Transcriptomic Analysis of Diabetic Erectile Dysfunction Rats After Red Blood Cell Exosome Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Main Instruments and Reagents
2.3. Isolation, Identification and Uptake of Red Blood Cell Exosomes
2.4. Establishment and Identification of DMED Rats Model
2.5. Grouping and Administration of Animals
2.6. Tissue Sampling and Testing
2.6.1. Observation of Masson Staining and H&E Staining
2.6.2. Immunofluorescence Staining
2.6.3. Western Blot
2.6.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Transcriptomic Analysis
2.8. Data Analysis
3. Result
3.1. Exosome Isolation and Identification
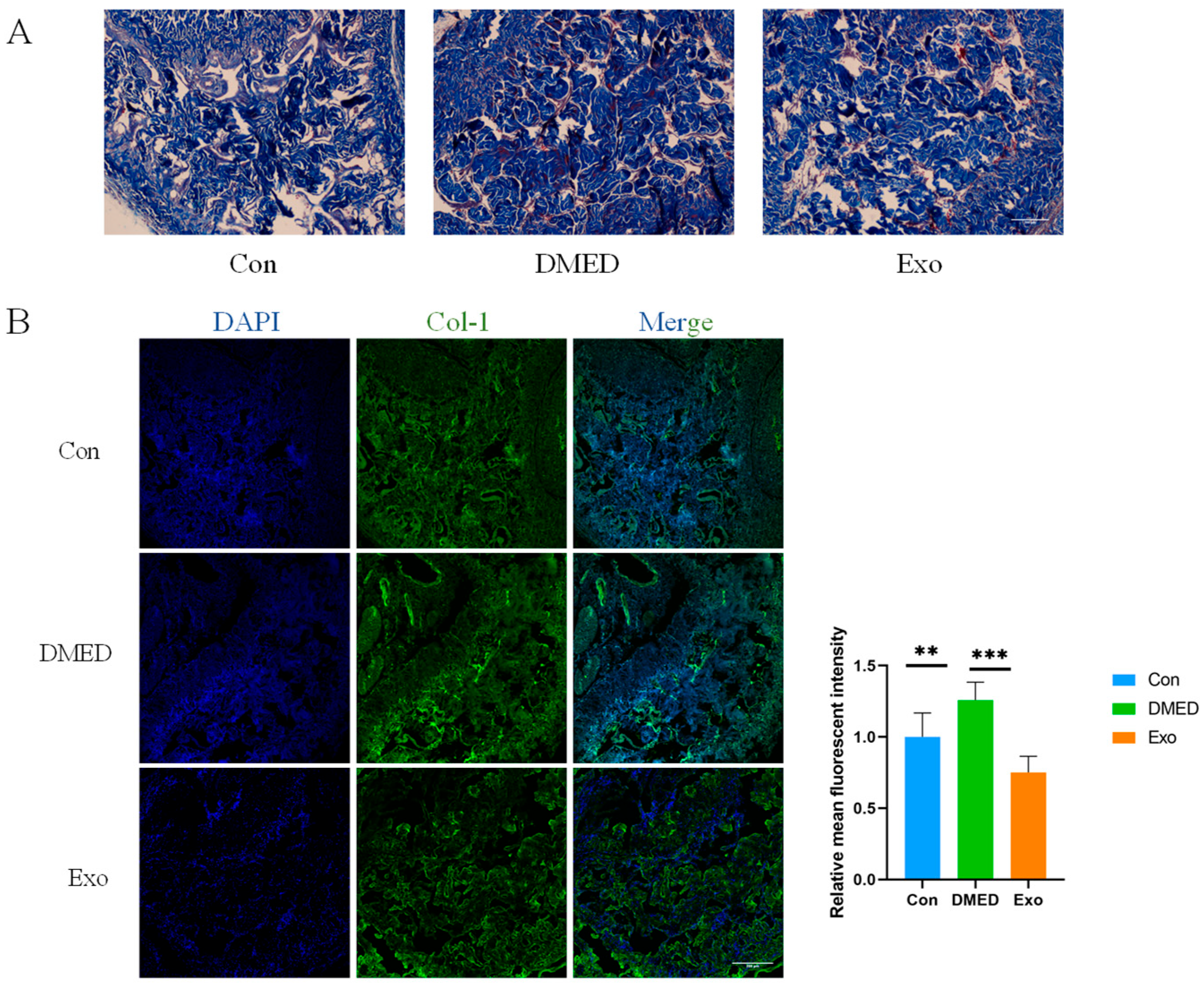
3.2. Collagen Deposition in the Penile Cavernous Bodies of DMED Rats Decreased Following Exosome Treatment
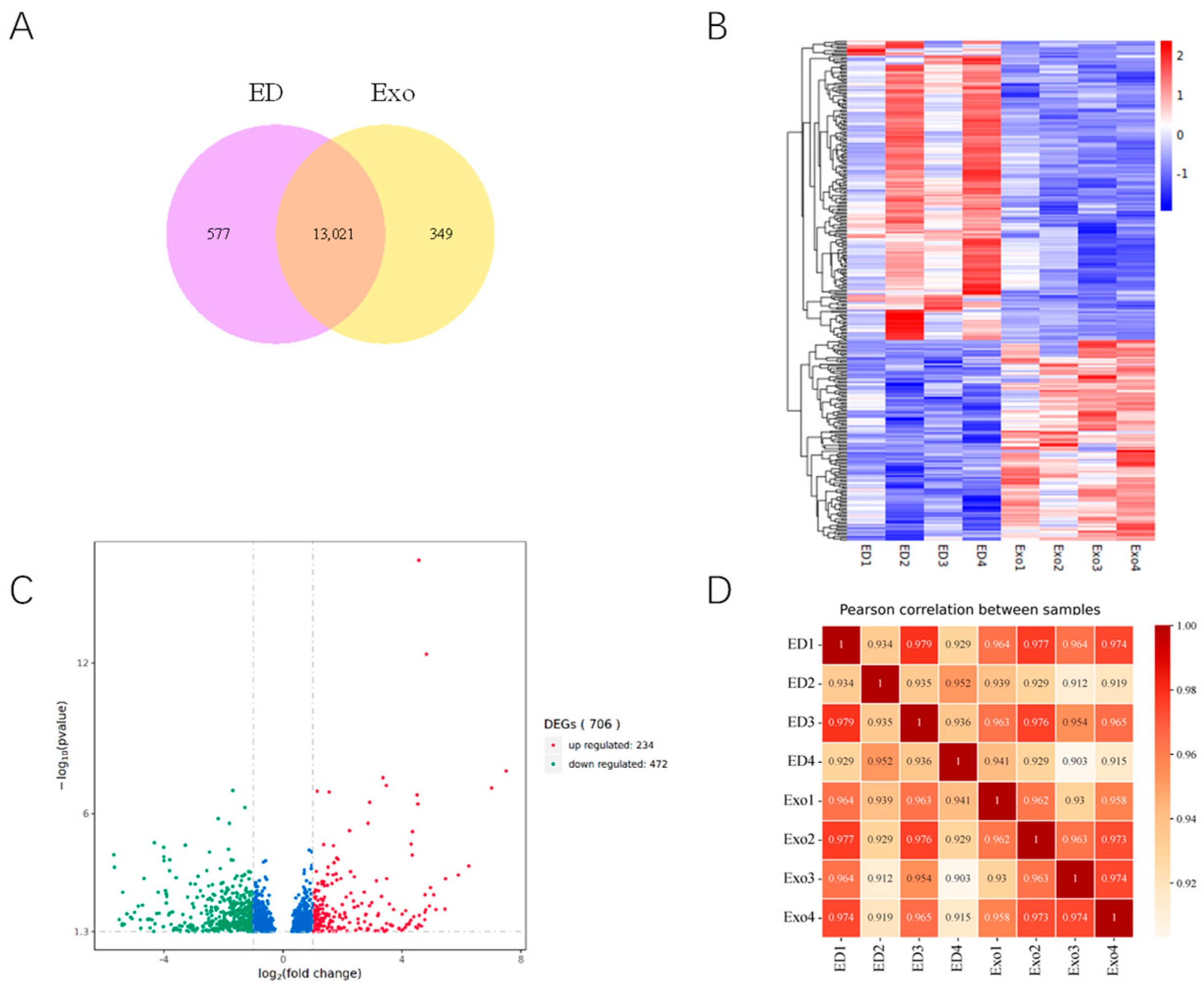
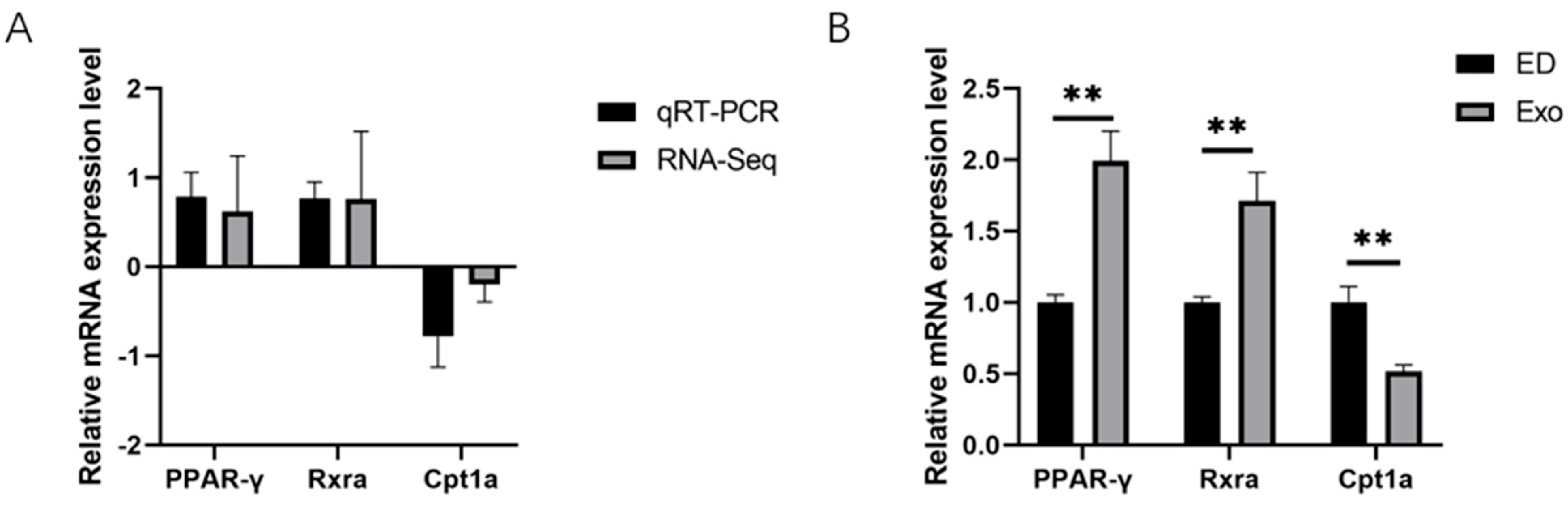
3.3. Expression Profiles of Differentially Expressed Genes (DEGs) in the DMED Group and Exosome Group
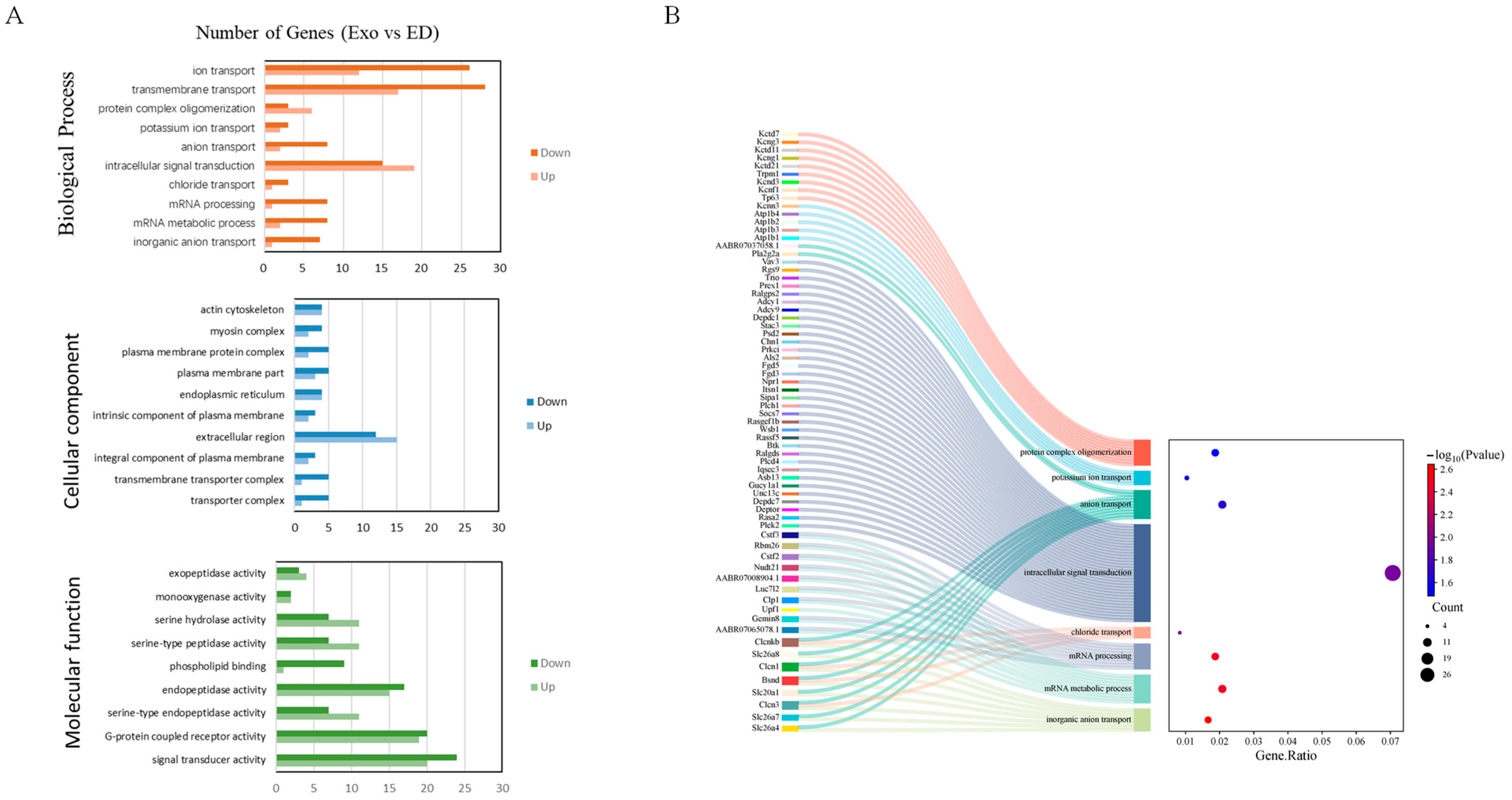
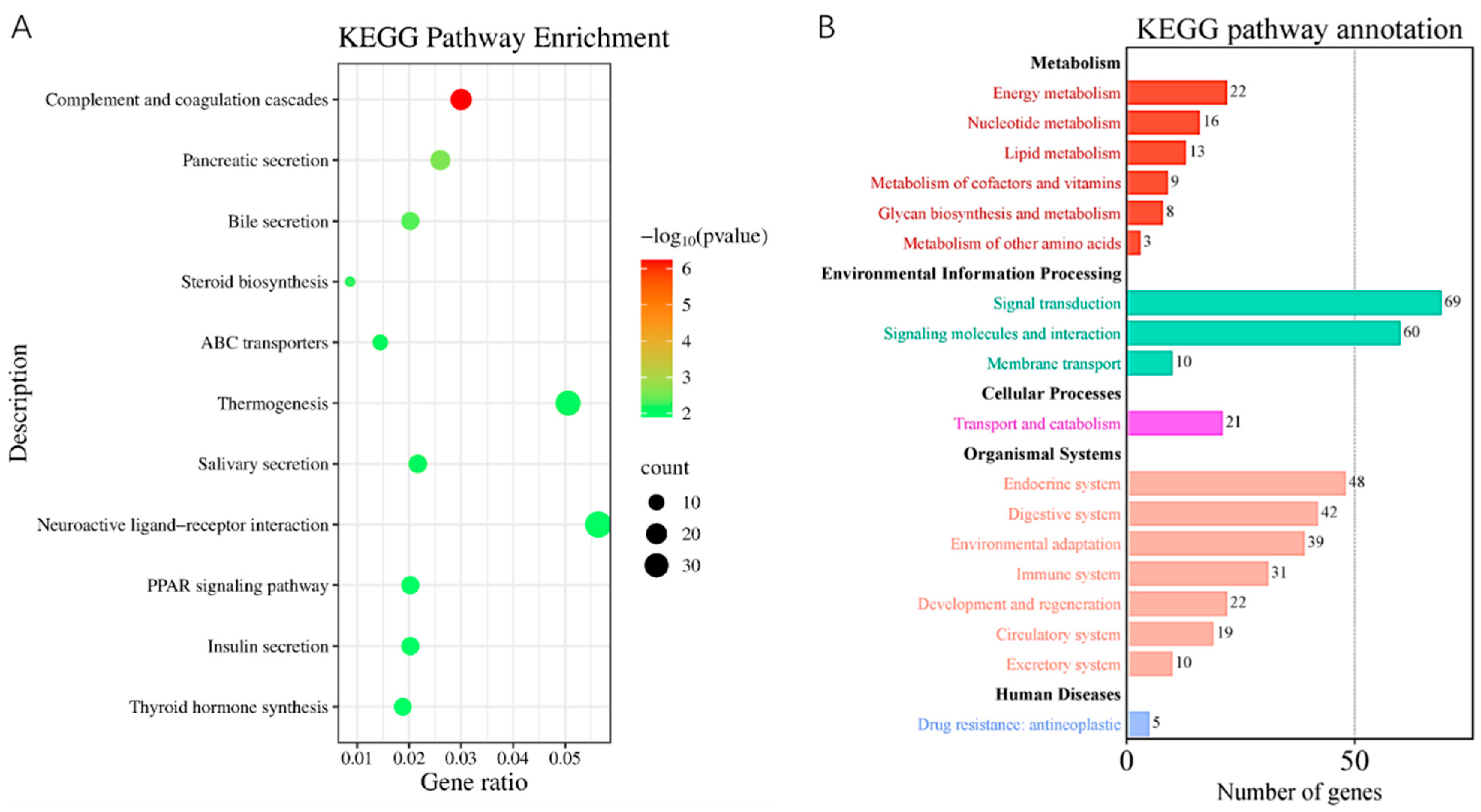
