Abstract
Background: Phytoremediation is an efficient approach for remediating heavy metal-contaminated soils. Heavy metal-associated isoprenylated plant proteins (HIPPs)—crucial for metal ion homeostasis—are unique to vascular plants, featuring a heavy metal-associated (HMA) domain and an isoprenylated CaaX motif. However, ZmHIPP genes have not been systematically or functionally characterized in maize. Methods: This study characterizes ZmHIPP at the genome-wide level, including phylogenetic classification, motif/gene structure, chromosome location, gene duplication events, promoter elements, and tissue expression patterns. Cadmium (Cd) responses were evaluated by specific ZmHIPP expression and Cd accumulation in shoots and roots under Cd treatment. Results: A total of 66 ZmHIPPs were distributed unevenly across ten chromosomes, classified into five phylogenetic groups phylogenetically. Gene collinearity revealed 26 pairs of segmental duplications in ZmHIPPs. Numerous synteny genes were detected in rice and sorghum, but none in Arabidopsis, suggesting high conservation of HIPP genes in crop evolution. Transcriptomic analysis revealed tissue-specific expression patterns of ZmHIPP members in maize. Cis-acting element analysis linked several binding elements to abscisic acid, MeJA response, and MYB and MYC transcription factors. Under Cd stress, 53 out of 66 ZmHIPP genes were significantly induced, exhibiting three expression patterns. Cd exposure confirmed that the expression of ZmHIPP11, ZmHIPP30, and ZmHIPP48 was generally higher in shoots than roots, while ZmHIPP02 and ZmHIPP57 exhibited the opposite. Cd accumulation was higher in roots than shoots, peaking at 72 h (96 mg/kg) in shoots and exceeding 1000 mg/kg in roots after 120 h. Conclusions: This study not only provides fundamental genetic and molecular insights into HIPP function in maize but also identifies specific ZmHIPP genes as promising genetic resources for breeding Cd-tolerant maize, aiding in phytoremediation of Cd-contaminated soils.
1. Introduction
Recently, soil contamination by heavy metals has increased mainly due to human activities, such as the direct discharge of industrial waste residue, fossil fuel combustion, extensive use of fertilizers and pesticides, and improper handling of urban domestic waste. This pollution inhibits plant growth, reducing crop yield and quality. Hazardous heavy metals, including cadmium (Cd), arsenic (As), copper (Cu), and lead (Pb), accumulate in crops, threatening human and animal health. Cd, a non-essential and highly toxic inorganic pollutant with high soil mobility and relative non-degradability, is particularly concerning [1]. Even trace amounts of Cd are toxic to plants, reducing enzyme activity, denaturing proteins, altering RNA synthesis, suppressing DNA repair, and inducing oxidative stress, culminating in cell or plant death [2].
Plants counter Cd stress through various mechanisms, including binding of Cd to cell walls and root exudates, suppression of root-to-shoot translocation via phloem restriction, Cd sequestration into vacuoles, and chelator-mediated Cd complexation for detoxification [3]. These processes are orchestrated via interconnected regulatory networks. Heavy metal-associated proteins (HMPs) in plants contribute to metal ion absorption, transport, and distribution, mitigating stress caused by their accumulation in cells [4]. HMPs include heavy metal-associated plant proteins (HPPs) and heavy metal-associated isoprenylated plant proteins (HIPPs), distinguished by a conserved CaaX motif (C = cysteine; a = aliphatic amino acid; X = any amino acid) at the C-terminus of HIPPs [5]. The CaaX motif is essential for carboxyl-terminal isoprenylation, a typical post-translational modification of many regulatory proteins. It binds proteins via hydrophobic side chains, facilitating specific interactions with the endomembrane system or other transport proteins. HIPPs contain at least one heavy metal-associated (HMA) domain (pfam00403) that sequesters heavy metals to prevent cytotoxicity [6].
HIPPs were first identified in Arabidopsis and are exclusive to vascular plants. They are crucial for plant development and environmental adaptation, and extensively participate in regulating responses to pathogens and abiotic stresses, including heavy metal toxicity [7]. However, research on HIPPs has focused primarily on Arabidopsis and rice, with limited functions explored. In Arabidopsis, the transcription factor AtMYB49 interacts with the promoters of AtHIPP22 and AtHIPP44, enhancing their transcription and mediating subsequent Cd accumulation [8]. Heterologous expression of AtHIPP20 and AtHIPP22 restores Cd tolerance in Δycf1 yeast mutants, whereas AtHIPP21 does not confer similar resistance. The hipp20/21/22 triple mutant in Arabidopsis exhibits increased Cd sensitivity and accumulates less Cd than the wild type [9]. AtHIPP06 is induced by Cd and Cu, and its HMA domain in AtHIPP06 can bind Cd and Cu. AtHIPP06 overexpression enhances Cd tolerance in yeast and Arabidopsis [10]. In rice, OsHIPP29 [11] and OsHIPP56 [12] are induced by Cd stress in roots. Overexpressing individuals exhibit enhanced Cd tolerance and reduced Cd content, while mutants display the opposite trend. This suggests that OsHIPP29 and OsHIPP56 mitigate Cd toxicity by decreasing its accumulation. Meanwhile, overexpression of OsHIPP24 enhances Cu and Cd accumulation in the wild-type yeast BY4741; however, overexpression and knockout OsHIPP24 lines exhibit stunted growth with reduced plant height in rice [13]. Cd, Mn, and Cu induce the expression of OsHIPP42 and OsHIPP34 in roots, as well as OsHIPP16 and OsHIPP28 in whole plants. Individual overexpression of OsHIPP42, OsHIPP34, or OsHIPP60 specifically enhances yeast resistance to Cd, Cu, and Zn, respectively [4,14,15]. In maize, ZmHIPP21, ZmHIPP09, and ZmHIPP02 are induced by Cd stress in roots [16]. Beyond heavy metal stress, HIPPs also aid in plant adaptation to other abiotic stresses, including cold and drought, as demonstrated by the inducible expression of AtHIPP26 in Arabidopsis and OsHIPP41 in rice. In terms of biotic stress, AtHIPP27 is a host susceptibility factor for sugar beet cyst nematode infection, with reduced susceptibility in AtHIPP27 knockout mutants [17].
To date, 59, 45, 45, 23, 56, and 26 HIPP genes have been identified in Oryza sativa, Arabidopsis thaliana, Sorghum bicolor, Medicago sativa, Camellia sinensis, and Citrus sinensis, respectively. These HIPPs are categorized into five clusters [5,9,18,19,20,21]. However, systematic genome-wide identification and functional characterization of the HIPP gene family remain unexplored in maize (Zea mays L.), a globally vital staple crop with significant importance in Cd accumulation and phytoremediation potential. Given the critical role of HIPPs in heavy metal detoxification and their differential expression patterns under Cd stress, it is plausible that specific ZmHIPP members in maize may function as key regulators of Cd tolerance and accumulation. In the current study, the ZmHIPP gene family was characterized in maize at the genome-wide level. The analysis of phylogeny, motif/gene structure, and gene duplication events indicates that the ZmHIPP gene family has undergone evolutionary expansion to enhance functional diversity under Cd stress. Cis-acting elements analysis suggests stress-responsive elements in ZmHIPP promoters help drive spatiotemporal expression during Cd exposure. Additionally, expression patterns in leaves and roots at multiple time points under Cd stress and the corresponding Cd content were examined to define the relationship between ZmHIPP expression and Cd accumulation. Collectively, the results of this study provide a basis for elucidating the biological functions of ZmHIPP genes in response to Cd stress and identify potential targets for developing Cd-tolerant maize.
