, J.; Paes, V.M.; Correia, H.H.V.; Ferreira, A.C.A.; Canafístula, F.G.; Bezerra, M.J.B.; et al. Comprehensive proteomic profiling of early antral follicles from sheep. Anim. Reprod. Sci. 2023, 248, 107153. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Zhang, L. The impact of follicular fluid oxidative stress levels on the outcomes of assisted reproductive therapy. Antioxidants 2023, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. Altered energy metabolism, mitochondrial dysfunction, and redox imbalance influencing reproductive performance in granulosa cells and oocyte during aging. Reprod. Sci. 2024, 31, 906–916. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.G.; Portela, V.M.; Dos Santos, E.C.; Aires, K.V.; Ferreira, R.; Missio, D.; da Silva, Z.; Koch, J.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; et al. FSH regulates YAP-TEAD transcriptional activity in bovine granulosa cells to allow the future dominant dollicle to exert Its augmented estrogenic capacity. Int. J. Mol. Sci. 2022, 23, 14160. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Crépieux, P. Molecular mechanisms of action of FSH. Front. Endocrinol. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yan, H.; He, M.; Zhang, T.; Mu, X.; Wang, H.; Zhang, H.; Xia, G.; Wang, C. GSK-3β protects fetal oocytes from premature death via modulating TAp63 expression in mice. BMC Biol. 2019, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Arvisais, E.W.; Davis, J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010, 151, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Z.B.; Jiang, Z.Z.; Hu, M.W.; Lin, F.; Zhang, Q.H.; Luo, Y.B.; Hou, Y.; Zhao, Y.; Fan, H.Y.; et al. Specific disruption of Tsc1 in ovarian granulosa cells promotes ovulation and causes progressive accumulation of corpora lutea. PLoS ONE 2013, 8, e54052. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Hämäläinen, T.; Cooney, A.J.; Huhtaniemi, I.; Lan, Z.J.; Liu, K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 2010, 19, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.S.; Findlay, G.M.; Gray, A.; Tolkacheva, T.; Wigfield, S.; Rebholz, H.; Barnett, J.; Leslie, N.R.; Cheng, S.; Shepherd, P.R.; et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 2004, 166, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Y.; Ma, L.; Xu, J.; Lv, C.; Deng, L.; Zhu, G. LINC00857 regulated by ZNF460 enhances the expression of CLDN12 by sponging miR-150-5p and recruiting SRSF1 for alternative splicing to promote epithelial-mesenchymal transformation of pancreatic adenocarcinoma cells. RNA Biol. 2022, 19, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Araujo, V.R.; Gastal, M.O.; Wischral, A.; Figueiredo, J.R.; Gastal, E.L. In vitro development of bovine secondary follicles in two- and three-dimensional culture systems using vascular endothelial growth factor, insulin-like growth factor-1, and growth hormone. Theriogenology 2014, 82, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cheng, J.; Yu, H.; Wang, Z.; Bai, Y.; Xia, C.; Xu, C. Early warning for ovarian diseases based on plasma non-esterified fatty acid and calcium concentrations in dairy cows. Front. Vet. Sci. 2021, 8, 792498. [Google Scholar] [CrossRef] [PubMed]
- Beam, S.W.; Butler, W.R. Effects of Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. Suppl. 1999, 54, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Jonard, S.; Dewailly, D. The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum. Reprod. Update 2004, 10, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Totty, M.L.; Morrell, B.C.; Spicer, L.J. Fibroblast growth factor 9 (FGF9) regulation of cyclin D1 and cyclin-dependent kinase-4 in ovarian granulosa and theca cells of cattle. Mol. Cell. Endocrinol. 2017, 440, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Christakou, C.; Kandarakis, H. Polycystic ovarian syndrome: The commonest cause of hyperandrogenemia in women as a risk factor for metabolic syndrome. Minerva Endocrinol. 2007, 32, 35–47. [Google Scholar] [PubMed]
