Transcriptomic Analysis Reveals the Molecular Mechanisms of Prolactin in Regulating Porcine Follicular Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Experimental Design
2.3. Collection of Ovaries
2.4. Collection of Follicular Fluid and Follicular Tissue Cells
2.5. ELISA
2.6. Culture of Granulosa Cells
2.7. Prolactin Treatment of Granulosa Cells
2.8. Cell Proliferation Assay
2.9. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.10. Western Blotting (WB)
2.11. RNA Extraction, Library Preparation, and Sequencing
2.12. Differential Expression and Functional Enrichment Analysis of mRNAs
2.13. Statistical Analysis
3. Results
3.1. Prolactin Concentrations in the Follicular Fluid of Different Follicle Sizes
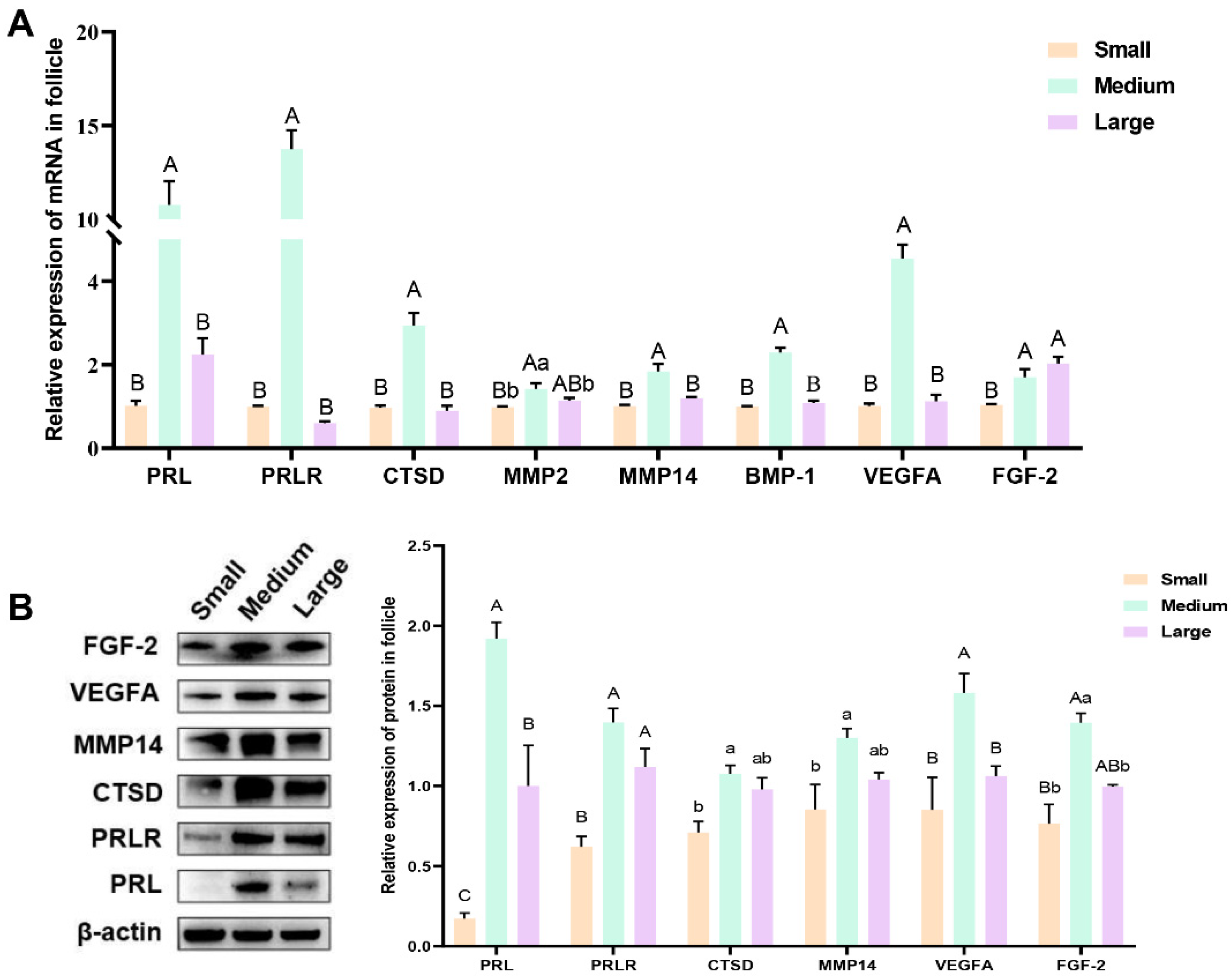
3.2. The Expression of Prolactin, Prolactin Receptor, Proteolytic Enzymes, and Key Angiogenesis Factors in Follicular Tissue Cells
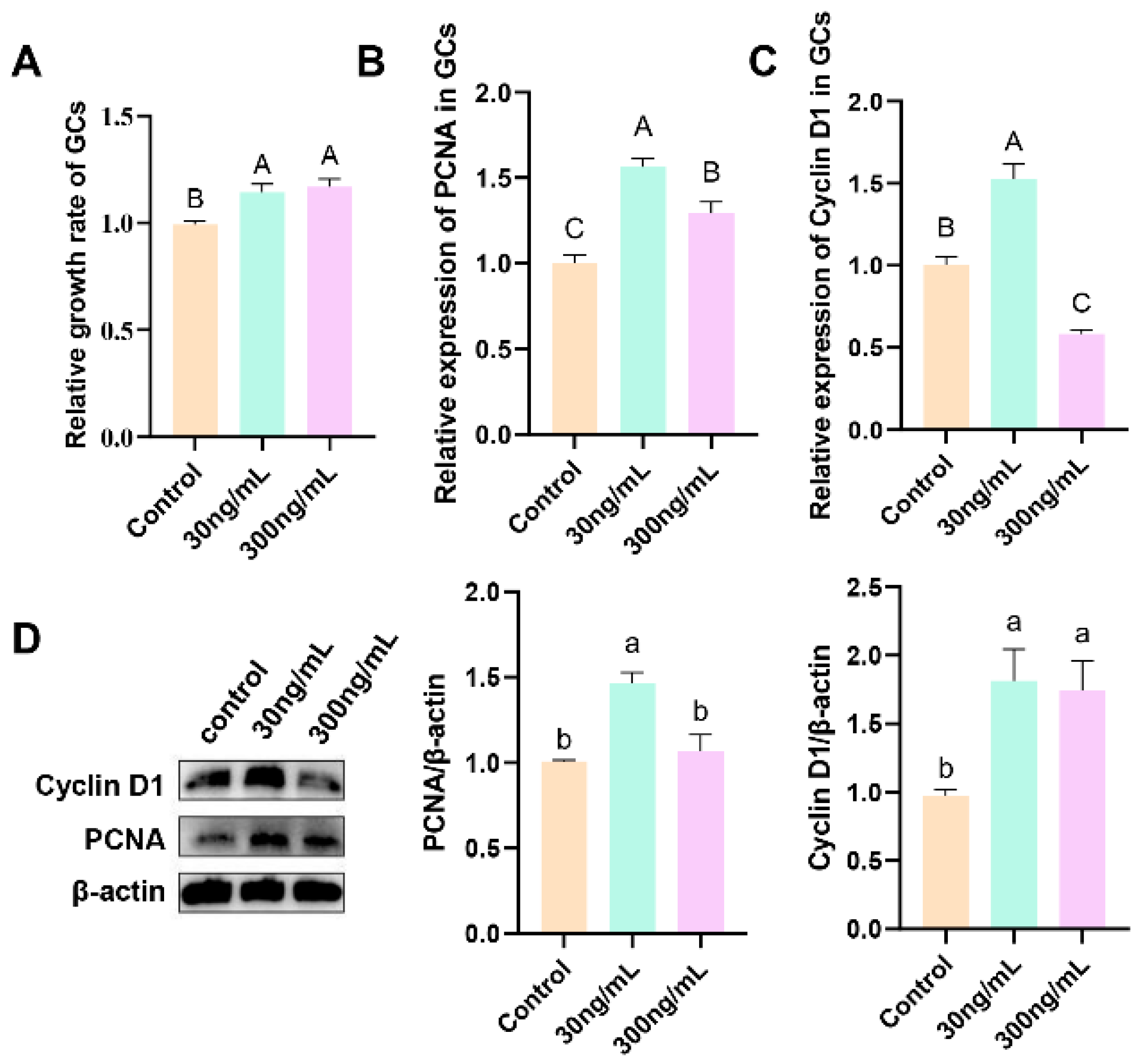
3.3. Effect of Different Concentrations of Prolactin on Granulosa Cell Proliferation
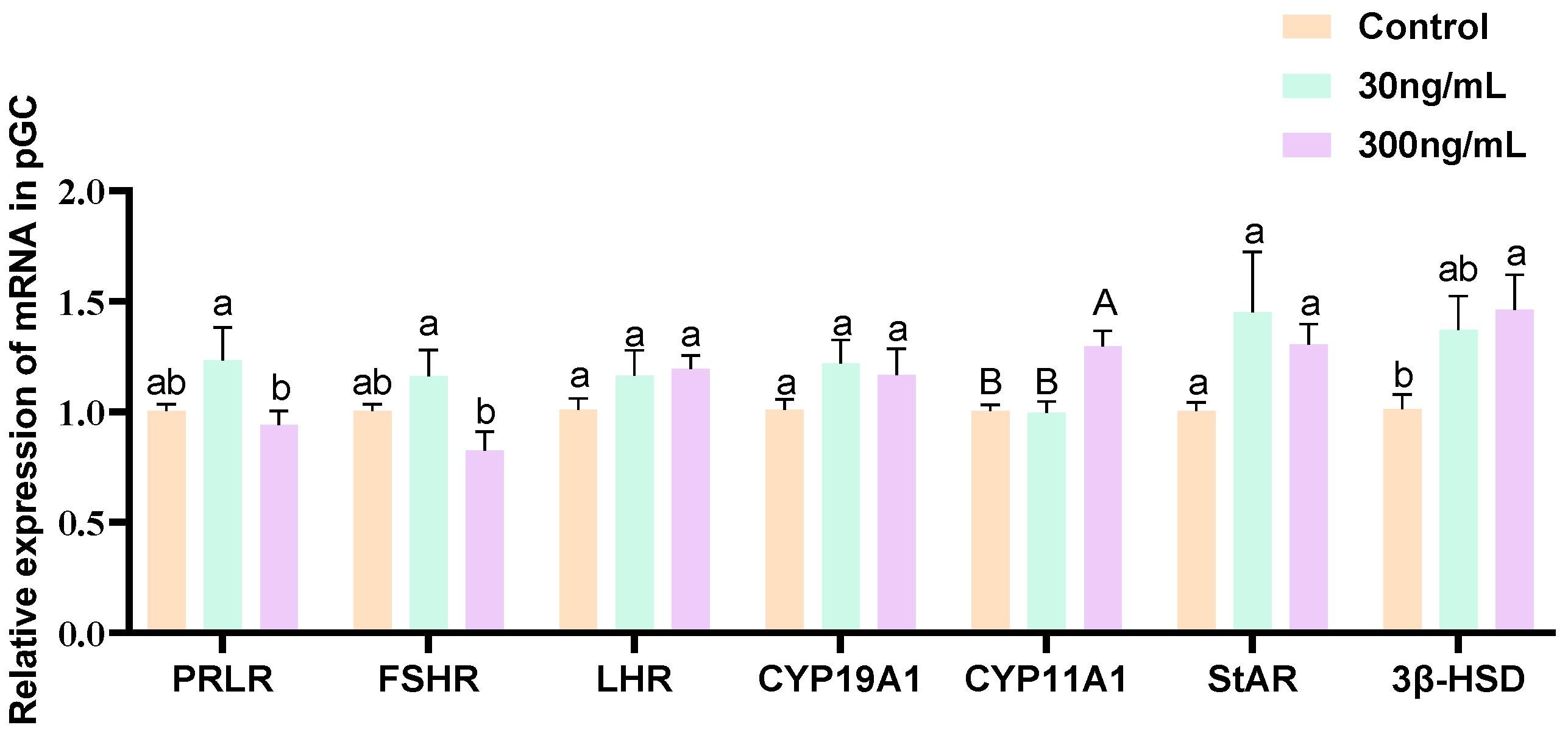
3.4. Effect of Different Concentrations of Prolactin on the Expression of PRLR and Steroidogenesis-Related Genes in Granulosa Cells
3.5. Effect of Prolactin on the Expression of Key Angiogenesis Factors in Granulosa Cells
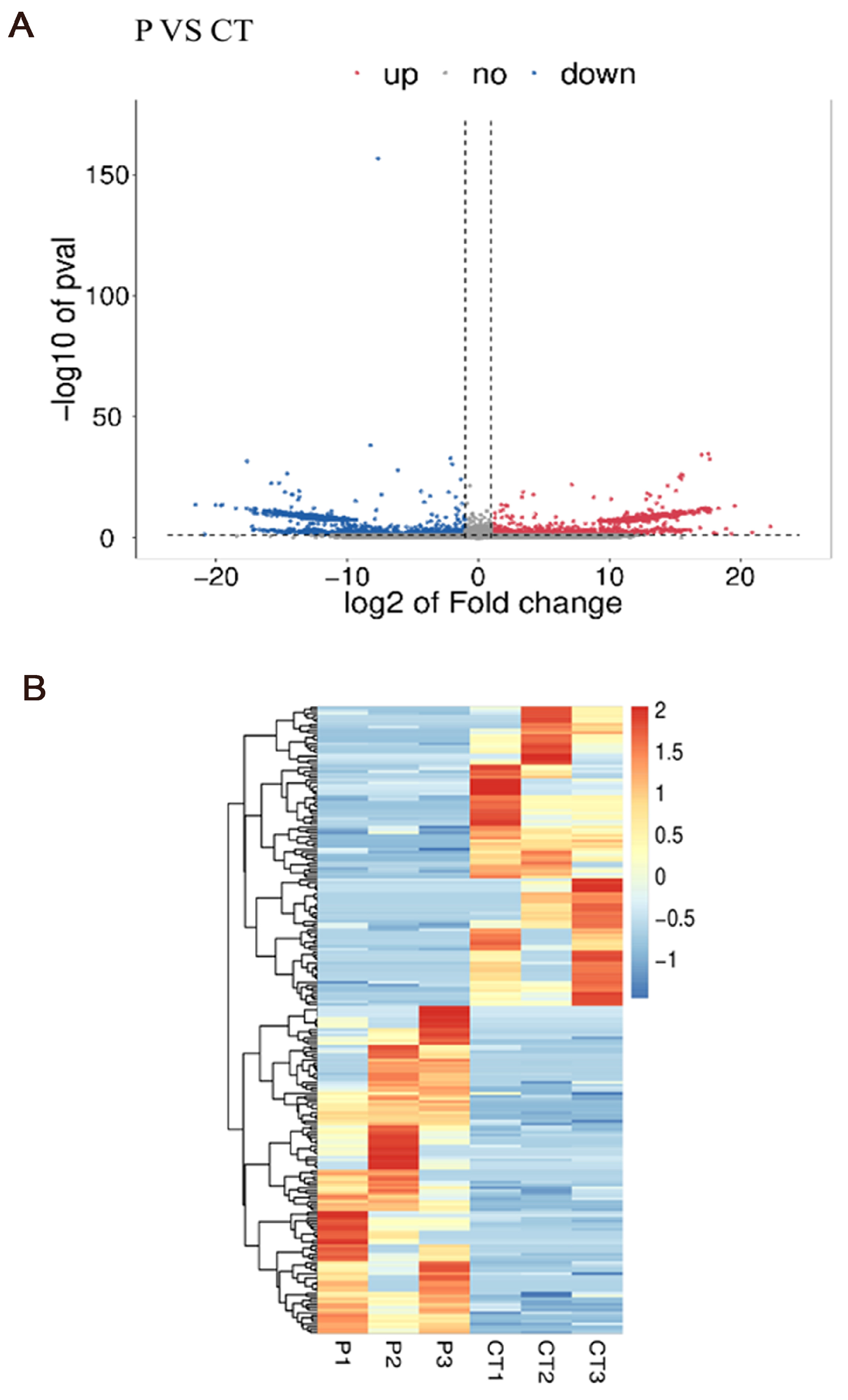
3.6. Differentially Expressed Genes Analysis
3.7. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analysis
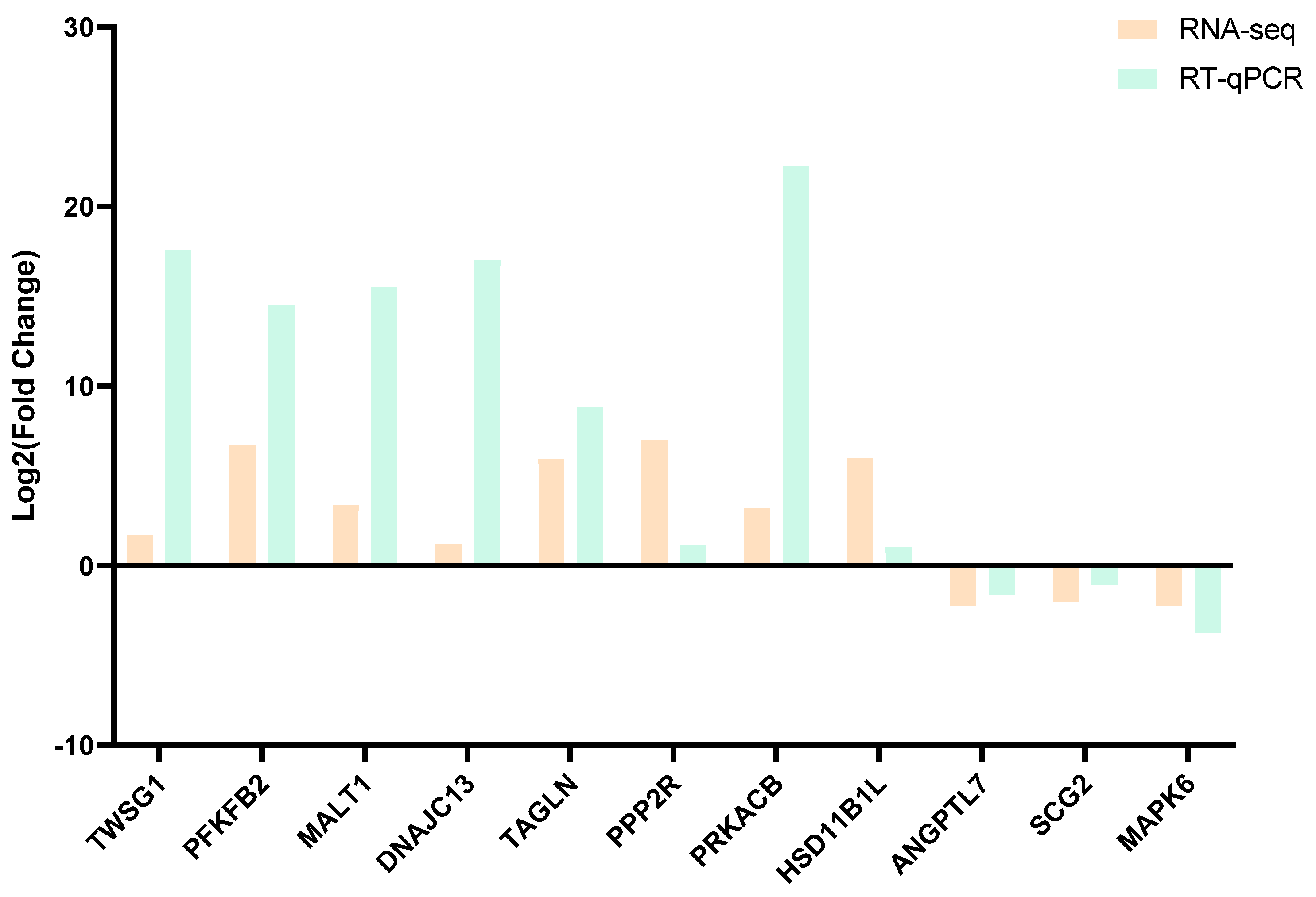
3.8. Real-Time Quantitative PCR Confirmation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dompe, C.; Kulus, M.; Stefańska, K.; Kranc, W.; Chermuła, B.; Bryl, R.; Pieńkowski, W.; Nawrocki, M.J.; Petitte, J.N.; Stelmach, B.; et al. Human Granulosa Cells—Stemness Properties, Molecular Cross-Talk and Follicular Angiogenesis. Cells 2021, 10, 1396. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Q.; Zadworny, D. Effects of nonglycosylated and glycosylated prolactin on basal and gonadotropin-stimulated steroidogenesis in chicken ovarian follicles. Domest. Anim. Endocrinol. 2017, 61, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Duan, C.; Zhang, S.; Liu, Y.; Zhang, Y. Prolactin Regulates Ovine Ovarian Granulosa Cell Apoptosis by Affecting the Expression of MAPK12 Gene. Int. J. Mol. Sci. 2023, 24, 10629. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Duan, C.; Zhang, S.; Guo, Y.; Shan, X.; Chen, M.; Yue, S.; Zhang, Y.; Liu, Y. High Prolactin Concentration Induces Ovarian Granulosa Cell Oxidative Stress, Leading to Apoptosis Mediated by L-PRLR and S-PRLR. Int. J. Mol. Sci. 2023, 24, 14407. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respir. Med. 2019, 156, 33–46. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Lapensee, C.R.; Lapensee, E.W. What can we learn from rodents about prolactin in humans? Endocr. Rev. 2008, 29, 1–41. [Google Scholar] [CrossRef]
- Dorshkind, K.; Horseman, N.D. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays 2001, 23, 288–294. [Google Scholar] [CrossRef]
- Bernichtein, S.; Touraine, P.; Goffin, V. New concepts in prolactin biology. J. Endocrinol. 2010, 206, 1–11. [Google Scholar] [CrossRef]
- Corbacho, A.M.; Martinez, D.L.E.G.; Clapp, C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J. Endocrinol. 2002, 173, 219–238. [Google Scholar] [CrossRef]
- Basini, G.; Baioni, L.; Bussolati, S.; Grolli, S.; Grasselli, F. Prolactin is a potential physiological modulator of swine ovarian follicle function. Regul. Pept. 2014, 189, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.F.; Shimasaki, S. The role of the oocyte in folliculogenesis. Trends Endocrinol. Metab. 2000, 11, 193–198. [Google Scholar] [CrossRef]
