Aluminum Stress Response Is Regulated Through a miR156/SPL13 Module in Medicago sativa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Generation of 35S:SPL13-OE Constructs and Plant Transformation
2.3. Al Treatment
2.4. Histochemical Staining
2.5. Nutrient Analysis
2.6. RNA Extraction and Sequencing
2.7. RNA-Seq Analysis
2.8. Differential Gene Expression, Gene Ontology, and GTAC Motif Analyses
2.9. Validation of RNA-Seq Results by RT-qPCR
2.10. Identification of Transcription Factors (TFs) Under Al Stress
2.11. Identification and SBP Domain Analysis of SPL13 Candidates
2.12. Analysis of the SPL13 Coexpression Network Under Al Stress
2.13. Small RNA Target Analysis in Alfalfa
2.14. Chromatin Immunoprecipitation (ChIP) Assay
2.15. ChIP-Seq and Data Analyses
2.16. Statistical Analysis
3. Results
3.1. miR156 Exacerbates Al Toxicity
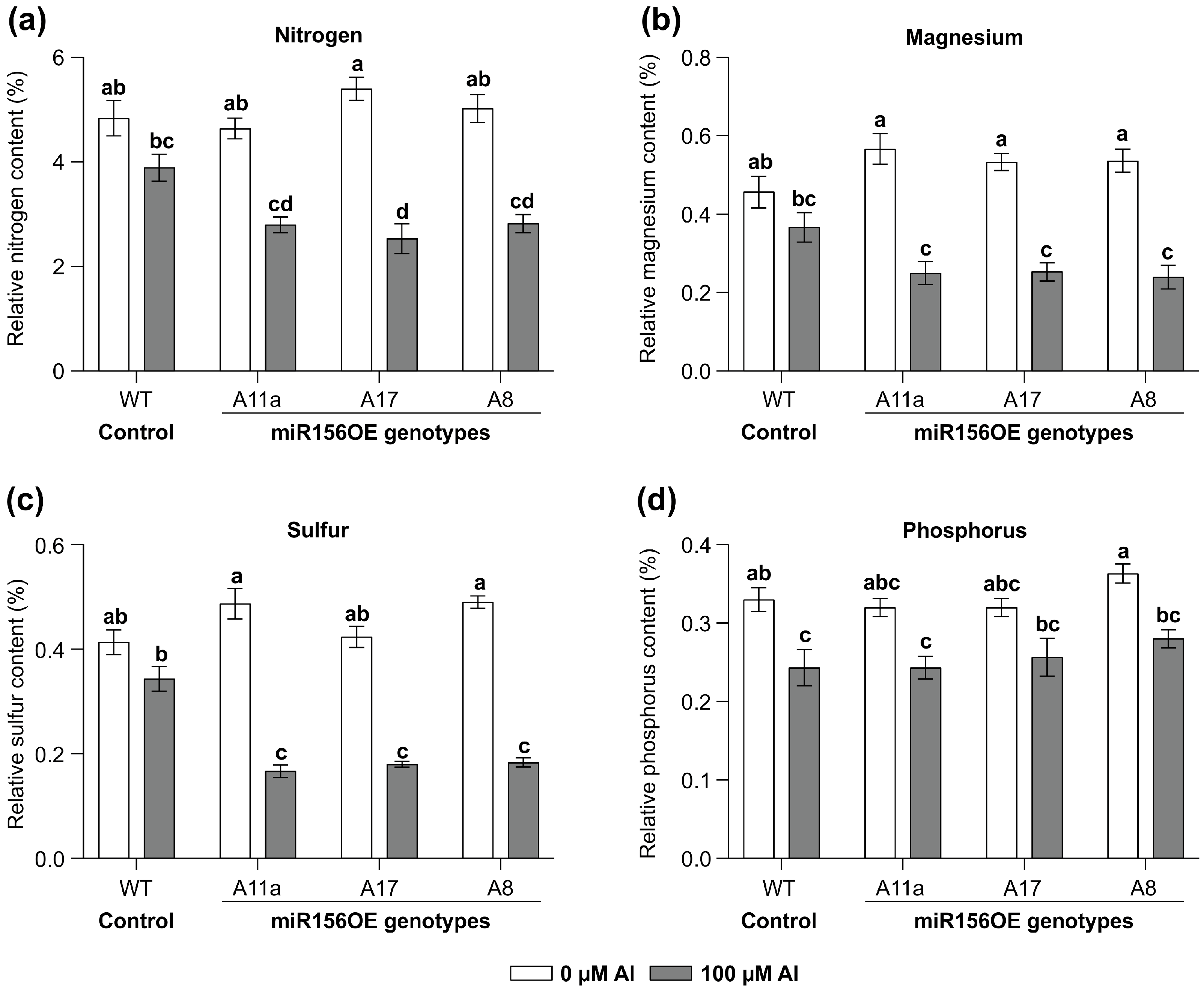
3.2. miR156 Affects Nutrient Uptake Under Al Stress
3.3. MsmiR156 Regulates MsSPL13 in Response to Al
3.4. SPL13 Regulates Root Growth Under Al Stress
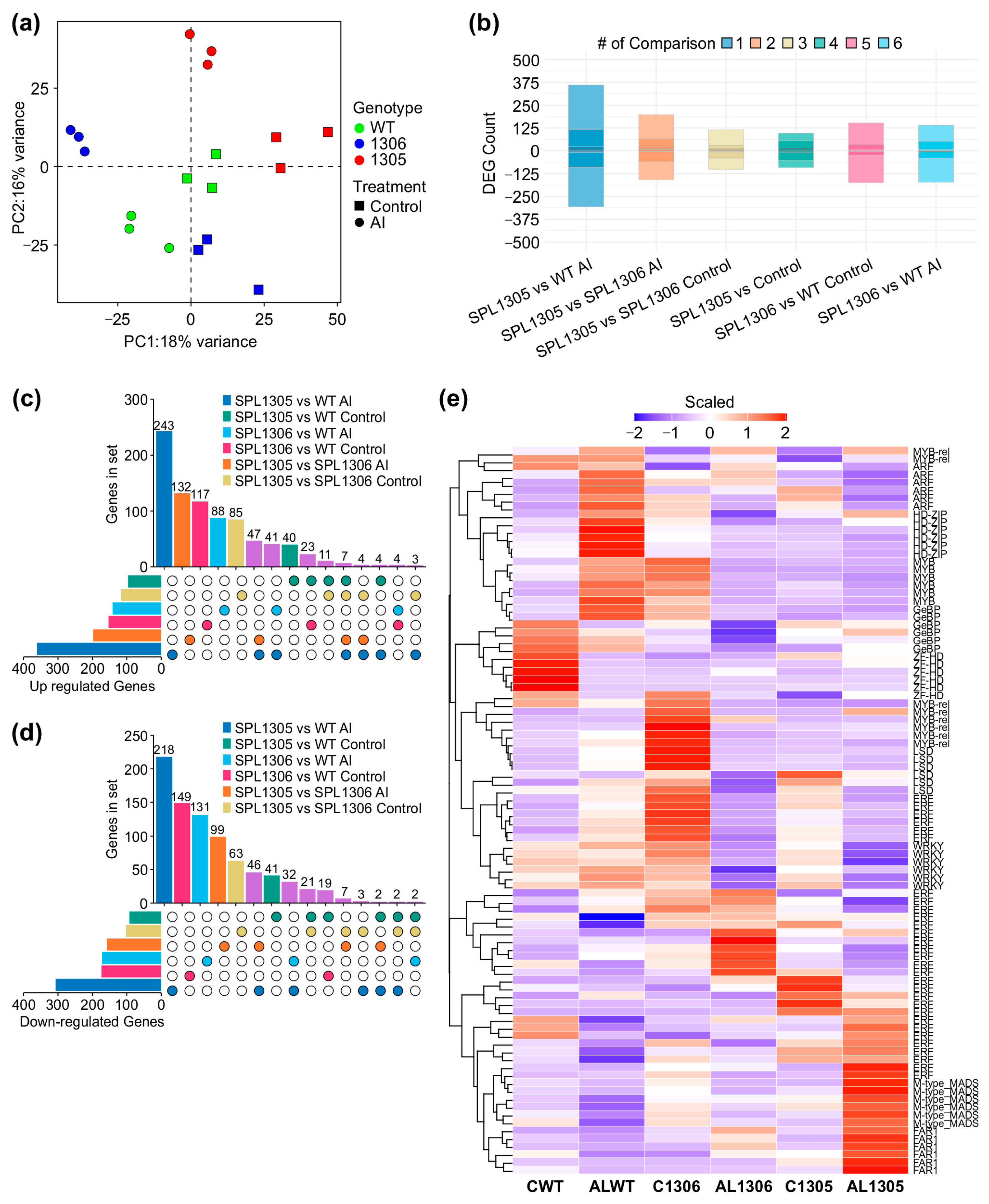
3.5. Global Changes in Gene Expression in MsSPL13-RNAi Plants Under Al Stress