3.4. The Biological Activities Decreased in the ED Group Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Defeudis, G.; Mazzilli, R.; Tenuta, M.; Rossini, G.; Zamponi, V.; Olana, S.; Faggiano, A.; Pozzilli, P.; Isidori, A.M.; Gianfrilli, D. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. Rev. 2022, 38, e3494. [Google Scholar] [CrossRef]
- Efficacy and safety of PDE5 inhibitors in the treatment of diabetes mellitus erectile dysfunction: Protocol for a systematic review: Erratum. Medicine 2018, 97, e13203. [CrossRef]
- Swiecicka, A. The efficacy of PDE5 inhibitors in diabetic patients. Andrology 2023, 11, 245–256. [Google Scholar] [CrossRef]
- Balhara, Y.P.; Sarkar, S.; Gupta, R. Phosphodiesterase-5 inhibitors for erectile dysfunction in patients with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Indian J. Endocrinol. Metab. 2015, 19, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Lebleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Feng, H.; Peng, W.; Deng, Z.; Liu, J.; Wang, T. Erectile dysfunction and exosome therapy. Front. Endocrinol. 2023, 14, 1123383. [Google Scholar] [CrossRef]
- Feng, H.; Liu, Q.; Deng, Z.; Li, H.; Zhang, H.; Song, J.; Liu, X.; Liu, J.; Wen, B.; Wang, T. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res. Ther. 2022, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, P.; Liu, K.; Xu, W.; Wang, J.; Li, Q.; Chen, B.; Deng, Y.; Han, C.; Sun, T.; et al. MiRNA-100 ameliorates diabetes mellitus-induced erectile dysfunction by modulating autophagy, anti-inflammatory, and antifibrotic effects. Andrology 2024, 12, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, S.; Luo, L.; Wang, J.; Zhu, Z.; Xiang, Q.; Deng, Y.; Zhao, Z. Mesenchymal stem cell-derived exosomes ameliorate erection by reducing oxidative stress damage of corpus cavernosum in a rat model of artery injury. J. Cell Mol. Med. 2019, 23, 7462–7473. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, S.; Wang, J.; Luo, L.; Li, E.; Zhu, Z.; Liu, Y.; Kang, R.; Zhao, Z. Bone marrow mesenchymal stem cells reduce ureteral stricture formation in a rat model via the paracrine effect of extracellular vesicles. J. Cell Mol. Med. 2018, 22, 4449–4459. [Google Scholar] [CrossRef]