- Summers, A.F.; Pohlmeier, W.E.; Sargent, K.M.; Cole, B.D.; Vinton, R.J.; Kurz, S.G.; McFee, R.M.; Cushman, R.A.; Cupp, A.S.; Wood, J.R. Altered theca and cumulus oocyte complex gene expression, follicular arrest and reduced fertility in cows with dominant follicle follicular fluid androgen excess. PLoS ONE 2014, 9, e110683. [Google Scholar] [CrossRef] [PubMed]
- Abedal-Majed, M.A.; Cupp, A.S. Livestock animals to study infertility in women. Anim. Front. 2019, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.R.; Ho, C.K.; Nelson-Degrave, V.L.; McAllister, J.M.; Strauss, J.F., 3rd. The molecular signature of polycystic ovary syndrome (PCOS) theca cells defined by gene expression profiling. J. Reprod. Immunol. 2004, 63, 51–60. [Google Scholar] [CrossRef] [PubMed]
- McFee, R.M.; Romereim, S.M.; Snider, A.P.; Summers, A.F.; Pohlmeier, W.E.; Kurz, S.G.; Cushman, R.A.; Davis, J.S.; Wood, J.R.; Cupp, A.S. A high-androgen microenvironment inhibits granulosa cell proliferation and alters cell identity. Mol. Cell. Endocrinol. 2021, 531, 111288. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Harada, M.; Hirota, Y.; Nose, E.; Azhary, J.M.; Koike, H.; Kunitomi, C.; Yoshino, O.; Izumi, G.; Hirata, T.; et al. Activation of endoplasmic reticulum stress in granulosa cells from patients with polycystic ovary syndrome contributes to ovarian fibrosis. Sci. Rep. 2017, 7, 10824. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 2008, 14, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Pratama, G.; Wiweko, B.; Widyahening, I.S.A.; Andraini, T.; Bayuaji, H.; Hestiantoro, A. Mechanism of elevated LH/FSH ratio in lean PCOS revisited: A path analysis. Sci. Rep. 2024, 14, 8229. [Google Scholar] [CrossRef] [PubMed]
- Merza, W.M.; Yaseen, A.K.; Mahmood, M.A. FSH, LH, lipid and adipokines in Polycystic Ovary Syndrome: Clinical biochemistry insights for diagnosis and management. J. Steroid Biochem. Mol. Biol. 2025, 251, 106773. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Chen, C.; Sun, Y. Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 2025, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major nutritional metabolic alterations influencing the reproductive system of postpartum dairy cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.; Lim, S.; Alycia, C.; Pirotta, S.; Thomson, R.; Gibson-Helm, M.; Blackmore, R.; Naderpoor, N.; Bennett, C.; Ee, C.; et al. Lifestyle management in polycystic ovary syndrome—Beyond diet and physical activity. BMC Endocr. Disord. 2023, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Bates, G.W.; Legro, R.S. Longterm management of Polycystic Ovarian Syndrome (PCOS). Mol. Cell. Endocrinol. 2013, 373, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, A.; Maftoohi, M.; Samarin, M.E.; Barani, S.; Banimohammad, M.; Samie, R. The last update on Polycystic Ovary Syndrome (PCOS), diagnosis criteria, and novel treatment. Endocr. Metab. Sci. 2025, 17, 100228. [Google Scholar] [CrossRef]
- Rapani, A.; Nikiforaki, D.; Karagkouni, D.; Sfakianoudis, K.; Tsioulou, P.; Grigoriadis, S.; Maziotis, E.; Pantou, A.; Voutsina, A.; Pantou, A.; et al. Reporting on the role of miRNAs and affected pathways on the molecular backbone of ovarian insufficiency: A systematic review and critical analysis mapping of future research. Front. Cell Dev. Biol. 2021, 8, 590106. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zheng, Y.; Tang, X.; Gao, H.; Liu, N.; Gao, Y.; Hao, L.; Liu, S.; Jiang, Z. miR-21-3p inhibits autophagy of bovine granulosa cells by targeting VEGFA via PI3K/AKT signaling. Reproduction 2019, 158, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.; Liu, H.; Pan, Z. MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, L.; Feng, G.; Xiang, W.; Zhang, K.; Chu, M.; Wang, P. MicroRNA mediating networks in granulosa cells associated with ovarian follicular development. BioMed Res. Int. 2017, 2017, 4585213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Heneidi, S.; Lee, J.M.; Layman, L.C.; Stepp, D.W.; Gamboa, G.M.; Chen, B.S.; Chazenbalk, G.; Azziz, R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 2013, 62, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.D.; Carletti, M.Z.; Hong, X.; Christenson, L.K. Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008, 79, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Nouri, N.; Shareghi-Oskoue, O.; Aghebati-Maleki, L.; Danaii, S.; Ahmadian Heris, J.; Soltani-Zangbar, M.S.; Kamrani, A.; Yousefi, M. Role of miRNAs interference on ovarian functions and premature ovarian failure. Cell Commun. Signal. 2022, 20, 198. [Google Scholar] [CrossRef] [PubMed]