3.6. Differential Expression of Transcription Factors in MsSPL13-RNAi Alfalfa Under Al Stress
3.7. Genotype-Specific Response of Alfalfa to Al Stress
3.8. GO Analysis of Genotype-Specific Comparison of DEG Pathways Modulating Al Stress Response in MsSPL13-RNAi Alfalfa
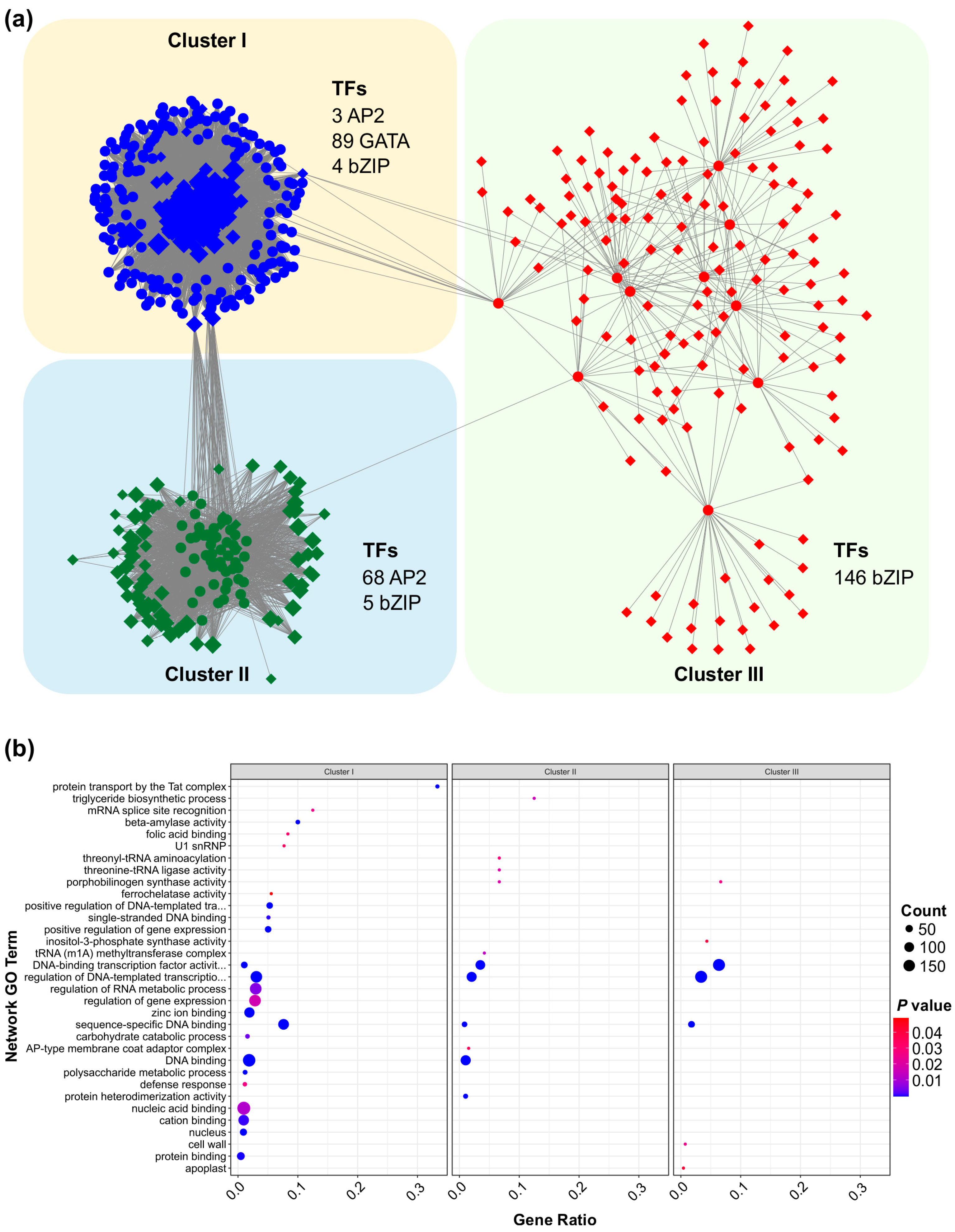
3.9. Regulatory Network and Functional Enrichment of SPL13-Associated Genes Under Al Stress
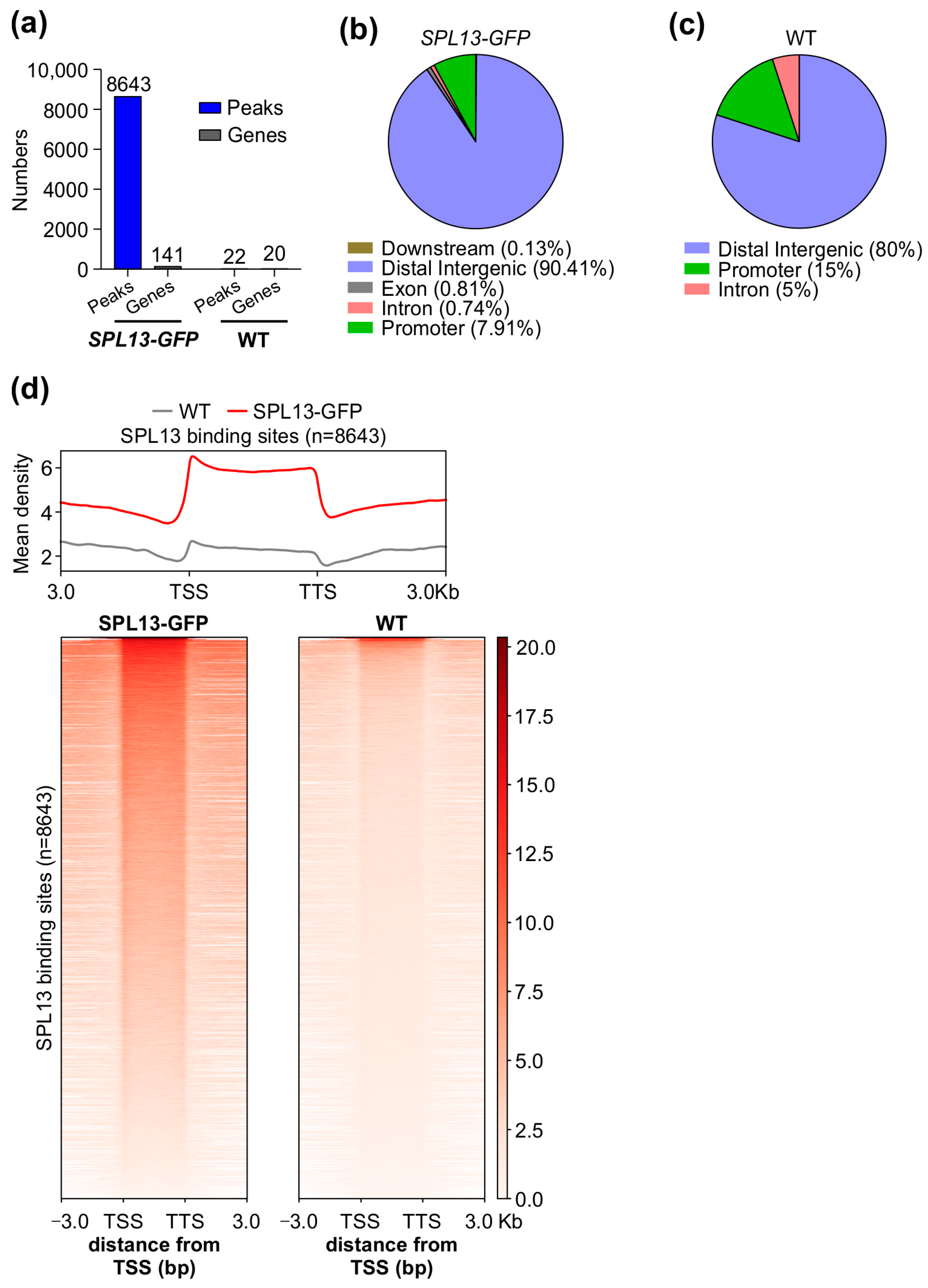
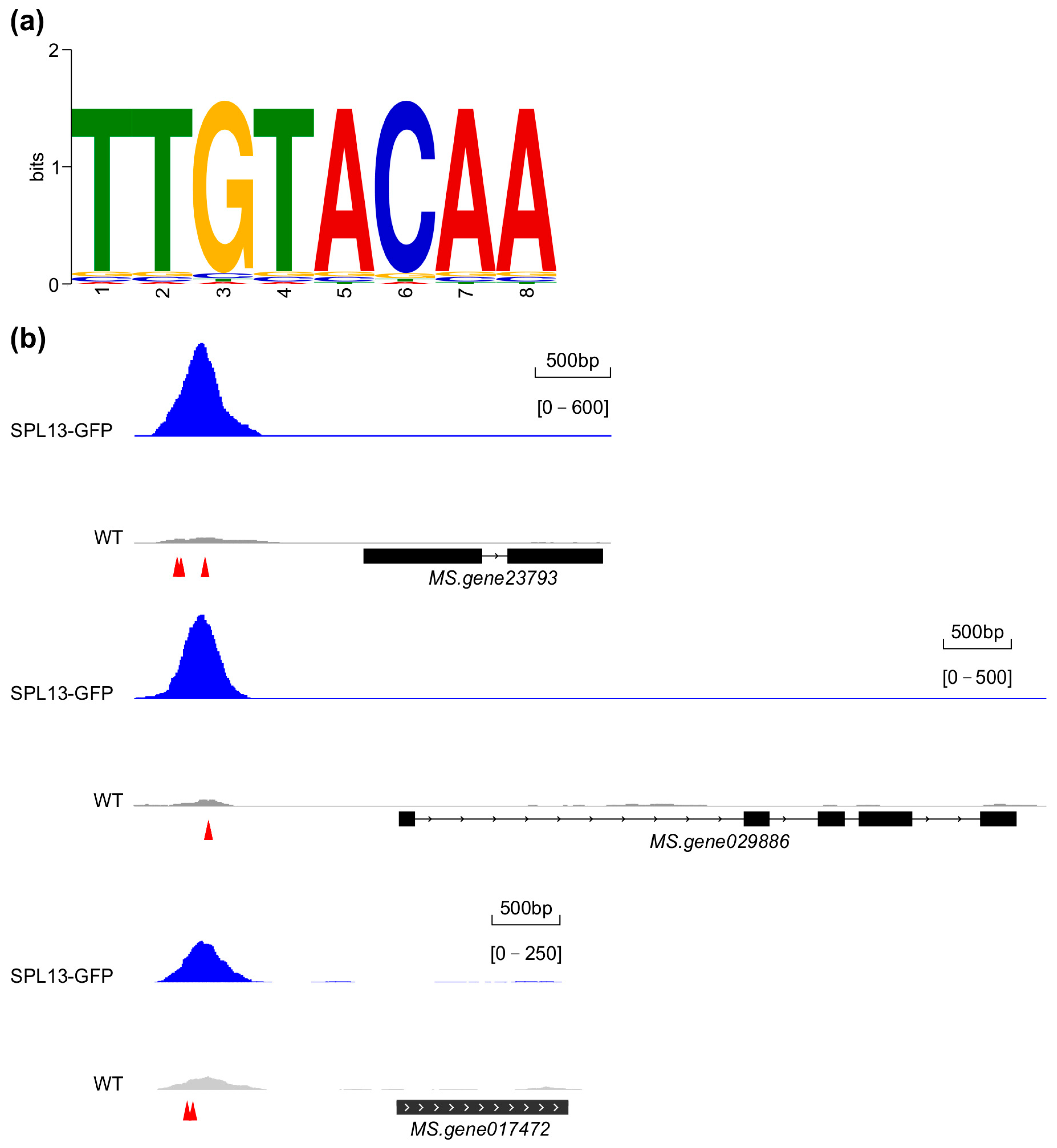
3.10. Genome-Wide Identification of Genes Regulated Directly by SPL13 in Alfalfa
4. Discussion
4.1. MsmiR156-OE Exacerbates Al Toxicity in Alfalfa
4.2. MsmiR156-OE Impairs Nutrient Uptake Under Aluminum Stress
4.3. MsSPL13 and Root Growth Under Al Stress
4.4. SPL13-Regulated Transcriptomic Changes in Alfalfa Under Al Stress
4.5. MsSPL13 Silencing Alters TFs Under Al Stress in Alfalfa
4.6. SPL13 Regulates Kinase-Mediated Signaling Under Al Stress
4.7. MsSPL13 Silencing Disrupts a Key Transcriptional Network for Al Detoxification
4.8. SPL13 Directly Binds to Al-Responsive Genes in Alfalfa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | Aluminum |