- Song, J.; Sun, T.; Tang, Z.; Ruan, Y.; Liu, K.; Rao, K.; Lan, R.; Wang, S.; Wang, T.; Liu, J. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J. Cell Mol. Med. 2020, 24, 13289–13302. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Ioakeimidis, N.; Terentes-Printzios, D.; Stefanadis, C. The triad: Erectile dysfunction--endothelial dysfunction--cardiovascular disease. Curr. Pharm. Des. 2008, 14, 3700–3714. [Google Scholar] [CrossRef]
- Miner, M.; Parish, S.J.; Billups, K.L.; Paulos, M.; Sigman, M.; Blaha, M.J. Erectile Dysfunction and Subclinical Cardiovascular Disease. Sex. Med. Rev. 2019, 7, 455–463. [Google Scholar] [CrossRef]
- Montorsi, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Margonato, A.; Macchi, A.; Galli, S.; Ravagnani, P.M.; Montorsi, P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur. Urol. 2003, 44, 360–364; discussion 364–365. [Google Scholar] [CrossRef]
- Ouyang, B.; Xie, Y.; Zhang, C.; Deng, C.; Lv, L.; Yao, J.; Zhang, Y.; Liu, G.; Deng, J.; Deng, C. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex. Med. 2019, 7, 241–250. [Google Scholar] [CrossRef]
- Ghosh, A.K. Pharmacological activation of PPAR-γ: A potential therapy for skin fibrosis. Int. J. Dermatol. 2021, 60, 376–383. [Google Scholar] [CrossRef]
