Identification of Immune Hub Genes in Obese Postmenopausal Women Using Microarray and Single-Cell RNA Seq Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Data Pre-Processing
2.4. Analysis of Differential Gene Expression
2.5. WGCNA Analysis
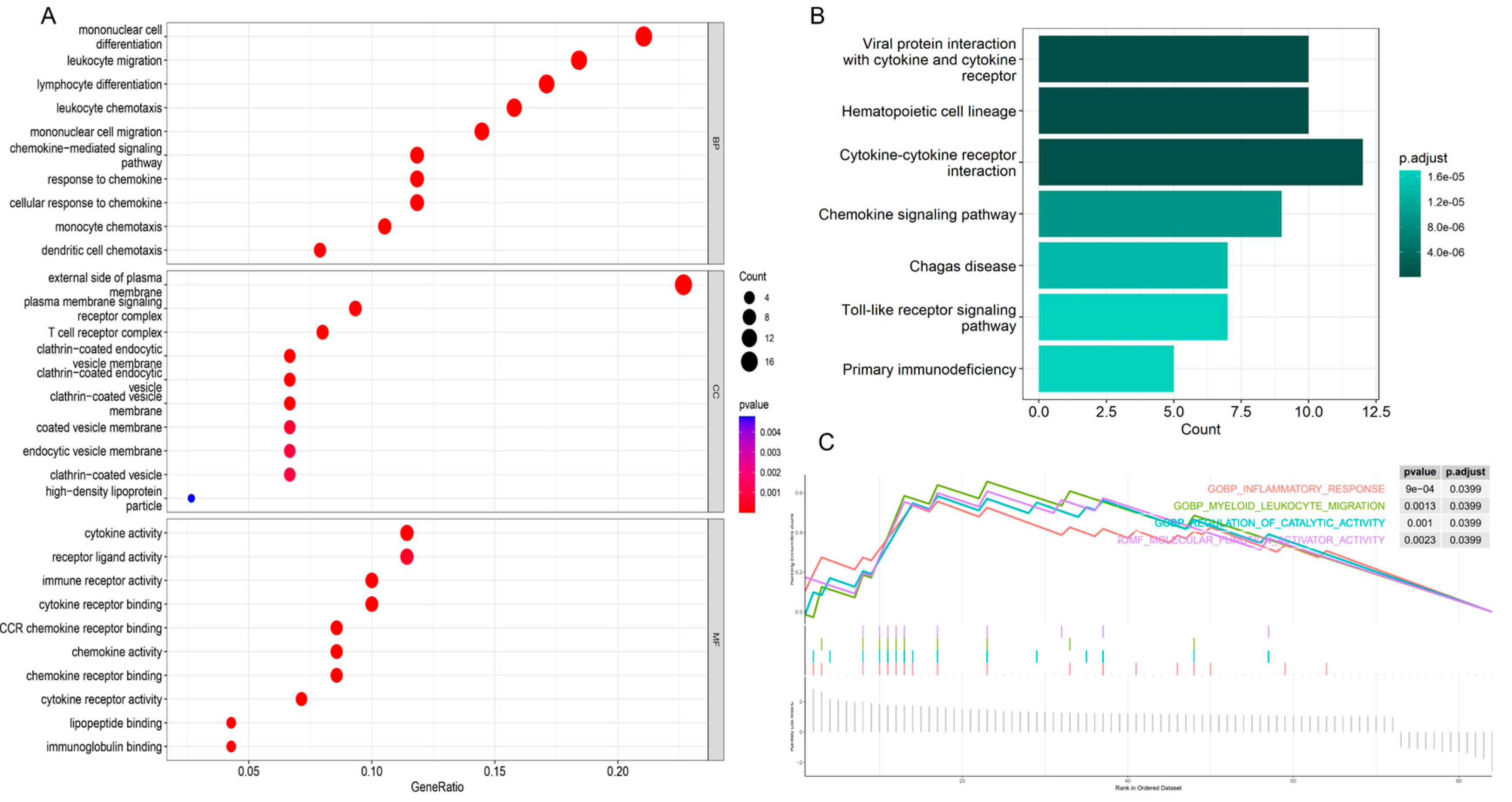
2.6. Functional Enrichment Analysis of Obesity-Related Genes
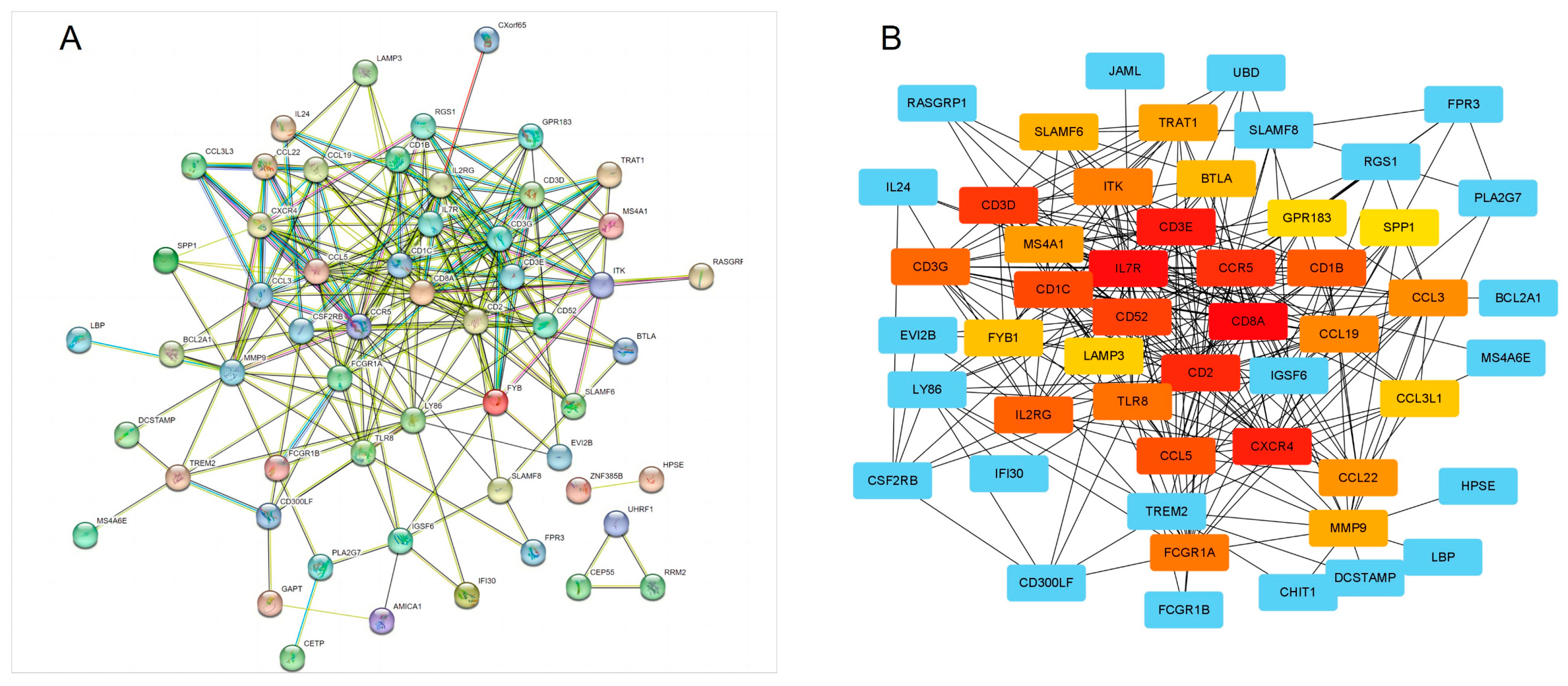
2.7. Construction of Protein–Protein Interaction Networks
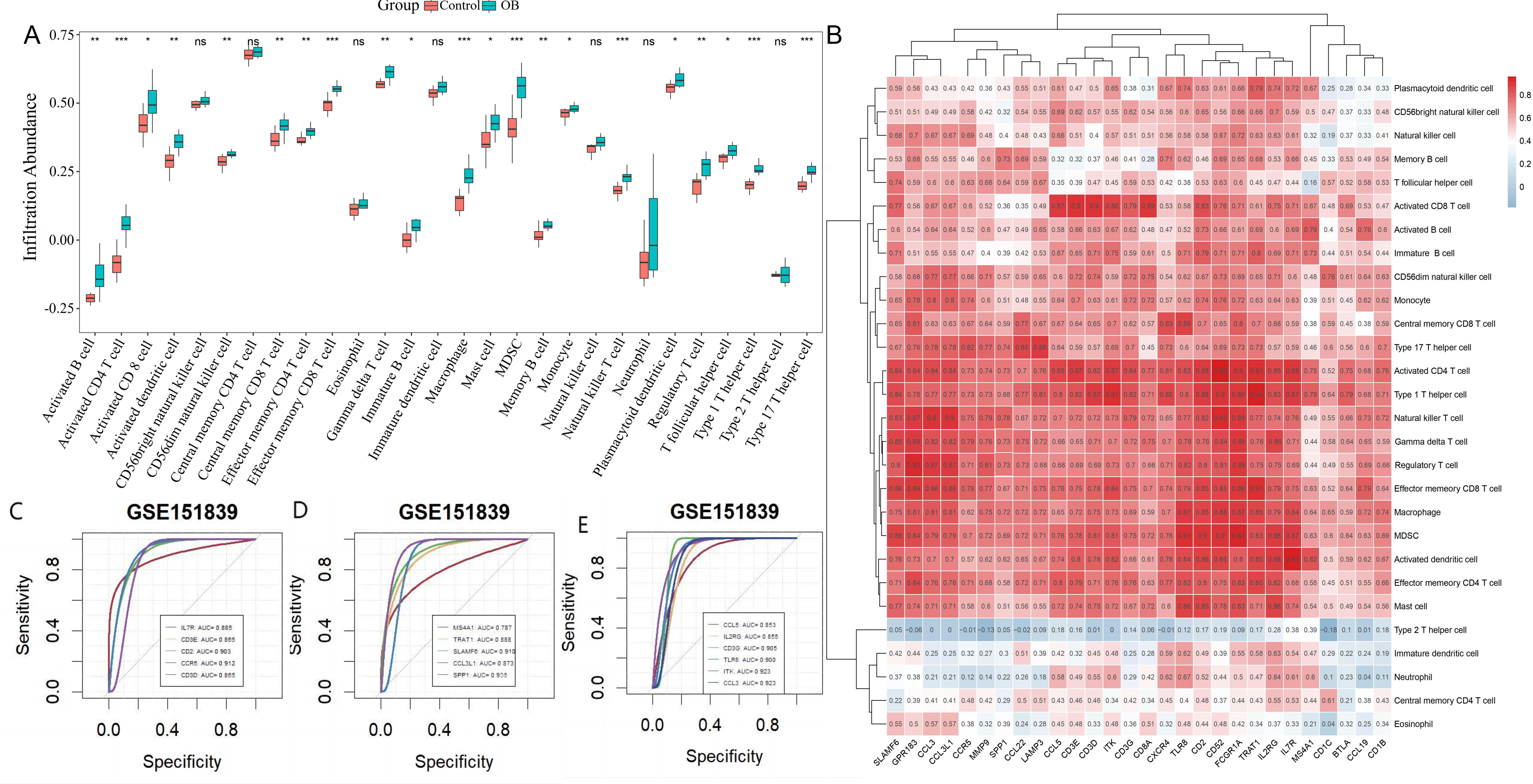
2.8. Immune Infiltration Analysis
2.9. Preliminary Screening of Immunity-Related Genes
2.10. Analysis of Single-Cell Datasets
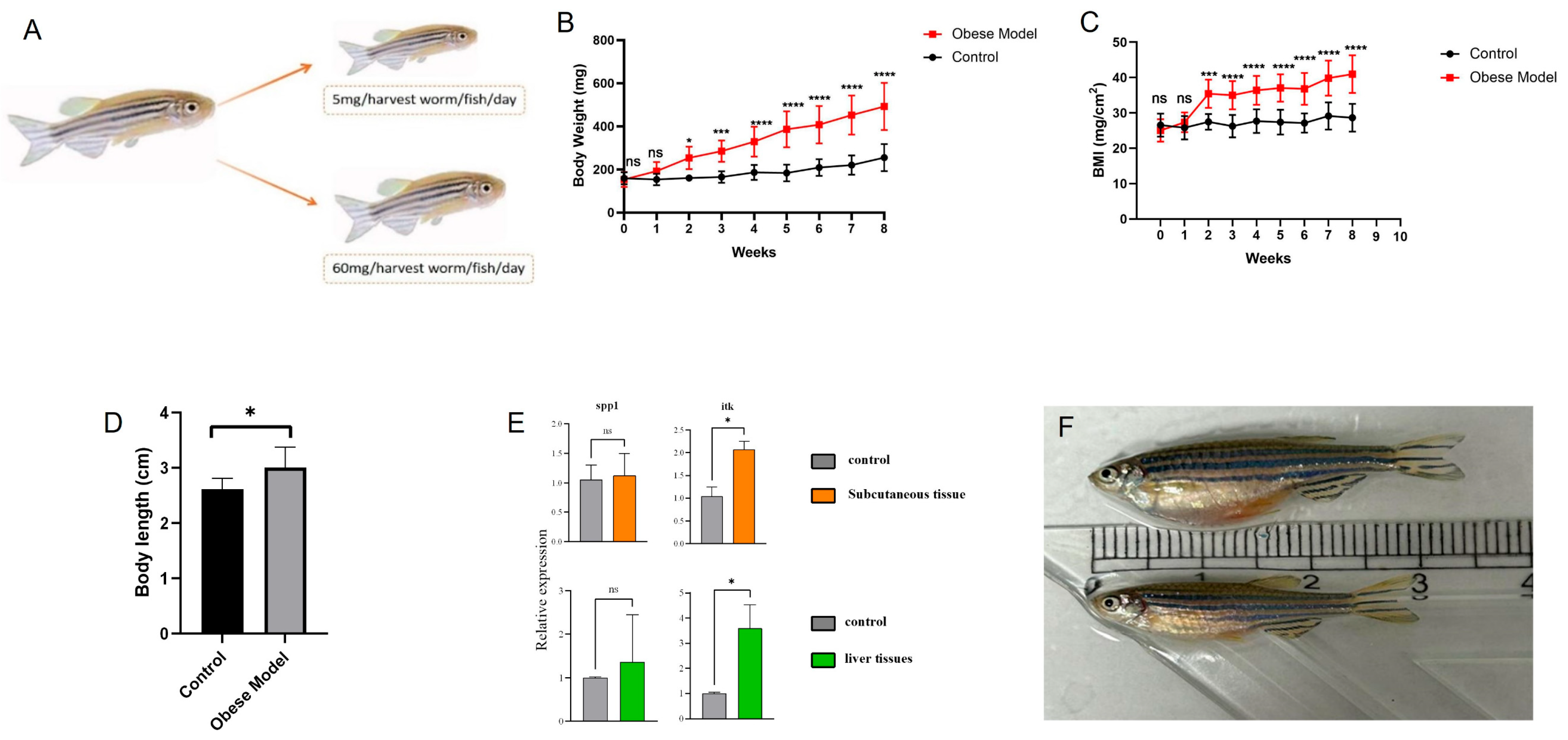
2.11. Zebrafish Experimental Validation
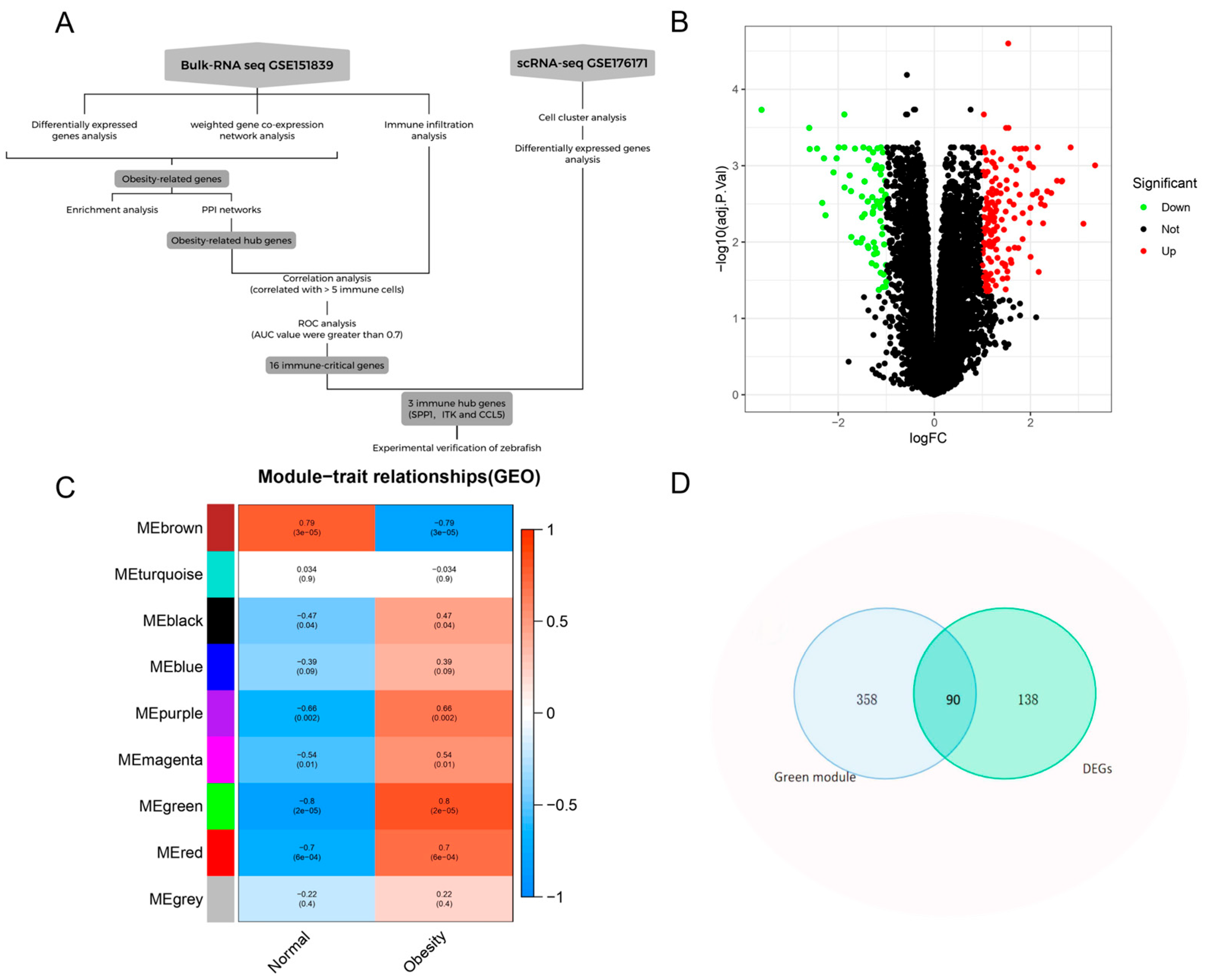
3. Results
3.1. Identify Genes Associated with Obesity
3.2. GO and KEGG Enrichment Analysis of Obesity-Related Genes
3.3. PPI Network Identifies Obesity-Related Hub Genes
3.4. Hub Gene-Immune Cell Correlation Analysis
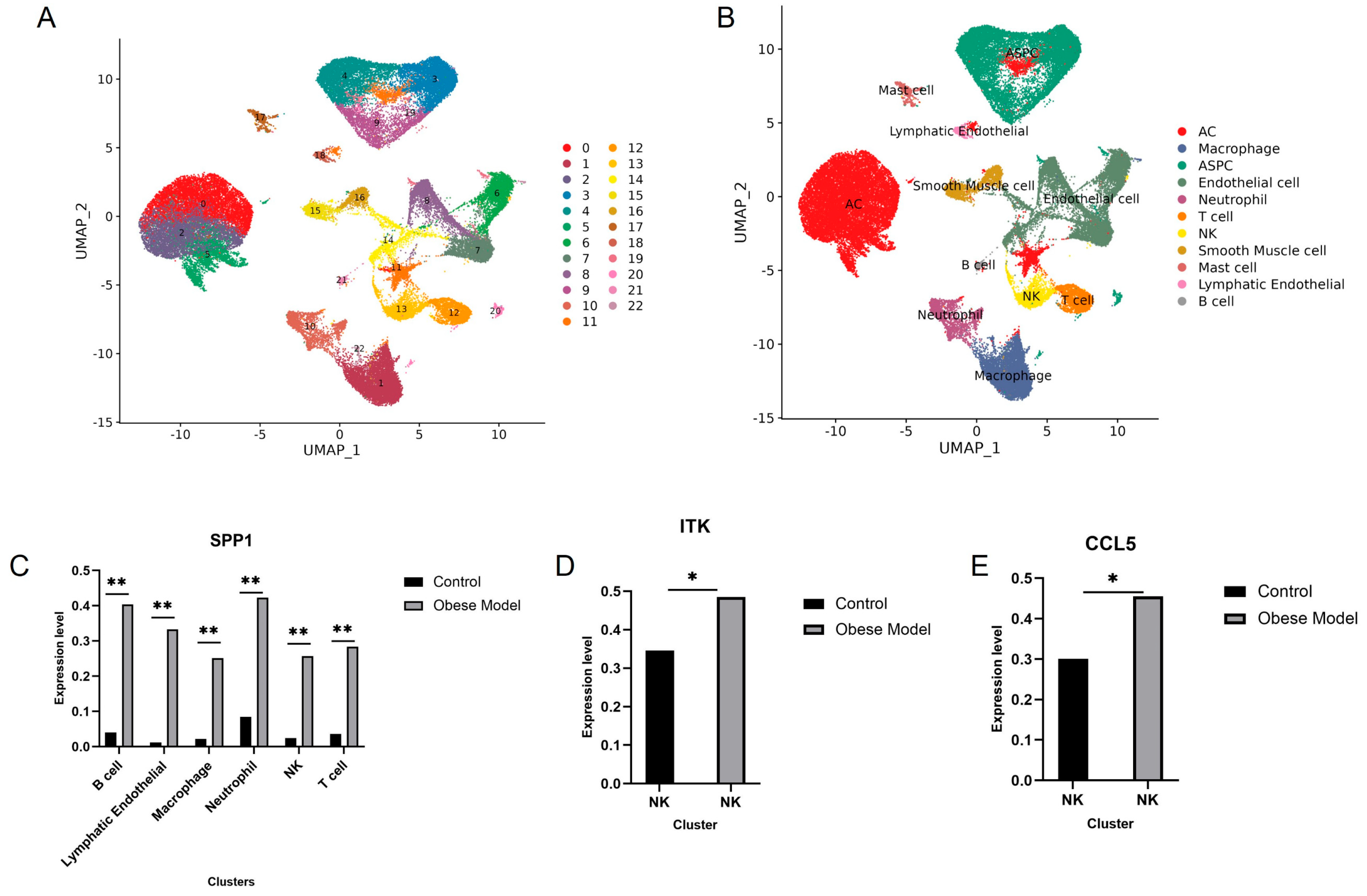
3.5. Validation of Immune-Critical Genes in scRNA-Seq Data
3.6. Validation of Immune Hub Genes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines For Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A. Metabolic Pathways in Obesity-Related Breast Cancer. Nat. Rev. Endocrinol. 2021, 17, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Brincat, M.; Erel, C.T.; Gambacciani, M.; Moen, M.H.; Schenck-Gustafsson, K.; Tremollieres, F.; Vujovic, S.; Rees, M.; Rozenberg, S. EMAS Position Statement: Managing Obese Postmenopausal Women. Maturitas 