- Robinson, R.S.; Woad, K.J.; Hammond, A.J.; Laird, M.; Hunter, M.G.; Mann, G.E. Angiogenesis and vascular function in the ovary. Reproduction 2009, 138, 869–881. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Martinez-Chequer, J.C.; Molskness, T.A.; Xu, F.; Hazzard, T.M. Regulation and action of angiogenic factors in the primate ovary. Arch. Med. Res. 2001, 32, 567–575. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W.; Engelse, M.A.; Quax, P.H. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 716–728. [Google Scholar] [CrossRef]
- Triebel, J.; Robles-Osorio, M.L.; Garcia-Franco, R.; Martinez, D.L.E.G.; Clapp, C.; Bertsch, T. From Bench to Bedside: Translating the Prolactin/Vasoinhibin Axis. Front. Endocrinol. 2017, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Lliberos, C.; Liew, S.H.; Zareie, P.; La Gruta, N.L.; Mansell, A.; Hutt, K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci. Rep. 2021, 11, 278. [Google Scholar] [CrossRef]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. Update 2004, 10, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Xie, S.; Han, B.; Gao, F.; Ma, Y.; Li, L.; Zhang, S.; Zou, X.; Wei, H. Eukaryotic expression and bioactivity verification of porcine prolactin. J. South China Agric. Univ. 2024, 45, 179–189. [Google Scholar]
- Qi, N.; Xing, W.; Li, M.; Liu, J. Quercetin Alleviates Toxicity Induced by High Levels of Copper in Porcine Follicular Granulosa Cells by Scavenging Reactive Oxygen Species and Improving Mitochondrial Function. Animals 2023, 13, 2745. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.B.; Brumsted, J.R.; Sites, C.K. Effect of prolactin on follicle-stimulating hormone receptor binding and progesterone production in cultured porcine granulosa cells. Fertil. Steril. 2000, 73, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Iancu, M.E.; Albu, A.I.; Albu, D.N. Prolactin Relationship with Fertility and In Vitro Fertilization Outcomes-A Review of the Literature. Pharmaceuticals 2023, 16, 122. [Google Scholar] [CrossRef]