| miRNA156 (miR156) | MicroRNA156 |
| SPL13 | SQUAMOSA promoter-binding protein-like 13 |
| ChIP-seq | Chromatin immunoprecipitation sequencing |
| WT | Wild type |
| GRAS | Gibberellic acid insensitive, repressor of GAI, and scarecrow |
| Myb-related proteins | Myb transcription factors |
| bHLH041 | Basic helix–loop–helix 041 |
| NAC | NAC domain-containing transcription factors |
| WRKY53 | WRKY transcription factor 53 |
| bZIP | Basic leucine zipper |
| AGAMOUS-like MADS-box | AGAMOUS-like MADS-box transcription factors |
| RT-qPCR | Reverse transcription quantitative PCR |
| FASTQ | Sequence file format for high-throughput sequencing data |
| PCA | Principal component analysis |
| DEGs | Differentially expressed genes |
| GO | Gene Ontology |
| FIMO | Find individual motif occurrences |
| SEA | Single element analysis |
| MEME | Multiple EM for Motif Elicitation |
| RISC | RNA-induced silencing complex |
| HPC | High-performance computing |
| RNA-seq | RNA sequencing |
| DE | Differentially expressed |
| MADS-box | MADS-domain transcription factors |
| LRR | Leucine-rich repeat |
| ABC | ATP-binding cassette |