2010, 66, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Study 2013 Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, Regional, and Global Trends in Body-Mass Index Since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 960 Country-Years and 9.1 Million Participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Tekeş, B.; Üzümcüoğlu, Y.; Hoe, C.; Özkan, T. The Relationship Between Hofstede’s Cultural Dimensions, Schwartz’s Cultural Values, and Obesity. Psychol. Rep. 2019, 122, 968–987. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Gao, L.; Pan, A.; Xue, H. Health Policy and Public Health Implications of Obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 446–461. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-Mass Index and Risk of 22 Specific Cancers: A Population-Based Cohort Study of 5.24 Million UK Adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Flint, A.J.; Berrington de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association Between Class III Obesity (BMI of 40–59 Kg/m2) and Mortality: A Pooled Analysis of 20 Prospective Studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef]
- Marozik, P.; Rudenka, A.; Kobets, K.; Rudenka, E. Vitamin D Status, Bone Mineral Density, and VDR Gene Polymorphism in a Cohort of Belarusian Postmenopausal Women. Nutrients 2021, 13, 837. [Google Scholar] [CrossRef]
- Alghanim, F.; Al-Hurani, I.; Qattous, H.; Al-Refai, A.; Batiha, O.; Alkhateeb, A.; Ikki, S. Machine Learning Model for Multiomics Biomarkers Identification for Menopause Status in Breast Cancer. Algorithms 2024, 17, 13. [Google Scholar] [CrossRef]