- Majewski, G.P.; Singh, S.; Bojanowski, K. Olive leaf-derived PPAR agonist complex induces collagen IV synthesis in human skin models. Int. J. Cosmet. Sci. 2021, 43, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.; Delvecchio, C.; Spitale, N.; Giesler, A.; Radford, K.; Bilan, P.; Cox, P.G.; Capone, J.P.; Nair, P. PPAR ligands decrease human airway smooth muscle cell migration and extracellular matrix synthesis. Eur. Respir. J. 2013, 41, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.F.; Kılıç, E.; Karabulut, D.; Çilenk, K.; Deligönül, E.; Dündar, M. Nitric oxide synthase in diabetic rat testicular tissue and the effects of pentoxifylline therapy. Syst. Biol. Reprod. Med. 2016, 62, 22–30. [Google Scholar] [CrossRef]
- Zhu, D.; Pham, Q.M.; Wang, C.; Colonnello, E.; Yannas, D.; Nguyen, B.H.; Zhang, Y.; Jannini, E.A.; Sansone, A. Erectile Dysfunction and Oxidative Stress: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 3073. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence (5′-3′) | Length (bp) |
|---|---|---|
| Rxra | Forward:ATTTCCTGCCGCTCGACTT Reverse:GCTGATGACCGAGAAGGGTG | 209 |