- Einspanier, R.; Pitzel, L.; Wuttke, W.; Hagendorff, G.; Preuss, K.D.; Kardalinou, E.; Scheit, K.H. Demonstration of mRNAs for oxytocin and prolactin in porcine granulosa and luteal cells. Effects of these hormones on progesterone secretion in vitro. FEBS Lett. 1986, 204, 37–40. [Google Scholar] [CrossRef]
- Henderson, K.M.; Mcneilly, A.S.; Swanston, I.A. Gonadotrophin and steroid concentrations in bovine follicular fluid and their relationship to follicle size. J. Reprod. Fertil. 1982, 65, 467–473. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, S.; Duan, C.; Guo, Y.; Shan, X.; Zhang, X.; Yue, S.; Zhang, Y.; Liu, Y. Effect of prolactin on cytotoxicity and oxidative stress in ovine ovarian granulosa cells. PeerJ 2023, 11, e15629. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.; Hughes, C.H.K.; Murphy, B.D. Orphan nuclear receptors in angiogenesis and follicular development. Reproduction 2021, 162, R35–R54. [Google Scholar] [CrossRef]
- Clapp, C.; Thebault, S.; Macotela, Y.; Moreno-Carranza, B.; Triebel, J.; Martinez, D.L.E.G. Regulation of blood vessels by prolactin and vasoinhibins. Adv. Exp. Med. Biol. 2015, 846, 83–95. [Google Scholar]
- Castilla, A.; Garcia, C.; Cruz-Soto, M.; Martinez, D.L.E.G.; Thebault, S.; Clapp, C. Prolactin in ovarian follicular fluid stimulates endothelial cell proliferation. J. Vasc. Res. 2010, 47, 45–53. [Google Scholar] [CrossRef]
- Zhao, H.; Gong, S.; Shi, Y.; Luo, C.; Qiu, H.; He, J.; Sun, Y.; Huang, Y.; Wang, S.; Miao, Y.; et al. The role of prolactin/vasoinhibins in cardiovascular diseases. Anim. Model. Exp. Med. 2023, 6, 81–91. [Google Scholar] [CrossRef]
- Nie, X.; Sheng, W.; Hou, D.; Liu, Q.; Wang, R.; Tan, Y. Effect of Hyperin and Icariin on steroid hormone secretion in rat ovarian granulosa cells. Clin. Chim. Acta 2019, 495, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Xie, Y.; Liang, H.; Lei, X.; Zhao, Z. Comparative profiling of differentially expressed microRNAs in estrous ovaries of Kazakh sheep in different seasons. Gene 2018, 664, 181–191. [Google Scholar] [CrossRef]