- Sam, S.; Mazzone, T. Adipose Tissue Changes in Obesity and the Impact on Metabolic Function. Transl. Res. 2014, 164, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; de Courten, M.P.J.; Stojanovska, L.; Blatch, G.L.; Tangalakis, K.; de Courten, B. The Complex Immunological and Inflammatory Network of Adipose Tissue in Obesity. Mol. Nutr. Food Res. 2016, 60, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Cildir, G.; Akıncılar, S.C.; Tergaonkar, V. Chronic Adipose Tissue Inflammation: All Immune Cells on the Stage. Trends Mol. Med. 2013, 19, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Kang, S.; Kumanogoh, A. The Spectrum of Macrophage Activation by Immunometabolism. Int. Immunol. 2020, 32, 467–473. [Google Scholar] [CrossRef]
- Walker, J.M.; Garcet, S.; Aleman, J.O.; Mason, C.E.; Danko, D.; Butler, D.; Zuffa, S.; Swann, J.R.; Krueger, J.; Breslow, J.L.; et al. Obesity and Ethnicity Alter Gene Expression in Skin. Sci. Rep. 2020, 10, 14079. [Google Scholar] [CrossRef]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A Single-Cell Atlas of Human and Mouse White Adipose Tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Xiong, Y.; Yuan, L.; Chen, L.; Zhu, Y.; Zhang, S.; Liu, X.; Xiao, Y.; Wang, X. Identifying a Novel Biomarker TOP2A of Clear Cell Renal Cell Carcinoma (ccRCC) Associated with Smoking by Co-Expression Network Analysis. J. Cancer 2018, 9, 3912–3922. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein-Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal Dynamics of Intratumoral Immune Cells Reveal the Immune Landscape in Human Cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, B.; Zhang, Y.; Tian, Y.; Yang, S.; Chen, Y.; Wu, H. Combination of Single-Cell and Bulk RNA Seq Reveals the Immune Infiltration Landscape and Targeted Therapeutic Drugs in Spinal Cord Injury. Front. Immunol. 2023, 14, 1068359. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.-R.; Raychaudhuri, S. Fast, Sensitive and Accurate Integration of Single-Cell Data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-Induced Obesity in Zebrafish Shares Common Pathophysiological Pathways with Mammalian Obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together. BioMed Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef]
- Villa, E.; Vukotic, R.; Cammà, C.; Petta, S.; Di Leo, A.; Gitto, S.; Turola, E.; Karampatou, A.; Losi, L.; Bernabucci, V.; et al. Reproductive Status Is Associated with the Severity of Fibrosis in Women with Hepatitis C. PLoS ONE 2012, 7, e44624. [Google Scholar] [CrossRef]
- Shimada, Y.; Kuninaga, S.; Ariyoshi, M.; Zhang, B.; Shiina, Y.; Takahashi, Y.; Umemoto, N.; Nishimura, Y.; Enari, H.; Tanaka, T. E2F8 Promotes Hepatic Steatosis Through FABP3 Expression in Diet-Induced Obesity in Zebrafish. Nutr. Metab. 2015, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Karvonen-Gutierrez, C.; Kim, C. Association of Mid-Life Changes in Body Size, Body Composition and Obesity Status with the Menopausal Transition. Healthcare 2016, 4, 42. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, G.; Dong, B.; Huang, L.; Zhang, C.; Sheng, Y.; Wu, B.; Han, L.; Wu, C.; Qi, Y. A Pan-Cancer Analysis of the Oncogenic Role of Secreted Phosphoprotein 1 (SPP1) in Human Cancers. Ann. Transl. Med. 2022, 10, 279. [Google Scholar] [CrossRef]
- Tardelli, M.; Zeyda, K.; Moreno-Viedma, V.; Wanko, B.; Grün, N.G.; Staffler, G.; Zeyda, M.; Stulnig, T.M. Osteopontin Is a Key Player for Local Adipose Tissue Macrophage Proliferation in Obesity. Mol. Metab. 2016, 5, 1131–1137. [Google Scholar] [CrossRef]
- Zeyda, M.; Gollinger, K.; Todoric, J.; Kiefer, F.W.; Keck, M.; Aszmann, O.; Prager, G.; Zlabinger, G.J.; Petzelbauer, P.; Stulnig, T.M. Osteopontin Is an Activator of Human Adipose Tissue Macrophages and Directly Affects Adipocyte Function. Endocrinology 2011, 152, 2219–2227. [Google Scholar] [CrossRef]
- Rojas, I.Y.; Moyer, B.J.; Ringelberg, C.S.; Tomlinson, C.R. Reversal of Obesity and Liver Steatosis in Mice via Inhibition of Aryl Hydrocarbon Receptor and Altered Gene Expression of CYP1B1, PPARα, SCD1, and Osteopontin. Int. J. Obes. 2020, 44, 948–963. [Google Scholar] [CrossRef]
- Choi, L.Y.; Kim, M.H.; Yang, W.M. Promotion of Osteogenesis by Sweroside via BMP2-Involved Signaling in Postmenopausal Osteoporosis. Phytother. Res. 2021, 35, 7050–7063. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Roda, J.M.; Parihar, R.; Magro, C.; Nuovo, G.J.; Tridandapani, S.; Carson, W.E. Natural Killer Cells Produce T Cell-Recruiting Chemokines in Response to Antibody-Coated Tumor Cells. Cancer Res. 2006, 66, 517–526. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Viel, S.; Besson, L.; Charrier, E.; Marçais, A.; Disse, E.; Bienvenu, J.; Walzer, T.; Dumontet, C. Alteration of Natural Killer Cell Phenotype and Function in Obese Individuals. Clin. Immunol. 2017, 177, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic Reprogramming of Natural Killer Cells in Obesity Limits Antitumor Responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, X.; Castillo, E.F.; Luo, Y.; Liu, M.; Yang, X.O. Leptin Enhances TH2 and ILC2 Responses in Allergic Airway Disease. J. Biol. Chem. 2016, 291, 22043–22052. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L.; Pietra, G.; Vacca, P.; Pende, D.; Moretta, F.; Bertaina, A.; Mingari, M.C.; Locatelli, F.; Moretta, A. Human NK Cells: From Surface Receptors to Clinical Applications. Immunol. Lett. 2016, 178, 15–19. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; White, A.E.; Metcalf, M.D.; Winters, B.R.; Diggs, B.S.; Zhu, X.; Marks, D.L. Systemic Inflammation and Insulin Sensitivity in Obese IFN-γ Knockout Mice. Metabolism 2012, 61, 1152–1161. [Google Scholar] [CrossRef]
- Boulenouar, S.; Michelet, X.; Duquette, D.; Alvarez, D.; Hogan, A.E.; Dold, C.; O’Connor, D.; Stutte, S.; Tavakkoli, A.; Winters, D.; et al. Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis Through Targeted Cytotoxicity. Immunity 2017, 46, 273–286. [Google Scholar] [CrossRef]