2. Materials and Methods
2.1. Plant Materials and Experimental Treatments
The maize (Z. mays inbred B73) plants were cultivated in a culture chamber at 30 °C/25 °C under a 16 h light/8 h dark photoperiod. Maize seedlings were subjected to Cd stress via application of 200 mg/L CdCl2 at ten days post-germination. The shoots and roots were sampled separately at 0, 12, 24, 48, 72, and 120 h after the onset of Cd exposure. Samples were rapidly frozen and stored at −80 °C pending further analyses.
2.2. RNA Isolation and Real-Time RT-PCR Analysis
Total RNA from maize shoots and roots was isolated using the RNAiso Plus reagent (Takara). The upper extract was precipitated by isopropanol and washed twice with 75% ethanol. The first-strand cDNA was synthesised in one step with a reagent kit (TransScript® All-in-One First-Strand cDNA Synthesis SuperMix) from TransGen Biotech Co., Ltd., Beijing, China.
Expression analysis of ZmHIPPs transcripts in response to Cd stress was conducted by quantitative real-time PCR (qRT-PCR) in both shoots and roots. cDNA templates were amplified employing the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China). PCR amplifications were carried out on a BIO-RAD CFX Connect instrument under the following cycling conditions: 40 cycles of denaturation at 95 °C for 10 s, annealing at 58 °C for 10 s, and extension at 72 °C for 30 s. Each cDNA sample was assayed in triplicate. Relative gene expression levels were calculated using the 2−ΔΔCt method. ZmTUB served as the reference gene for normalization. The sequences of all primers used are provided in Table S1.
2.3. Identification and Physicochemical Properties of HIPP Genes in Maize
Genome sequences, protein sequences, and genome annotation of maize were obtained from the Ensembl database. The 45 Arabidopsis and 59 rice HIPP protein sequences were obtained from the Arabidopsis Information Resource database and Rice Genome Annotation Project, respectively. The OsHIPP protein sequences were used as a reference to obtain the homologous genes from maize using TBtools software (v2.310). The hidden Markov model (HMM) of HMA (PF00403) was also obtained from the Pfam database, and then the maize protein sequences were searched using TBtools. The data obtained via BLAST (version 2.14.0+) and HMM analyses were compared, and duplicate genes were removed. The candidate ZmHIPPs were screened according to whether the proteins contained the isoprenylation motif CaaX at the C-terminus. The amino acid number, molecular mass, theoretical pI, instability index, aliphatic index, and grand average of hydropathicity (GRAVY) of ZmHIPP proteins were computed via the ExPASy online platform (https://www.expasy.org/, accessed on 10 February 2025). Additionally, predictions regarding the subcellular localization of ZmHIPPs were generated using the WoLF PSORT web server.
2.4. Phylogenetic Analysis and Classification
The HIPP proteins of maize, Arabidopsis, and rice were compared using ClustalW with MEGA 7 software (version 7.0.26). The phylogenetic tree was constructed using the neighbor-joining method in MEGA7. The bootstrap value was set at 1000. The draft was optimized using the Interactive Tree of Life website (https://itol.embl.de/, accessed on 12 February 2025).
2.5. Conserved Motif, Domain, and Gene Structure Analysis of ZmHIPPs
The identified ZmHIPP proteins were analyzed for conserved motifs and functional domains using TBtools with motifs set at 10 and leaving the other parameters set to default. The conserved structural domains of ZmHIPPs proteins were analyzed using the Batch CD-search module from the National Center for Biotechnology Information. The Gene Structure View module from TBtools was used for visualization.
2.6. Chromosome Distribution and Synteny Analysis in ZmHIPP Gene Family
The chromosomal localization of the ZmHIPP gene family members within the maize genome was analyzed and visualized using GTF/GFF from TBtools. The genome sequences and the annotated files for maize, Arabidopsis, rice, and sorghum were retrieved from Ensembl Plants (https://plants.ensembl.org, accessed on 13 February 2025). Synteny analyses for maize, Arabidopsis, rice, and sorghum were performed using One Step MCScanX in TBtools. Gene duplication and collinearity were visualized using TBtools.
2.7. Analysis of ZmHIPP Cis-Acting Elements
The promoter sequences 2000 bp upstream of ZmHIPP genes were analyzed using TBtools, and the cis-acting elements were predicted using the online tool PlantCARE. Some elements, such as TATA or CAAT boxes, were excluded. A large number of repeated elements with related annotations were selected and visualized using the Simple BioSequence Viewer from TBtools.
2.8. Expression Profiling of ZmHIPPs
Based on the published transcriptomic data [22], the expression patterns of ZmHIPPs were analyzed across 23 tissues and organs at different developmental stages. The female spikelets and silk were collected on the same day. FPKM values were transformed by log2 (FPKM + 1) and then used to construct the heatmap. Previous researchers have conducted a transcriptomic analysis for ten-day maize seedlings exposed to 200 mg/L CdCl2 [23]. The aboveground parts of B73 seedlings were sampled at different time intervals (0 h, 12 h, and 72 h) after Cd treatment for RNA-seq analysis. The mean FPKM value of three biological replicates was calculated for any given gene. Genes were considered expressed if the average FPKM value was not less than 0.5. Thus, 25,907 genes that were expressed in at least one sample were obtained, and we screened the upregulated or downregulated ZmHIPP genes among these genes. Heatmaps were generated from the collected FPKM values of the control and treatment groups using the Lianchuan Bio online platform (https://www.omicstudio.cn/tool/, accessed on 15 February 2025).
2.9. Determination of Cd Concentration
To determine the Cd content in the aboveground parts and roots, dried samples were subjected to mixed acid digestion. First, 9 mL of HNO and 3 mL of HF were added to a digestion tube containing 0.20 g of dry sample. The tightly capped digestion tubes were placed into a hot plate, heated to 200 °C, and maintained for 4 h. After digestion, the tube lids were opened and placed at 150 °C for 2 h until approximately 1 mL remained. The mixture was then transferred to a 50-mL volumetric flask, supplemented with 0.50 mol/L nitric acid and 100 μg/L Au solution, and brought to volume (50 mL) with deionized water. Finally, determination of Cd content was performed using an inductively coupled plasma mass spectrometer (ICP-MS, Agilent7800; Agilent Technologies, Santa Clara, CA, USA).