- Emmen, J.M.; Couse, J.F.; Elmore, S.A.; Yates, M.M.; Kissling, G.E.; Korach, K.S. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)alpha and ERbeta null mice indicate a role for ERbeta in follicular maturation. Endocrinology 2005, 146, 2817–2826. [Google Scholar] [CrossRef]
- Graham, J.D.; Clarke, C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar]
- Nakamura, E.; Otsuka, F.; Inagaki, K.; Miyoshi, T.; Yamanaka, R.; Tsukamoto, N.; Suzuki, J.; Ogura, T.; Makino, H. A Novel Antagonistic Effect of the Bone Morphogenetic Protein System on Prolactin Actions in Regulating Steroidogenesis by Granulosa Cells. Endocrinology 2010, 151, 5506–5518. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Su, X.; Yang, H.; Zhang, Y.; Duan, C.; Yang, R.; Xie, F.; Liu, Y.; Liu, W. Effects of prolactin on the proliferation and hormone secretion of ovine granulosa cells in vitro. Anim. Biosci. 2024, 37, 1712–1725. [Google Scholar] [CrossRef]
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Arensburg, J.; Orly, J.; Payne, A.H. The murine 3β-hydroxysteroid dehydrogenase (3β-HSD) gene family: A postulated role for 3β-HSD VI during early pregnancy. Mol. Cell. Endocrinol. 2002, 187, 213–221. [Google Scholar] [CrossRef]
- Ciereszko, R.; Opalka, M.; Kaminska, B.; Gorska, T.; Dusza, L. Prolactin signalling in porcine theca cells: The involvement of protein kinases and phosphatases. Reprod. Fertil. Dev. 2003, 15, 27–35. [Google Scholar] [CrossRef]