- Gadue, P.; Stein, P.L. NK T Cell Precursors Exhibit Differential Cytokine Regulation and Require Itk for Efficient Maturation. J. Immunol. 2002, 169, 2397–2406. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, X.-L.; Wang, Y.-J.; Fang, Y.; Li, M.-L.; Liu, X.-Y.; Luo, H.-Y.; Tian, Y. The Regulatory Network of the Chemokine CCL5 in Colorectal Cancer. Ann. Med. 2023, 55, 2205168. [Google Scholar] [CrossRef]
- Chan, P.-C.; Lu, C.-H.; Chien, H.-C.; Tian, Y.-F.; Hsieh, P.-S. Adipose Tissue-Derived CCL5 Enhances Local Pro-Inflammatory Monocytic MDSCs Accumulation and Inflammation via CCR5 Receptor in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14226. [Google Scholar] [CrossRef]
- Chan, P.-C.; Hung, L.-M.; Huang, J.-P.; Day, Y.-J.; Yu, C.-L.; Kuo, F.-C.; Lu, C.-H.; Tian, Y.-F.; Hsieh, P.-S. Augmented CCL5/CCR5 Signaling in Brown Adipose Tissue Inhibits Adaptive Thermogenesis and Worsens Insulin Resistance in Obesity. Clin. Sci. 2022, 136, 121–137. [Google Scholar] [CrossRef]
- Wu, H.; Ghosh, S.; Perrard, X.D.; Feng, L.; Garcia, G.E.; Perrard, J.L.; Sweeney, J.F.; Peterson, L.E.; Chan, L.; Smith, C.W.; et al. T-Cell Accumulation and Regulated on Activation, Normal T Cell Expressed and Secreted Upregulation in Adipose Tissue in Obesity. Circulation 2007, 115, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Prendeville, H.; Raverdeau, M.; Wilk, M.M.; Loftus, R.M.; Douglas, A.; McCormack, J.; Moran, B.; Wilkinson, M.; Mills, E.L.; et al. Suppressive Effects of the Obese Tumor Microenvironment on CD8 T Cell Infiltration and Effector Function. J. Exp. Med. 2022, 219, e20210042. [Google Scholar] [CrossRef]