3. Results
3.1. Characterization and Physicochemical Properties of HIPP Genes in Maize
A total of 66 ZmHIPPs were identified based on the CysXXCys motif in the HMA domain and a C-terminal isoprenylation CaaX motif. These genes were designated ZmHIPP1 to ZmHIPP66 based on their chromosomal locations (Figure 1). Chromosomal localization analysis showed that the 66 ZmHIPP genes were unevenly distributed across the ten maize chromosomes, with significant enrichment in distal chromosomal regions. Chromosome 2 had the most ZmHIPP genes (15 genes), followed by Chromosomes 1 and 5 with ten ZmHIPP genes each. Chromosomes 3, 10, 7, 4, and 9 possessed eight, seven, five, four, and three ZmHIPP genes, respectively. Meanwhile, Chromosomes 6 and 8 each carried two ZmHIPP genes. This distribution pattern suggested no significant correlation between chromosome length and ZmHIPP gene density.
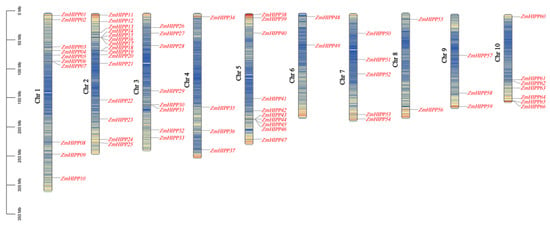
Figure 1.
Distribution of the HIPP gene family on maize chromosomes. Genomic distributions of 66 HIPP genes across the 10 maize chromosomes. The physical coordinates are accurately depicted on the chromosome.
The lengths of proteins encoded by the 66 ZmHIPPs ranged from 99 to 551 amino acids, with corresponding molecular weights varying between 11.57 kDa (ZmHIPP35) and 55.21 kDa (ZmHIPP60; Table S2). The theoretical pI values varied from 5.00 (ZmHIPP13) to 9.83 (ZmHIPP8). Among the 66 ZmHIPP proteins, 49 were categorized as alkaline proteins (pI > 7). Instability coefficients ranged from 17.05 (ZmHIPP12) to 85.13 (ZmHIPP10); 19 proteins, including ZmHIPP1, ZmHIPP2, and ZmHIPP9, exhibited higher stability with coefficients below 40. The aliphatic index varied between 32.85 (ZmHIPP60) and 87.59 (ZmHIPP44). All proteins were hydrophilic, as indicated by negative GRAVY values. Predicted subcellular localizations revealed that most ZmHIPP proteins were in the cytoplasm (23), nucleus (20), and chloroplasts (19).
3.2. Phylogenetic Analysis of ZmHIPP Proteins Based on Conserved Motifs and Gene Structure
Phylogenetic analyses can elucidate evolutionary relationships between homologous HIPP proteins across species. A phylogenetic tree was constructed using MEGA software (version 7.0) by combining 66 ZmHIPPs with 45 AtHIPPs and 59 OsHIPPs (Figure 2). Based on the topology of the phylogenetic tree, the ZmHIPP family was divided into five groups (I: 9, II: 9, III: 17, IV: 13, and V: 18 genes), consistent with rice and Arabidopsis. ZmHIPP members within each group exhibit close evolutionary relationships, implying similar functional roles.
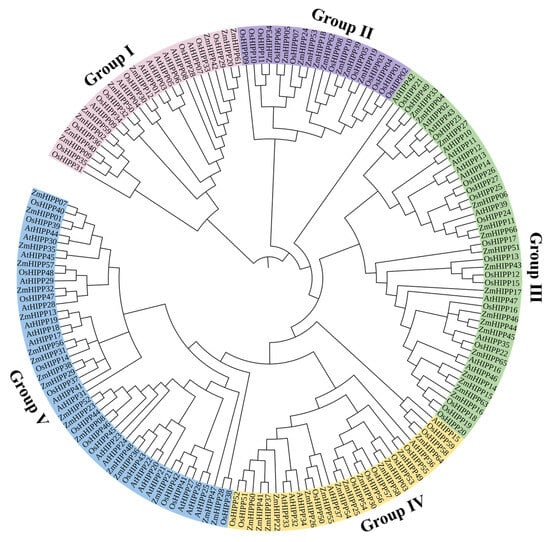
Figure 2.
Phylogenetic analysis and classification of HIPPs in Z. mays, A. thaliana, and O. sativa.
MEME analysis of ZmHIPP identified ten conserved motifs; motifs 2 and 3 were in all ZmHIPPs. ZmHIPPs within a group shared similar motifs (Figure 3). Analysis of the amino acid sequences revealed that motif 3 was an HMA domain, while motif 2 was an isoprenylated CaaX motif at the C-terminus. Nine ZmHIPPs in group I contained two HMA domains, whereas the remaining ZmHIPPs contained one HMA domain. HMA domains enable HIPPs to fold βαββαβ secondary structures for binding heavy metals, maintaining metal homeostasis in cells. The isoprenylation site affects HIPPs’ localization and interactions [19]. Additionally, ZmHIPPs in the same group displayed similar exon/intron structures. ZmHIPPs in group I had four or six exons, more than the other groups (one to four exons). ZmHIPP08 and ZmHIPP24 contained a particularly long intron. Additionally, group IV ZmHIPPs were longer, while those in group III were the shortest.
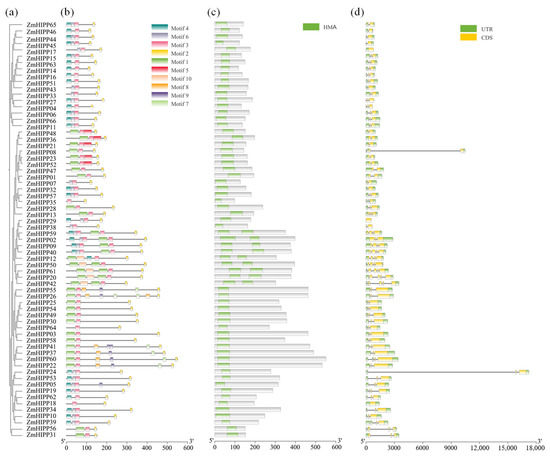
Figure 3.
Analysis of the gene structure, conserved motifs, and protein-coding domains of the HIPP gene family in maize. (a) The phylogenetic tree was constructed using ClustalW based on the amino acid sequences of HIPP genes. (b) Composition and distribution of conserved motifs in the ZmHIPP proteins, with 10 motifs listed above. (c) Conserved domain of the ZmHIPP family genes. (d) Gene structure of the ZmHIPP gene family. Coding sequences and introns are depicted using yellow boxes and black lines, respectively. The HIPP gene family is divided into five groups based on characteristics of the above aspects.