- Ciereszko, R.E.; Petroff, B.K.; Ottobre, A.C.; Guan, Z.; Stokes, B.T.; Ottobre, J.S. Assessment of the mechanism by which prolactin stimulates progesterone production by early corpora lutea of pigs. J. Endocrinol. 1998, 159, 201–209. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Oklinski, M.K.; Nielsen, S.; Skowronska, A. Pituitary Gonadotropins, Prolactin and Growth Hormone Differentially Regulate AQP1 Expression in the Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2018, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.M.; Duncan, W.C. SRB Reproduction, Fertility and Development Award Lecture 2008 Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod. Fertil. Dev. 2009, 21, 377–392. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Kano, M.R.; Morishita, Y.; Iwata, C.; Iwasaka, S.; Watabe, T.; Ouchi, Y.; Miyazono, K.; Miyazawa, K. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRβ signaling. J. Cell Sci. 2005, 118, 3759–3768. [Google Scholar] [CrossRef]
- Mishra, S.R.; Bharati, J.; Rajesh, G.; Chauhan, V.S.; Taru, S.G.; Bag, S.; Maurya, V.P.; Singh, G.; Sarkar, M. Fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor A (VEGFA) synergistically promote steroidogenesis and survival of cultured buffalo granulosa cells. Anim. Reprod. Sci. 2017, 179, 88–97. [Google Scholar] [CrossRef]
- Wu, J.F.; Liu, Y.; Gong, S.N.; Zi, X.D.; Tan, Y.G. Effects of vascular endothelial growth factor (VEGF) on the viability, apoptosis and steroidogenesis of yak (Bos grunniens) granulosa cells. Theriogenology 2023, 207, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gorvin, C.M. The prolactin receptor: Diverse and emerging roles in pathophysiology. J. Clin. Transl. Endocrinol. 2015, 2, 85–91. [Google Scholar] [CrossRef][Green Version]
- Jaskiewicz, A.; Pajak, B.; Orzechowski, A. The Many Faces of Rap1 GTPase. Int. J. Mol. Sci. 2018, 19, 2848. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Haquet, M.V.; Santana-Sanchez, P.; Chavez-Sanchez, L.; Chavez-Rueda, A.K. The effect of prolactin on immune cell subsets involved in SLE pathogenesis. Front. Immunol. 2022, 13, 1016427. [Google Scholar] [CrossRef]