| SAUR | Small auxin-up RNA |
| TCA | Tricarboxylic acid cycle |
| PSII | Photosystem II |
| ROS | Reactive oxygen species |
| SPL | SQUAMOSA promoter-binding protein-like |
| STOP1 | Stress-responsive element binding protein 1 |
| GTAC | Core motif in SPL binding |
| ACC1 | Acetyl-CoA carboxylase 1 |
| ACC2 | Acetyl-CoA carboxylase 2 |
| Bp | Base pair |
| cDNA | Complementary DNA |
| DETF(s) | Differentially expressed transcription factor(s) |
| FLA | Fasciclin-like arabinogalactan protein |
| h | Hour(s) |
| HPC | High-performance computing |
| ICP-OES | Inductively coupled plasma optical emission spectroscopy |
| PCR | Polymerase chain reaction |
| psRNATarget | Plant small RNA target analysis |
| SEM | Standard error of the mean |
| TCA | Tricarboxylic acid cycle |
| TSS | Transcription start site |
| TF(s) | Transcription factor(s) |
| Tris-HCl | Tris(hydroxymethyl)aminomethane hydrochloride |
| UTR | Untranslated region |
References
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Yang, Z.; Chen, C.; Yang, F.; Wang, S.; Dong, R. Identification and characterization of long noncoding RNAs involved in the aluminum stress response in Medicago truncatula via genome-wide analysis. Front. Plant Sci. 2022, 13, 1017869. [Google Scholar] [CrossRef]