- Tai, Y.; Tian, H.; Yang, X.; Feng, S.; Chen, S.; Zhong, C.; Gao, T.; Gang, X.; Liu, M. Identification of Hub Genes and Candidate Herbal Treatment in Obesity through Integrated Bioinformatic Analysis and Reverse Network Pharmacology. Sci. Rep. 2022, 12, 17113. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.-R.; Lu, X.; Li, J.-L.; Li, Y.-X.; Pang, W.-W.; Wang, N.; Liu, K.; Zhang, Q.-Q.; Deng, Y.; Zeng, Q.; et al. Identification of Immune Hub Genes in Obese Postmenopausal Women Using Microarray and Single-Cell RNA Seq Data. Genes 2025, 16, 783. https://doi.org/10.3390/genes16070783
Zhang F-R, Lu X, Li J-L, Li Y-X, Pang W-W, Wang N, Liu K, Zhang Q-Q, Deng Y, Zeng Q, et al. Identification of Immune Hub Genes in Obese Postmenopausal Women Using Microarray and Single-Cell RNA Seq Data. Genes. 2025; 16(7):783. https://doi.org/10.3390/genes16070783
Chicago/Turabian StyleZhang, Fu-Rong, Xuan Lu, Jia-Li Li, Yu-Xin Li, Wei-Wei Pang, Ning Wang, Kun Liu, Qian-Qian Zhang, Yun Deng, Qin Zeng, and et al. 2025. "Identification of Immune Hub Genes in Obese Postmenopausal Women Using Microarray and Single-Cell RNA Seq Data" Genes 16, no. 7: 783. https://doi.org/10.3390/genes16070783
APA StyleZhang, F.-R., Lu, X., Li, J.-L., Li, Y.-X., Pang, W.-W., Wang, N., Liu, K., Zhang, Q.-Q., Deng, Y., Zeng, Q., Qu, X.-C., Chen, X.-D., Deng, H.-W., & Tan, L.-J. (2025). Identification of Immune Hub Genes in Obese Postmenopausal Women Using Microarray and Single-Cell RNA Seq Data. Genes, 16(7), 783. https://doi.org/10.3390/genes16070783