- Matalka, K.Z. Prolactin enhances production of interferon-gamma, interleukin-12, and interleukin-10, but not of tumor necrosis factor-alpha, in a stimulus-specific manner. Cytokine 2003, 21, 187–194. [Google Scholar] [CrossRef]
- Ellah, N.H.A.; Ahmed, E.A.; Abd-Ellatief, R.B.; Ali, M.F.; Zahrans, A.M.; Hetta, H.F. Metoclopramide nanoparticles modulate immune response in a diabetic rat model: association with regulatory T cells and proinflammatory cytokines. Int. J. Nanomed. 2019, 14, 2383–2395. [Google Scholar] [CrossRef] [PubMed]
- Naldini, A.; Pucci, A.; Bernini, C.; Carraro, F. Regulation of angiogenesis by Th1- and Th2-type cytokines. Curr. Pharm. Des. 2003, 9, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Lange, T.; Fehm, H.L.; Born, J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain. Behav. Immun. 2004, 18, 368–374. [Google Scholar] [CrossRef] [PubMed]




| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Q20 | Q30 | GC (%) |
|---|---|---|---|---|---|---|---|
| CT1 | 45,683,498 | 6.85 | 44,556,134 | 6.68 | 99.98 | 96.33 | 51 |
| CT2 | 46,050,912 | 6.91 | 44,719,212 | 6.71 | 99.97 | 96.28 | 51 |
| CT3 | 45,777,432 | 6.87 | 44,505,288 | 6.68 | 99.98 | 96.51 | 51 |
| P1 | 45,994,936 | 6.90 | 44,730,614 | 6.72 | 99.97 | 96.39 | 51 |
| P2 | 38,084,340 | 5.71 | 37,043,024 | 5.56 | 99.98 | 96.68 | 51 |
| P3 | 40,008,974 | 6.00 | 38,965,208 | 5.84 | 99.98 | 96.67 | 51.5 |
| Gene Function | Genes | Description | Log2FC |
|---|---|---|---|
| Cell proliferation-related differential genes | PRKACB | Protein kinase cAMP-activated catalytic subunit beta | 22.28 |
| DNAJC13 | DNAJ heat shock protein family (Hsp40) member C13 | 17.03 | |
| PFKFB2 | 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 2 | 14.47 | |
| TAGLN | Transgelin | 8.83 | |
| PCNA | Proliferating cell nuclear antigen | 3.34 | |
| MAPK3 | Mitogen-activated protein kinase 3 | 1.32 | |