3.3. Synteny Analysis in HIPP Genes
Gene duplications generate new genes throughout evolution. Given the importance of tandem and segmental duplication events in gene families, ZmHIPP gene duplication events were investigated, and 26 segmental duplication events were identified. As shown in Figure 4, four groups of tandemly duplicated genes were detected in the ZmHIPP gene family: (1) ZmHIPP14 and ZmHIPP15, (2) ZmHIPP16 and ZmHIPP17, (3) ZmHIPP18 and ZmHIPP19, and (4) ZmHIPP43, ZmHIPP44, and ZmHIPP45. Additionally, the maize HIPP members contained 56 and 51 collinear gene pairs with the monocots sorghum and rice, respectively. However, no synteny genes were detected in dicot Arabidopsis (Figure 5). This suggests that ZmHIPP genes may have originated from monocot divergence.
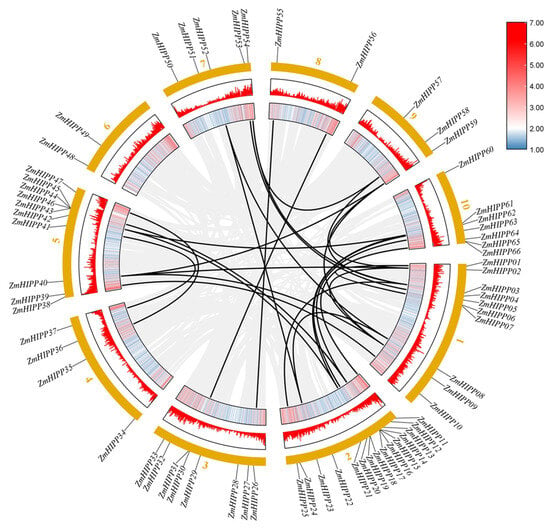
Figure 4.
Intraspecific synteny analysis in the HIPP gene family of maize. The middle grey lines indicate all collinear blocks in the maize genome as background. The red lines represent duplicated HIPP gene pairs in maize.
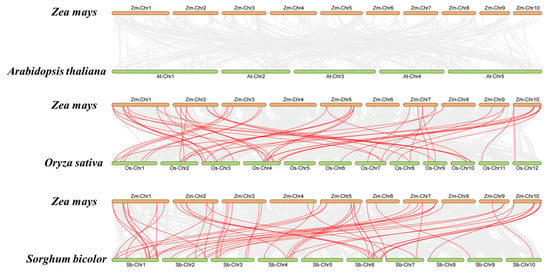
Figure 5.
Synteny analysis in the HIPP gene family of Z. mays, S. bicolor, A. thaliana, and O. sativa. Gray lines in the background present all synteny modules in the genome, and the blue lines indicate the collinearity module of the HIPP gene pairs across different species.
3.4. Analyses of ZmHIPP Gene Promoter Cis-Regulatory Elements
To investigate the transcriptional regulation of ZmHIPPs in maize, their promoters were analyzed by examining the 2000 bp region upstream of the transcription start site and predicting cis-acting regulatory elements using PlantCARE (Figure 6). A total of 3290 cis-elements were annotated: 905 transcription factor binding sites, 596 stress-responsive elements, 1097 hormone-related elements, 450 light-responsive elements, and 242 plant growth-associated elements. Notably, each ZmHIPP contained 5–9 MYB-binding elements. Massive biotic and abiotic stress-related elements were presented in ZmHIPPs, including the stress response promoter element (STRE), GC-motif (related to anoxic-specific inducibility), anaerobic induction element (ARE), dehydration-responsive element (DRE), and wound-responsive element (WRE3). Cis-acting elements that respond to MeJA, such as the TGACG-motif and CGTCA motifs, were prevalent, alongside other hormone-related elements like ABRE, as-1, TGA, and P-box—related to abscisic acid, salicylic acid, auxin, and gibberellin, respectively. Light-responsive elements included BOX 4, Sp1, G-box, and GT1-motif, whereas growth-associated elements comprised CCGTCC motif, AAGAA motif, CAT-box (related to meristem expression), and RY element (related to seed-specific regulation). Collectively, cis-acting element analysis of the ZmHIPP gene family revealed numerous elements related to light responsiveness, hormone and stress responsiveness, and growth and development, suggesting that ZmHIPPs play integrated roles in stress response and growth regulation.
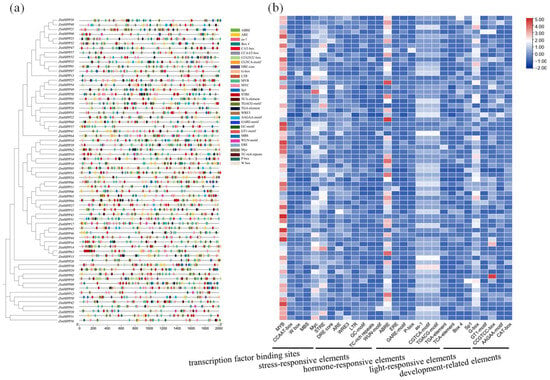
Figure 6.
Cis-acting elements analysis in the promoter region of the ZmHIPP gene family. The figure presents the main types of cis-acting elements and their locations in the 2000 bp upstream region of the ZmHIPP transcription start site. (a) The distribution of cis-acting elements on the promoter. (b) Statistics on the number of cis-acting elements classified.
3.5. Expression Profiling of ZmHIPP Genes in Maize
To investigate the tissue-specific expression patterns of ZmHIPPs, transcript abundances were analyzed in 23 maize tissues and organs. The clustering heatmap revealed that the expression of ZmHIPP04, ZmHIPP07, ZmHIPP11, ZmHIPP12, ZmHIPP18, ZmHIPP51, ZmHIPP57, ZmHIPP64, and ZmHIPP65 was significantly higher in roots than in other tissues (Figure 7). Meanwhile, ZmHIPP06, ZmHIPP14, ZmHIPP19, ZmHIPP28, ZmHIPP34, ZmHIPP35, ZmHIPP36, and ZmHIPP48 were upregulated in leaves compared with the roots and stems. Additionally, ZmHIPP02 and ZmHIPP23 were predominantly expressed in embryos, while ZmHIPP13, ZmHIPP44, ZmHIPP45, and ZmHIPP47 were highly expressed in other reproductive organs, including pollen, silk, spikelets, and ears, respectively. Overall, ZmHIPP family members exhibit distinct tissue-specific expression patterns throughout the life cycle of maize.
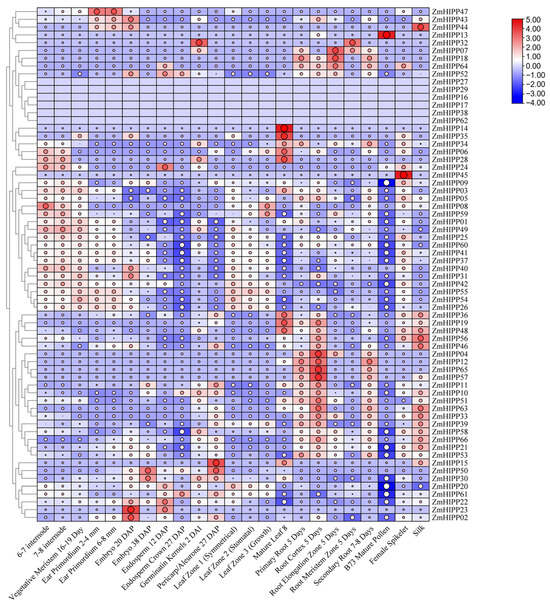
Figure 7.