| Cpt1a | Forward:GGCTTGGGACTTGGGCTTAC Reverse:CTTCTCTGCAACCCGGTAGG | 158 |
| PPAR-γ | Forward:GCAGAAACTGGGAGTAGCCTG Reverse:ATGGCATCTCTGTGTCAACCA | 223 |
| GADPH | Forward:CGTAAAGACCTCTATGCCAACA Reverse:CGGACTCATCGTACTCCTGCT | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Quan, B.; Liu, X.; Cui, Q.; Yin, X.-J. Transcriptomic Analysis of Diabetic Erectile Dysfunction Rats After Red Blood Cell Exosome Treatment. Genes 2025, 16, 768. https://doi.org/10.3390/genes16070768
Lv Y, Quan B, Liu X, Cui Q, Yin X-J. Transcriptomic Analysis of Diabetic Erectile Dysfunction Rats After Red Blood Cell Exosome Treatment. Genes. 2025; 16(7):768. https://doi.org/10.3390/genes16070768
Chicago/Turabian StyleLv, Yantong, Biaohu Quan, Xinyue Liu, Qichao Cui, and Xi-Jun Yin. 2025. "Transcriptomic Analysis of Diabetic Erectile Dysfunction Rats After Red Blood Cell Exosome Treatment" Genes 16, no. 7: 768. https://doi.org/10.3390/genes16070768
APA StyleLv, Y., Quan, B., Liu, X., Cui, Q., & Yin, X.-J. (2025). Transcriptomic Analysis of Diabetic Erectile Dysfunction Rats After Red Blood Cell Exosome Treatment. Genes, 16(7), 768. https://doi.org/10.3390/genes16070768