- Chandran, D.; Sharopova, N.; Vandenbosch, K.A.; Garvin, D.F.; Samac, D.A. Physiological and molecular characterization of aluminum resistance in Medicago truncatula. BMC Plant Biol. 2008, 8, 89. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status and opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.R.; Zhang, G.P.; Zhou, M.X.; Wu, F.B.; Chen, J.X. Influence of Aluminum and Cadmium Stresses on Mineral Nutrition and Root Exudates in Two Barley Cultivars. Project supported by the Chinese Ministry of Science and Technology (China-Australian Special Link Research Program) and the Grains Research and Development Corporation of Australia (No. UT-8). Pedosphere 2007, 17, 505–512. [Google Scholar] [CrossRef]
- Mariano, E.D.; Pinheiro, A.S.; Garcia, E.E.; Keltjens, W.G.; Jorge, R.A.; Menossi, M. Differential aluminium-impaired nutrient uptake along the root axis of two maize genotypes contrasting in resistance to aluminium. Plant Soil. 2015, 388, 323–335. [Google Scholar] [CrossRef]
- Moustaka, J.; Ouzounidou, G.; Bayçu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. BioMetals 2016, 29, 611–623. [Google Scholar] [CrossRef]
- Kichigina, N.E.; Puhalsky, J.V.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Loskutov, S.I.; Safronova, V.I.; Tikhonovich, I.A.; Vishnyakova, M.A.; Semenova, E.V. Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants 2017, 23, 851–863. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.P.; Cai, Y.T.; Yang, T.Y.; Yang, L.T.; Huang, Z.R.; Chen, L.S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef]
- Borges, C.E.; Cazetta, J.O.; de Sousa, F.B.F.; Oliveira, K.S. Aluminum toxicity reduces the nutritional efficiency of macronutrients and micronutrients in sugarcane seedlings. Cienc. E Agrotecnologia 2020, 44, 1–18. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Szutu, D.J.; Verfaillie, J.G.; Baldocchi, D.D.; Silver, W.L. Carbon-sink potential of continuous alfalfa agriculture lowered by short-term nitrous oxide emission events. Nat. Commun. 2023, 14, 1926. [Google Scholar] [CrossRef]