Analysis of the expression patterns of ZmHIPP genes across different tissues and organs. The abundance of transcript levels is represented by a color gradient from red to blue. “NA” (not available) was replaced by FPKM = 0. The values of log2 (FPKM + 1) are visualized as a heatmap.
3.6. ZmHIPP Expression Under Cd Stress and Corresponding Cd Accumulation in Maize
When maize seedlings were subjected to Cd stress, 53 of the 66 ZmHIPP genes were significantly induced at 12 h or 72 h (Figure 8). Three expression patterns emerged: (1) expression increased after 72 h of Cd stress (e.g., HIPP02, HIPP48); (2) responded within 12 h to Cd stress (e.g., ZmHIPP11 and ZmHIPP30); (3) high expression under normal conditions, decreased after 12 h of Cd stress, and increased at 72 h (e.g., ZmHIPP34 and ZmHIPP40).
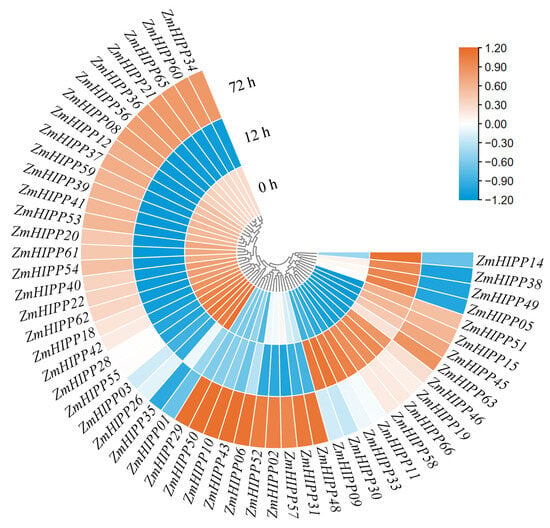
Figure 8.
Expression profiles of ZmHIPP genes under Cd exposure (100 µM).
B73 plants were assessed under Cd stress to verify gene expression. Six ZmHIPP genes were selected for expression analysis in shoots and roots. The expression patterns of the six genes were consistent with the transcriptome data (Figure 9). The expression of ZmHIPP11, ZmHIPP30, and ZmHIPP48 was generally higher in leaves than roots, while ZmHIPP02 and ZmHIPP57 showed the opposite pattern. ZmHIPP48 was induced only in leaves. The expression of ZmHIPP11 and ZmHIPP30 in leaves rapidly increased within 12 h of Cd stress, similar to ZmHIPP40 in roots.
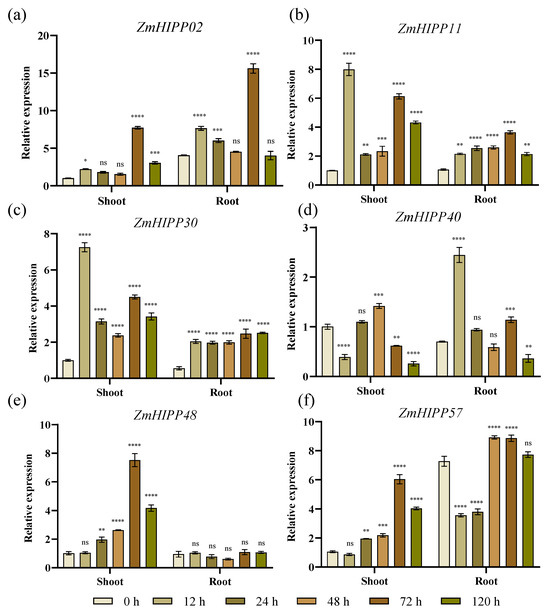
Figure 9.
The expression changes of the ZmHIPPs gene family in maize under Cd stress were analyzed using qRT-PCR. (a) ZmHIPP02; (b) ZmHIPP11; (c) ZmHIPP30; (d) ZmHIPP40; (e) ZmHIPP48; (f) ZmHIPP57. Each experiment included three biological replicates and three technical replicates. Data are presented as the mean ± SD. Statistical significance is denoted as follows: “****” for p < 0.00001, “***” for p < 0.0001, “**” for p < 0.001, “*” for p < 0.05, and “ns” for no significant difference.
Cd content was measured in the aboveground parts and roots of treated plants. Cd was transported from the soil to the shoots via root absorption. Cd accumulation was markedly higher in roots than leaves (Figure 10). At 12 and 120 h post-treatment, roots exhibited a significant increase in Cd accumulation. In shoots, Cd accumulation peaked at 72 h and decreased at 120 h. Cd toxicity caused yellowing of leaves in the later stage of treatment, as they exhibited a degree of tolerance. However, ultimately, Cd severely impaired plant growth.
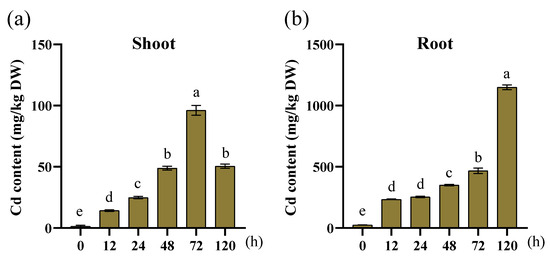
Figure 10.
Cd accumulation in the shoot and root tissues of maize seedlings under Cd stress. (a) Cd content in the shoot tissue; (b) Cd content in the root tissue. Each experiment was conducted in triplicate, and the data are presented as the mean ± SEM. Statistical significance was determined using the Tukey multiple comparison test (p < 0.05).
4. Discussion
In the current study, genomic analysis identified 66 ZmHIPPs containing HMA domains and CaaX motifs, which is more than the majority of plants, including those recently identified in sorghum, alfalfa, and citrus [18,19,21]. Based on the conserved motifs and domains, ZmHIPP proteins were classified into five groups similar to the gene family in Arabidopsis and rice [24], with protein structures varying among groups. Group I of ZmHIPPs contained two HMA domains that facilitate binding to more metal ions, promoting metal ion uptake and maintaining metal ion homeostasis, similar to AtHIPP07 [25] and OsHIPP33 [26]. In contrast, the HIPPs in Group IV were relatively large and could bind heavy metal ions through HMA motifs, sequestering them in the cell wall. This prevents the penetration of heavy metals into other organelles, thus mitigating the toxicity of free heavy metal ions on cells, as reported for ZmHIPP22 [27].