- Ban, E.; Kwon, T.H.; Kim, A. Delivery of therapeutic miRNA using polymer-based formulation. Drug Deliv. Transl. Res. 2019, 9, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Carrillo, E.; Liu, Y.P.; Berkhout, B. Improving miRNA delivery by optimizing miRNA expression cassettes in diverse virus vectors. Hum. Gene Ther. Methods 2017, 28, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent advances in miRNA delivery systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Lee, O.; Alizadeh, A.H.; LaMarre, J.; Koch, T.G. Why the hype—What are microRNAs and why do they provide unique investigative, diagnostic, and therapeutic opportunities in veterinary medicine? Can. Vet. J. 2020, 61, 845–852. [Google Scholar] [PubMed]
- Myrzabekova, M.; Labeit, S.; Niyazova, R.; Akimniyazova, A.; Ivashchenko, A. Identification of bovine miRNAs with the potential to affect human gene expression. Front. Genet. 2022, 12, 705350. [Google Scholar] [CrossRef] [PubMed]
- Zachut, M.; Sood, P.; Levin, Y.; Moallem, U. Proteomic analysis of preovulatory follicular fluid reveals differentially abundant proteins in less fertile dairy cows. J. Proteom. 2016, 139, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Cao, X.X.; Hua, H.; Zhu, H. The functions and implications of microRNAs in premature ovarian insufficiency. Mol. Genet. Genom. Med. 2025, 13, e70074. [Google Scholar] [CrossRef] [PubMed]

| Gene ID | Degree | Betweenness |
|---|---|---|
| Upregulated | ||
| NEAT1 | 7 | 83,015.76 |
| KCNQ1OT1 | 6 | 59,933.59 |
| AGO2 | 5 | 61,059.11 |
| NUFIP2 | 5 | 41,540.83 |
| PTEN | 5 | 34,929.53 |
| TUG1 | 5 | 38,981.89 |
| XIST | 5 | 43,828.79 |
| HCG18 | 5 | 41,237.25 |
| DICER1 | 4 | 22,365.89 |
| E2F3 | 4 | 21,041.56 |
| FZD6 | 4 | 27,243.63 |
| HIF1A | 4 | 21,041.56 |
| MYCN | 4 | 23,217 |
| REL | 4 | 23,217 |
| SNHG1 | 4 | 28,911.3 |
| VEGFA | 4 | 20,431.3 |
| CDK6 | 4 | 22,842.08 |
| SIRT1 | 4 | 26,887.84 |
| EGFR | 4 | 22,653.18 |
| PSMA3-AS1 | 4 | 24,494.06 |
| Downregulated | ||
| KCNQ1OT1 | 6 | 62,381.99 |
| NEAT1 | 6 | 62,381.99 |
| OIP5-AS1 | 5 | 40,151.9 |
| RNF138 | 5 | 22,458.95 |
| CCND1 | 4 | 38,215.53 |
| CCND2 | 4 | 37,271.94 |
| CDK6 | 4 | 37,271.94 |
| HNRNPM | 4 | 37,271.94 |
| SERBP1 | 4 | 33,966.8 |
| XIST | 6 | 33,717.75 |
| EEF1A1 | 4 | 31,595 |
| ATP2A2 | 4 | 26,749.51 |
| CREG1 | 4 | 26,749.51 |
| KLHDC10 | 4 | 26,749.51 |
| MLXIP | 4 | 26,749.51 |
| PPTC7 | 4 | 26,749.51 |
| PSMD7 | 4 | 26,749.51 |
| SRPR | 4 | 26,749.51 |
| SRPRA | 4 | 26,749.51 |
| Gene ID | circRNA |
|---|---|
| Upregulated | |
| GTF2I | hsa_circ_0006944 |
| AMMECR1L | hsa_circ_0005892 |
| GTF2H2C | hsa_circ_0004914 |
| EXTL3 | hsa_circ_0003885 |
| GPAM | - |
| DNAJC10 | hsa_circ_0057256 |
| CUL3 | hsa_circ_0008309 |
| GLUD1 | hsa_circ_0019034 |
| CTC1 | hsa_circ_0008041 |
| ATP1A1 | hsa_circ_0013692 |
| RCBTB1 | hsa_circ_0030262 |
| DDX3X | hsa_circ_0090290 |
| CTDSPL | hsa_circ_0064843 |
| AP2B1 | hsa_circ_0043121 |
| Downregulated | |
| ATM | hsa_circ_0007694 |
| AFF4 | hsa_circ_0007945 |
| AP3S2 | hsa_circ_0009156 |
| ADAM15 | hsa_circ_0014480 |
| ABR | hsa_circ_0041188 |
| snRNA | Degree | Betweenness |
|---|---|---|
| RN7SL585P | 9 | 894.1123 |
| RN7SKP91 | 11 | 827.2224 |
| SNORD17 | 9 | 802.2572 |
| RN7SKP22 | 8 | 777.9545 |
| RNA5SP524 | 6 | 733.8872 |
| RN7SKP232 | 8 | 727.4715 |
| RNA5SP404 | 3 | 677.4443 |
| RNA5SP29 | 3 | 601.4439 |
| SNORD69 | 3 | 587.1188 |
| RN7SKP8 | 8 | 581.8583 |
| lncRNA | Degree | Betweenness |
|---|---|---|