- Ning, J.; He, X.Z.; Hou, F.; Lou, S.; Chen, X.; Chang, S.; Zhang, C.; Zhu, W. Optimizing alfalfa productivity and persistence versus greenhouse gases fluxes in a continental arid region. PeerJ 2020, 2020, e8738. [Google Scholar] [CrossRef]
- Li, J.; Su, L.; Lv, A.; Li, Y.; Zhou, P.; An, Y. MsPG1 alleviated aluminum-induced inhibition of root growth by decreasing aluminum accumulation and increasing porosity and extensibility of cell walls in alfalfa (Medicago sativa). Environ. Exp. Bot. 2020, 175, 104045. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brummer, E.C. Applied genetics and genomics in alfalfa breeding. Agronomy 2012, 2, 40–61. [Google Scholar] [CrossRef]
- Nichols, N.N.; Dien, B.S.; Cotta, M.A. Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour. Technol. 2010, 101, 7545–7550. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Adler, P.R. Perennial forages as second generation bioenergy crops. Int. J. Mol. Sci. 2008, 9, 768–788. [Google Scholar] [CrossRef]
- Imarc, Global Alfalfa Hay Market to Reach 430.7 Million Metric Tons by 2033, Impelled by Growing Consciousness Regarding Animal Nutrition. Tranforming into Impact: 2024. Available online: https://www.imarcgroup.com/global-alfalfa-hay-market (accessed on 15 April 2025).