Isoprenylation is a post-translational modification that covalently attaches hydrophobic groups to target proteins, allowing them to interact specifically with membrane and transporter proteins, thereby altering protein localization [28]. After entering the cells, metal ions are relocated via metal transporter proteins, such as movement from the cytoplasm to vacuoles [29]. ZmHIPP22 [27], SbHIPP40 [18], CsHIPP10, CsHIPP19, CsHIPP22 [21], OsHIPP16 [30], OsHIPP33 [26], OsHIPP29 [11], OsHIPP56 [12], and OsHIPP42 [14] are localized in the nucleus and plasma membrane, while CsHIPP19 and CsHIPP22 [21] are within the nucleus and microtubules, and TaHIPP1 [31] and AtHIPP3 [32] are specifically localized in the nucleus. Within the current study, most ZmHIPPs were predicted to be localized in the cytoplasm, nucleus, and chloroplasts. But this requires experimental confirmation.
In plants, gene duplication events such as tandem duplication and segmental duplication contribute significantly to genomic expansion, facilitating the emergence of new genes and evolutionary novelty [20]. Tandem duplication generates clustered gene families with high sequence homology and functional similarity on a single chromosome. Nine ZmHIPP genes (13.6%) are found to be tandem repeats in maize. There are four distinct pairs of tandemly duplicated genes, of which three pairs are located on Chromosome 2, and another group of 3 genes is located on Chromosome 5. Meanwhile, Chromosome 2 contains the most ZmHIPP genes (15), suggesting that this chromosome may be an evolutionary hotspot of the ZmHIPP family due to the local chromatin environment or selection pressure. A collinearity analysis of ZmHIPP family genes detects 26 segmental duplication events within 38 ZmHIPPs, suggesting that the abundance of chromosomal segment duplication may be a possible reason for the larger number of ZmHIPPs. Cross-species comparisons reveal that 40 and 34 ZmHIPP genes exhibit 56 and 51 collinear gene pairs with sorghum and rice, respectively. However, no collinear HIPP gene pairs are detected between maize and Arabidopsis. The exclusive presence of collinear ZmHIPP pairs in monocots, coupled with their absence in dicots, strongly supports their origin subsequent to the monocot-dicot divergence event during HIPP family evolution and suggests that HIPP genes might have evolved unique and essential functions in monocot environmental adaptation. Additionally, genes like ZmHIPP53 exhibit multiple collinear relationships (2–3 gene pairs) between maize and sorghum/rice, suggesting their potential role as evolutionary hubs in the diversification of the ZmHIPP gene family.
The cis-acting element analysis of ZmHIPP genes identifies numerous stress-responsive elements and hormone-related elements, indicating that these genes might be involved in stress tolerance and growth regulation. Notably, we identify 905 (27.5%) cis-acting sites that potentially interact with transcription factors, such as MYB, MYC, and WRKY (W box). Transcription factors like WRKY [33,34], MYB [35], bHLH [36,37], and ERF [38] have been reported to participate in modulating gene expression to counteract Cd toxicity. Therefore, further prediction and analysis of the transcriptional regulation of ZmHIPP genes are necessary, and the binding sites of MYB or WRKY represent preferred targets for screening the core promoter motifs of ZmHIPP genes.
Current research on HIPP functions focuses primarily on model plants, with limited studies on maize. In this study, gene expression dynamics in shoots reveal that 83.33% (55) of ZmHIPP genes respond to Cd stress at 0 h, 12 h, and 72 h, including ZmHIPP21, ZmHIPP09, and ZmHIPP02 (Figure 8). ZmHIPP22 functions as a hub gene conferring Pb tolerance in maize seedlings [27]. Knockout of ZmHIPP22 significantly inhibits the growth of maize seedlings under Pb stress and reduces Pb accumulation by blocking cellular uptake rather than facilitating cell wall sequestration. ZmHIPP22 expression shows significant down-regulation (nearly 18-fold) at 12 h of excess Cd exposure (Figure 8), indicating that ZmHIPP22 mitigates Cd toxicity by reducing its accumulation in shoots. A comparative transcriptomic analysis in roots of maize and rice subjected to Cd stress reveals rapid upregulation of ZmHIPP21, ZmHIPP09, and ZmHIPP02, as well as their rice counterparts. Notably, ZmHIPP02 and its homolog OsHIPP36 exhibit the most pronounced transcriptional activation. Furthermore, similar to its rice ortholog OsHIPP42, the common stress-responsive gene ZmHIPP21 participates in various abiotic stresses (drought, salt, and low temperature) [16]. Collectively, these induced ZmHIPP genes, such as ZmHIPP02, ZmHIPP21, and ZmHIPP22, play important roles in coping with Cd stress and can be used as valuable genetic resources for breeding Cd-tolerant maize in the future.
Cd enters root cells through transporter proteins. After entering the cytosol, it is chelated into the vacuole through transporters and stored as a complex, which reduces Cd mobility and transfer from roots to leaves, resulting in significantly higher Cd accumulation in roots than in the aboveground parts (Figure 10). Cd is transported to grains via the xylem-to-phloem pathway, posing significant risks to crop production and food safety due to excessive Cd accumulation [30]. Root metal transporters are crucial components in defense against soil metal toxicity. Numerous transporter families facilitating Cd movement have been identified, including NRAMP [39,40], HMA [41], ZIP [42], and ABC [43]. HIPPs are plant-specific metal chaperones involved in plant development and abiotic stress responses, with particular significance in heavy metal transport [44]. Maize faces concerns regarding Cd contamination. Identification and functional screening of ZmHIPP genes will provide a foundation for targeted breeding of Cd-tolerant maize varieties and soil ecological remediation.
5. Conclusions
This study comprehensively characterized the HIPP family in maize and identified 66 ZmHIPP genes, which were divided into five groups and distributed across ten chromosomes. ZmHIPP genes are particularly conserved in evolution and closely related to orthologs in rice. Most ZmHIPP genes exhibit tissue-specific expression patterns during development, and 53 ZmHIPP genes were induced by excess Cd exposure. qRT-PCR analysis confirmed that six induced ZmHIPPs coordinated in response to Cd stress as they functioned in different sections and stages. The accumulation of Cd in roots was significantly greater than in shoots. Cd levels in roots increased until 120 h post-treatment, while the accumulation in shoots peaked at 72 h. This study includes a genome-wide assessment of the HIPP gene family in maize and offers critical insights for functional analyses of Cd stress in maize, suggesting a potential strategy for breeding Cd-tolerant maize and phytoremediation of Cd-contaminated soils.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16070770/s1, Table S1: Primers used in qRT-PCR; Table S2: The sequence information and physicochemical properties of ZmHIPPs.