| KCNQ1OT1 | 507 | 50,840.21 |
| NEAT1 | 504 | 48,867.59 |
| XIST | 454 | 39,551.45 |
| OIP5-AS1 | 259 | 10,794.21 |
| HCG18 | 256 | 10,484.17 |
| MALAT1 | 249 | 9724.205 |
| GABPB1-AS1 | 245 | 9648.505 |
| HELLPAR | 215 | 7300.995 |
| TUG1 | 204 | 6706.267 |
| MIR29B2CHG | 171 | 4842.389 |
| miRNA | Function | Role in Ovarian Follicular Cysts | Functional Category |
|---|---|---|---|
| miR-26b | Promotes granulosa cell apoptosis via ATM, SMAD4, and HAS2 pathways | May impair follicle survival, promoting atresia and contributing to cyst persistence | Stress Response |
| miR-18a | Regulates TGF-β/SMAD signaling, induces apoptosis in granulosa cells | Dysregulation may disrupt follicular atresia or maturation, possibly leading to cystic changes | Stress Response |
| miR-30b/30e | Involved in granulosa cell proliferation, apoptosis, and autophagy | Altered expression may disturb follicular growth dynamics, favoring abnormal persistence | Stress Response |
| miR-15b | Regulates ovulation, follicular atresia, and steroidogenesis | Imbalance may impair ovulation timing, contributing to cyst formation | Steroidogenesis |
| miR-29a | Expressed in granulosa cells of mature dominant/subordinate follicles; ECM modulation | Dysregulation may influence dominance selection, leading to abnormal follicular development | ECM Remodeling |
| miR-191 | Involved in immune signaling (e.g., Interleukin 6 (IL6), Toll-Like Receptor 3 (TLR3); proinflammatory | Changes in expression may impact the inflammatory milieu, affecting follicular stability | Stress Response/Insulin Resistance (NEB) |
| miR-132 | Regulates hormone production, steroidogenesis, and NF-κB, IL8 activity | Dysregulation may disturb hormonal balance and granulosa function, promoting cysts | Steroidogenesis/Stress Response |
| miR-221 | Modulates ErbB, PI3K-Akt signaling, targets Fos Proto-Oncogene, AP-1 Transcription Factor Subunit (FOS), and Matrix Metallopeptidase 1 (MMP1) | Upregulation in subordinate follicles implies a role in follicular arrest and cystogenesis | Steroidogenesis/ECM Remodeling/Insulin Resistance (NEB) |
| miR-145 | Controls follicle formation, maintenance, and activation | Downregulation in hyperstimulated follicles may impair normal follicle development | ECM Remodeling |
| miR-103 | Involved in metabolic regulation; targets PLAG1 Zinc Finger (PLAG1), Cell Division Cycle-Associated 4 (CDCA4), Beta-Secretase 1 (BACE1) | Upregulation may alter granulosa cell metabolism and proliferation in hyperstimulated follicles | Negative Energy Balance/Insulin Resistance (NEB) |
| miR-101 | Targets Cyclooxygenase 2 (COX2); modulates inflammation and prostaglandin production | Dysregulated inflammation may interfere with ovulation and promote cyst development | Stress Response |
| miR-193a | Present in both mature dominant and subordinate follicle libraries | Possible role in follicle fate; altered expression may favor cyst persistence | Steroidogenesis |
| miRNA | Primary Function | Role in PCOS | Functional Category |
|---|---|---|---|
| miR-18a | Regulates TGF-β/SMAD signaling, induces apoptosis | Overexpression promotes follicular persistence and anovulation | Stress Response/Cell Survival |
| miR-103 | Influences insulin sensitivity; targets IRS pathway | Upregulated in PCOS; impairs insulin signaling in granulosa cells | Insulin Signaling |
| miR-221 | Modulates ErbB, PI3K-Akt signaling, MMP1, FOS | Promotes insulin resistance, alters steroidogenesis, and affects follicular growth | Insulin Signaling/Cell Survival |
| miR-30b/30e | Cell proliferation, apoptosis, autophagy | Regulates granulosa survival and stress response | Stress Response/Cell survival |
| miR-21 | Anti-apoptotic; inhibits caspase-3 | Overexpression delays follicular atresia, promoting anovulation | Stress Response/Cell Survival |
| miR-132/320 | Regulate steroidogenesis and granulosa cell function | Downregulation impairs estrogen synthesis, affecting follicle maturation | Steroidogenesis |
| miR-193a | Follicle fate regulation | Present in PCOS-related follicular environments | Cell Survival |