| PPP2R2B | Protein phosphatase 2 regulatory subunit beta | 1.12 | |
| HSD11B1 | Hydroxysteroid 11-beta dehydrogenase 1 | 1.03 | |
| DDX54 | DEAD-box helicase 5 | −16.44 | |
| HSD17B4 | Hydroxysteroid 17-beta dehydrogenase 4 | −13.06 | |
| FDFT1 | Farnesyl-diphosphate farnesyltransferase 1 | −12.59 | |
| Immune response-related differential genes | DKK3 | Dickkopf wnt signaling pathway inhibitor 3 | 19.57 |
| MALT1 | MALT1 paracaspase | 15.51 | |
| IL15 | Interleukin 15 | −9.54 | |
| TNFRSF9 | TNF receptor superfamily member 9 | −8.72 | |
| MAPK6 | Mitogen-activated protein kinase 6 | −3.75 | |
| Angiogenesis-related differential genes | TWSG1 | Twisted gastrulation BMP signaling modulator 1 | 17.56 |
| MRTFA | Myocardin-related transcription factor A | 17.65 | |
| SCG2 | Secretogranin II | −1.07 | |
| ANGPTL7 | Angiopoietin-like 7 | −1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Han, B.; He, Q.; Li, L.; Zhang, S.; Wei, H. Transcriptomic Analysis Reveals the Molecular Mechanisms of Prolactin in Regulating Porcine Follicular Development. Genes 2025, 16, 774. https://doi.org/10.3390/genes16070774
You Y, Han B, He Q, Li L, Zhang S, Wei H. Transcriptomic Analysis Reveals the Molecular Mechanisms of Prolactin in Regulating Porcine Follicular Development. Genes. 2025; 16(7):774. https://doi.org/10.3390/genes16070774
Chicago/Turabian StyleYou, Yubin, Beibei Han, Qiang He, Li Li, Shouquan Zhang, and Hengxi Wei. 2025. "Transcriptomic Analysis Reveals the Molecular Mechanisms of Prolactin in Regulating Porcine Follicular Development" Genes 16, no. 7: 774. https://doi.org/10.3390/genes16070774
APA StyleYou, Y., Han, B., He, Q., Li, L., Zhang, S., & Wei, H. (2025). Transcriptomic Analysis Reveals the Molecular Mechanisms of Prolactin in Regulating Porcine Follicular Development. Genes, 16(7), 774. https://doi.org/10.3390/genes16070774