Author Contributions
Experiment design and the manuscript written, C.G., J.C. and Y.Z. (Youcheng Zhu); Cd treatments and gene expression analysis, Y.Z. (Yuxuan Zhu) and C.G.; Determination of Cd concentration, J.T., K.Y. and D.L.; bioinformatic analyses, Z.Z., J.H. and C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Key Foundations of the Anhui Bureau of Education (2024AH051452), the Biological and Medical Sciences of Applied Summit Nurturing Disciplines in Anhui Province (Anhui Education Secretary Department [2023]13), the Science and Technology Research Project of Jilin Provincial Department of Education (JJKH20241311KJ) and the Fuyang Normal University doctoral talent introduction project (2023KYQD0028, 2024KYQD0089).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We thank our contributors for their dedication and compliance through the many stages of this research, as well as the editors and anonymous reviewers whose comments helped to greatly improve this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| As | Arsenic |
| Cd | Cadmium |
| Cu | Copper |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| GFF | General Feature Format |
| GTF | Gene Transfer Format |
| HIPP | Heavy metal-associated isoprenylated plant protein |
| HMA | Heavy metal-associated |
| HMM | Hidden Markov model |
| Pb | Lead |
| STRE | Stress response promoter element |
References
- Liu, C.C.; Wen, L.; Cui, Y.J.; Ahammed, G.J.; Cheng, Y. Metal transport proteins and transcription factor networks in plant responses to cadmium stress. Plant Cell Rep. 2024, 43, 218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.P.; Cao, L.; Zhang, C.; Pan, W.C.; Wang, W.; Yu, X.; Li, Y.P.; Fan, T.T.; Miao, M.; Tang, X.F.; et al. MYB43 as a novel substrate for CRL4PRL1 E3 ligases negatively regulates cadmium tolerance through transcriptional inhibition of HMAs in Arabidopsis. New Phytol. 2022, 234, 884–901. [Google Scholar] [CrossRef] [PubMed]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Khan, I.U.; Rono, J.K.; Zhang, B.Q.; Liu, X.S.; Wang, M.Q.; Wang, L.L.; Wu, X.C.; Chen, X.; Cao, H.W.; Yang, Z.M. Identification of novel rice (Oryza sativa) HPP and HIPP genes tolerant to heavy metal toxicity. Ecotoxicol. Environ. Saf. 2019, 175, 8–18. [Google Scholar] [CrossRef]
- de Abreu-Neto, J.B.; Turchetto-Zolet, A.C.; de Oliveira, L.F.V.; Zanettini, M.H.B.; Margis-Pinheiro, M. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J. 2013, 280, 1604–1616. [Google Scholar] [CrossRef]
- Parasyri, A.; Barth, O.; Zschiesche, W.; Humbeck, K. The Barley Heavy Metal Associated Isoprenylated Plant Protein HvFP1 Is Involved in a Crosstalk between the Leaf Development and Abscisic Acid-Related Drought Stress Responses. Plants 2022, 11, 21. [Google Scholar] [CrossRef]
- Wang, Z.K.; Zhang, H.; Li, Y.B.; Chen, Y.M.; Tang, X.; Zhao, J.; Yu, F.F.; Wang, H.Y.; Xiao, J.; Liu, J.; et al. Isoprenylation modification is required for HIPP1-mediated powdery mildew resistance in wheat. Plant Cell Environ. 2023, 46, 288–305. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, R.; Ju, Q.; Li, W.; Tran, L.; Xu, J. The R2R3-MYB Transcription Factor MYB49 Regulates Cadmium Accumulation. Plant Physiol. 2019, 180, 529–542. [Google Scholar] [CrossRef]
- Tehseen, M.; Cairns, N.; Sherson, S.; Cobbett, C.S. Metallochaperone-like genes in Arabidopsis thaliana. Metallomics 2010, 2, 556–564. [Google Scholar] [CrossRef]
- Suzuki, N.; Yamaguchi, Y.; Koizumi, N.; Sano, H. Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant. J. 2002, 32, 165–173. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Feng, S.; Zhao, Y.; Wang, L.; Rono, J.; Li, H.; Yang, Z. Developing a cadmium resistant rice genotype with OsHIPP29 locus for limiting cadmium accumulation in the paddy crop. Chemosphere 2020, 247, 125958. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Wang, M.Q.; Li, C.; Cao, H.W.; Rono, J.K.; Yang, Z.M. The metallochaperone OsHIPP56 gene is required for cadmium detoxification in rice crops. Environ. Exp. Bot. 2022, 193, 10. [Google Scholar] [CrossRef]
- Chen, K.; Chen, P.; Qiu, X.; Chen, J.; Gao, G.; Wang, X.; Zhu, A.; Yu, C. Regulating role of abscisic acid on cadmium enrichment in ramie (Boehmeria nivea L.). Sci. Rep. 2021, 11, 22045. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Rono, J.K.; Liu, X.; Feng, S.; Li, H.; Chen, X.; Yang, Z. Functional characterization of a new metallochaperone for reducing cadmium concentration in rice crop. J. Clean. Prod. 2020, 272, 11. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, M.; Zhang, J.; Huang, L.; Chen, X.; Jiang, M.; Tan, M. Profiling of rice Cd-tolerant genes through yeast-based cDNA library survival screening. Plant Physiol. Biochem. 2020, 155, 429–436. [Google Scholar] [CrossRef]
- Cheng, D.; Tan, M.; Yu, H.; Li, L.; Zhu, D.; Chen, Y.; Jiang, M. Comparative analysis of Cd-responsive maize and rice transcriptomes highlights Cd co-modulated orthologs. BMC Genom. 2018, 19, 709. [Google Scholar] [CrossRef]
- Radakovic, Z.S.; Anjam, M.S.; Escobar, E.; Chopra, D.; Cabrera, J.; Silva, A.C.; Escobar, C.; Sobczak, M.; Grundler, F.M.W.; Siddique, S. Arabidopsis HIPP27 is a host susceptibility gene for the beet cyst nematode Heterodera schachtii. Mol. Plant Pathol. 2018, 19, 1917–1928. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, G.W.; Ni, X.L.; Liu, Z.W.; Song, T.; Han, Y.; Wang, Y.; Shao, Y.; Wang, F.L.; Zou, G.H.; et al. Genome-wide identification of HIPP genes family in sorghum reveals the novel role of SbHIPP40 in accumulation of cadmium. J. Hazard. Mater. 2025, 494, 138478. [Google Scholar] [CrossRef]
- Xia, H.Y.; Jing, X.; He, H.Q.; Peng, J.W.; Liu, Y.Y.; Sun, W.Y.; Wang, X.Z.; Yuan, Z.; Wu, J.X.; Zhang, M.Y.; et al. Genome-wide identification of the HIPPs gene family and functional validation of MsHIPP12 in enhancing cadmium tolerance in Medicago sativa. J. Hazard. Mater. 2025, 491, 137894. [Google Scholar] [CrossRef]