| miR-132 | Regulates NF-κB, IL8, steroid hormone pathways | Downregulation impacts hormone synthesis and promotes abnormal follicular development | Steroidogenesis/Stress Response |
| miR-29a | Involved in extracellular matrix turnover and oocyte development | Dysregulation impairs follicle structure and ovulatory capacity | ECM Remodeling |
| miR-145 | Regulates folliculogenesis and cell cycle checkpoints | Downregulation impairs granulosa cell differentiation and follicle progression | ECM Remodeling/Cell Cycle |
| miR-221 | Modulates ErbB and PI3K-Akt signaling | Overexpression may block follicle maturation and arrest granulosa proliferation | Steroidogenesis/Insulin Signaling |
| miRNA | Function | In Bovine Follicular Cysts | In Human PCOS |
|---|---|---|---|
| miR-26b | Promotes granulosa cell apoptosis via ATM, SMAD4, HAS2 | Enhances atresia, reducing follicle survival, contributing to cyst formation | Not widely reported in PCOS |
| miR-21 | Anti-apoptotic; inhibits caspase-3 | Prevents apoptosis of granulosa cells in mature follicles | Overexpression promotes follicular persistence and anovulation |
| miR-18a | Regulates TGF-β/SMAD signaling, induces apoptosis | May disrupt follicular atresia and lead to cystic changes | Alters SMAD signaling; involved in follicular atresia |
| miR-30b/30e | Cell proliferation, apoptosis, autophagy | Dysregulation affects follicular dynamics and leads to abnormal persistence | Regulates granulosa survival and stress response |
| miR-15b | Ovulation, atresia, steroidogenesis | Impairs ovulation timing and contributes to cyst development | Associated with ovulatory dysfunction in PCOS |
| miR-29a | ECM remodeling and oocyte development | Dysregulation impairs mature dominant follicle selection | Disrupts follicular structure and ovulation |
| miR-132 | Hormone production, NF-κB, IL8 regulation | Disrupts steroid balance and granulosa cell function | Downregulated; contributes to low estrogen and inflammation |
| miR-221 | Modulates Erb-B2 Receptor Tyrosine Kinase (ErbB), PI3K-Akt signaling, MMP1, FOS | Linked to follicular arrest in subordinate follicles | Promotes insulin resistance, alters steroidogenesis, and affects follicular growth |
| miR-145 | Follicle maintenance and activation | Downregulated in hyperstimulated follicles, affects granulosa function | Downregulated in PCOS; impairs follicle activation |
| miR-103 | Metabolic regulation, insulin signaling | Upregulation alters granulosa metabolism | Contributes to insulin resistance and metabolic dysfunction |
| miR-101 | Targets COX2, inflammatory modulation | Disrupts ovulatory processes through inflammation | Inflammatory mediator; possible involvement in ovulatory failure |
| miR-193a | Follicle fate regulation | Associated with the persistence of subordinate follicles | Present in PCOS-related follicular environments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimanickam, R.; Kasimanickam, V.; Ferreira, J.; Kastelic, J.; de Souza, F. Regulatory RNA Networks in Ovarian Follicular Cysts in Dairy Cows: Implications for Human Polycystic Ovary Syndrome. Genes 2025, 16, 791. https://doi.org/10.3390/genes16070791
Kasimanickam R, Kasimanickam V, Ferreira J, Kastelic J, de Souza F. Regulatory RNA Networks in Ovarian Follicular Cysts in Dairy Cows: Implications for Human Polycystic Ovary Syndrome. Genes. 2025; 16(7):791. https://doi.org/10.3390/genes16070791
Chicago/Turabian StyleKasimanickam, Ramanathan, Vanmathy Kasimanickam, Joao Ferreira, John Kastelic, and Fabiana de Souza. 2025. "Regulatory RNA Networks in Ovarian Follicular Cysts in Dairy Cows: Implications for Human Polycystic Ovary Syndrome" Genes 16, no. 7: 791. https://doi.org/10.3390/genes16070791
APA StyleKasimanickam, R., Kasimanickam, V., Ferreira, J., Kastelic, J., & de Souza, F. (2025). Regulatory RNA Networks in Ovarian Follicular Cysts in Dairy Cows: Implications for Human Polycystic Ovary Syndrome. Genes, 16(7), 791. https://doi.org/10.3390/genes16070791