- Wei, Y.F.; Peng, X.Q.; Wang, X.J.; Wang, C. The heavy metal-associated isoprenylated plant protein (HIPP) gene family plays a crucial role in cadmium resistance and accumulation in the tea plant (Camellia sinensis L.). Ecotoxicol. Environ. Saf. 2023, 260, 115077. [Google Scholar] [CrossRef]
- Huang, G.Y.; Hu, Y.N.; Li, F.X.; Zuo, X.R.; Wang, X.Y.; Li, F.Y.; Li, R.M. Genome-wide characterization of heavy metal-associated isoprenylated plant protein gene family from Citrus sinensis in response to huanglongbing. Front. Plant Sci. 2024, 15, 1369883. [Google Scholar] [CrossRef] [PubMed]
- Walley, J.W.; Sartor, R.C.; Shen, Z.; Schmitz, R.J.; Wu, K.J.; Urich, M.A.; Nery, J.R.; Smith, L.G.; Schnable, J.C.; Ecker, J.R.; et al. Integration of omic networks in a developmental atlas of maize. Science 2016, 353, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, J.L.; Yin, Y.Z.; Zhang, X.Y.; Dai, Y.X.; Xing, Y.P.; Cheng, X.P.; Zhang, A.; Li, C.; Zhu, Y.S.; et al. Dynamic transcriptome profiling revealed a key gene ZmJMJ20 and pathways associated with cadmium stress in maize. Ecotoxicol. Environ. Saf. 2024, 277, 116352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, L.; Wang, H.; Xiao, J.; et al. Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. Int. J. Mol. Sci. 2020, 21, 6191. [Google Scholar] [CrossRef]
- Barr, Z.K.; Werner, T.; Tilsner, J. Heavy Metal-Associated Isoprenylated Plant Proteins (HIPPs) at Plasmodesmata: Exploring the Link between Localization and Function. Plants 2023, 12, 3015. [Google Scholar] [CrossRef]
- Cao, H.W.; Li, C.; Zhang, B.Q.; Rono, J.K.; Yang, Z.M. A Metallochaperone HIPP33 Is Required for Rice Zinc and Iron Homeostasis and Productivity. Agronomy 2022, 12, 488. [Google Scholar] [CrossRef]
- Ma, L.; An, R.; Jiang, L.; Zhang, C.; Li, Z.; Zou, C.; Yang, C.; Pan, G.; Lubberstedt, T.; Shen, Y. Effects of ZmHIPP on lead tolerance in maize seedlings: Novel ideas for soil bioremediation. J. Hazard. Mater. 2022, 430, 128457. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Z.; Yan, T.; Lu, R.; Peng, C.; Li, S.; Jing, Y. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Chang, J.; Xie, Y.; Zhang, H.; Zhang, S.; Zhao, F. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects Cd distribution to rice grain. Plant Soil. 2022, 476, 79–95. [Google Scholar] [CrossRef]
- Cao, H.W.; Zhao, Y.N.; Liu, X.S.; Rono, J.K.; Yang, Z.M. A metal chaperone OsHIPP16 detoxifies cadmium by repressing its accumulation in rice crops. Environ. Pollut. 2022, 311, 120058. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, H.; Feng, C.; Xu, H.; Huang, X.; Wang, Q.; Duan, X.; Wang, X.; Wei, G.; Huang, L.; et al. Isolation and characterisation of cDNA encoding a wheat heavy metal-associated isoprenylated protein involved in stress responses. Plant Biol. 2015, 17, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Zschiesche, W.; Barth, O.; Daniel, K.; Böhme, S.; Rausche, J.; Humbeck, K. The zinc-binding nuclear protein HIPP3 acts as an upstream regulator of the salicylate-dependent plant immunity pathway and of flowering time in Arabidopsis thaliana. New Phytol. 2015, 207, 1084–1096. [Google Scholar] [CrossRef]
- Sheng, Y.B.; Yan, X.X.; Huang, Y.; Han, Y.Y.; Zhang, C.; Ren, Y.B.; Fan, T.T.; Xiao, F.M.; Liu, Y.S.; Cao, S.Q. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Hou, Y.Y.; Sun, Y.Y.; Chen, X.X.; Wang, G.Y.; Wang, H.C.; Zhu, B.; Du, X.Y. The maize WRKY transcription factor ZmWRKY64 confers cadmium tolerance in Arabidopsis and maize (Zea mays L.). Plant Cell Rep. 2024, 43, 44. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Huang, Y.; Song, H.; Chen, F.; Geng, Q.L.; Hu, M.; Zhang, C.; Wu, X.; Fan, T.T.; Cao, S.Q. A MYB4-MAN3-Mannose-MNB1 signaling cascade regulates cadmium tolerance in arabidopsis. PLoS Genet. 2021, 17, e1009636. [Google Scholar] [CrossRef]
- Wei, X.; Geng, M.H.; Yuan, J.C.; Zhan, J.J.; Liu, L.S.; Chen, Y.L.; Wang, Y.; Qin, W.Q.; Duan, H.Y.; Zhao, H.; et al. GhRCD1 promotes cotton tolerance to cadmium by regulating the GhbHLH12-GhMYB44-GhHMA1 transcriptional cascade. Plant Biotechnol. J. 2024, 22, 1777–1796. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, J.Y.; Dong, S.C.; Chang, M.H.; Zhu, J.X.; Guo, D.L.; Guo, C.H.; Bi, Y.D. Alfalfa MsbHLH115 confers tolerance to cadmium stress through activating the iron deficiency response in Arabidopsis thaliana. Front. Plant Sci. 2024, 15, 1358673. [Google Scholar] [CrossRef]
- Anwar, A.; Wang, Y.D.; Chen, M.Q.; Zhang, S.W.; Wang, J.M.; Feng, Y.Q.; Xue, Y.X.; Zhao, M.F.; Su, W.; Chen, R.Y.; et al. Zero-valent iron (nZVI) nanoparticles mediate SlERF1 expression to enhance cadmium stress tolerance in tomato. J. Hazard. Mater. 2024, 468, 133829. [Google Scholar] [CrossRef]
- Chang, J.; Huang, S.; Konishi, N.; Wang, P.; Chen, J.; Huang, X.; Ma, J.; Zhao, F. Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J. Exp. Bot. 2020, 71, 5705–5715. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, M.; Chen, M.; Lin, X.; Xu, P.; Cao, Z. Mutation of OsNRAMP5 reduces cadmium xylem and phloem transport in rice plants and its physiological mechanism. Environ. Pollut. 2024, 341, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Z.; Jin, M.; Wang, Y.; Li, Y.; Wu, J.; Xiao, Y.; Peng, Y.; Lv, Q.; Gui, S.; et al. A heavy metal transporter gene ZmHMA3a promises safe agricultural production on cadmium-polluted arable land. J. Genet. Genomics 2023, 50, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Chai, J.; Zhu, P.; Shanklin, J.; Liu, Q. Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters. Nat. Commun. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, M.; Jiang, Y.; Wang, C.; Ow, D. Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.). Agronomy 2021, 11, 12. [Google Scholar] [CrossRef]
- Guo, T.; Weber, H.; Niemann, M.C.E.; Theisl, L.; Leonte, G.; Novak, O.; Werner, T. Arabidopsis HIPP proteins regulate endoplasmic reticulum-associated degradation of CKX proteins and cytokinin responses. Mol. Plant 2021, 14, 1918–1934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