- Willis, J.H. Inbreeding load, average dominance and the mutation rate for mildly deleterious alleles in Mimulus guttatus. Genetics 1999, 153, 1885–1898. [Google Scholar] [CrossRef]
- Aung; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Yuan, J.; Guo, T.; Xu, L.; Mu, Z.; Han, L. Analysis of transcripts and splice isoforms in Medicago sativa L. by single-molecule long-read sequencing. Plant Mol. Biol. 2019, 99, 219–235. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, Y.; Yang, Y.; Huang, L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.; Liu, Y.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The Chromosome-Level Genome Sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms Provide Genomic Resources for Alfalfa Research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Zhang, F.; Zhang, Z.; Li, M.; Chen, L.; Wang, X.; Liu, W.; Zhang, T.; Yu, L.X.; He, F.; et al. Genome Assembly of Alfalfa Cultivar Zhongmu-4 and Identification of SNPs Associated with Agronomic Traits. Genom. Proteom. Bioinform. 2022, 20, 14–28. [Google Scholar] [CrossRef]
- Jeyakumar, J.M.J.; Ali, A.; Wang, W.M.; Thiruvengadam, M. Characterizing the role of the miR156-SPL network in plant development and stress response. Plants 2020, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Cardon, G.; Höhmann, S.; Klein, J.; Nettesheim, K.; Saedler, H.; Huijser, P. Molecular characterisation of the Arabidopsis SBP-box genes. Gene 1999, 237, 91–104. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Nunokawa, E.; et al. A Novel Zinc-binding Motif Revealed by Solution Structures of DNA-binding Domains of Arabidopsis SBP-family Transcription Factors. J. Mol. Biol. 2004, 337, 49–63. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Mol. Plant 2015, 8, 677–688. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.H.; Rocha, G.H.B.; Polverari, L.; Pinheiro Brito, D.A.; Batista, D.S.; Notini, M.M.; Da Cruz, A.C.F.; Morea, E.G.O.; Sabatini, S.; Otoni, W.C.; et al. MiR156-targeted SPL10 controls Arabidopsis root meristem activity and root-derived de novo shoot regeneration via cytokinin responses. J. Exp. Bot. 2020, 71, 934–950. [Google Scholar] [CrossRef]
- Aung; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. Ectopic expression of LjmiR156 delays flowering, enhances shoot branching, and improves forage quality in alfalfa. Plant Biotechnol. Rep. 2015, 9, 379–393. [Google Scholar] [CrossRef]
- Sun, Z.; Su, C.; Yun, J.; Jiang, Q.; Wang, L.; Wang, Y.; Cao, D.; Zhao, F.; Zhao, Q.; Zhang, M.; et al. Genetic improvement of the shoot architecture and yield in soya bean plants via the manipulation of GmmiR156b. Plant Biotechnol. J. 2019, 17, 50–62. [Google Scholar] [CrossRef]
- Xie, K.; Wu, C.; Xiong, L. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, F.; Wang, J.; Zhang, C.; Xiao, F.; Ye, J.; Li, C.; Ye, Z.; Zhang, J. miR156a-targeted SBP-Box transcription factor SlSPL13 regulates inflorescence morphogenesis by directly activating SFT in tomato. Plant Biotechnol. J. 2020, 18, 1670–1682. [Google Scholar] [CrossRef]
- Cui; Liu, X.; Hao, Y. SCARECROW, SCR-LIKE 23 and SHORT-ROOT control bundle sheath cell fate and function in Arabidopsis thaliana. Plant J. 2014, 78, 319–327. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, X.; Liu, P.; Sun, J. miR156-targeted SBP-box transcription factors interact with DWARF53 to regulate teosinte branched1 and barren STALK1 expression in bread wheat. Plant Physiol. 2017, 174, 1931–1948. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, F.; Jiang, Q.; Yang, S.; Yue, P.; Wang, F.; Zhang, Y.; Li, L.; He, P.; et al. The miR156/SPL module regulates apple salt stress tolerance by activating MdWRKY100 expression. Plant Biotechnol. J. 2021, 19, 311–323. [Google Scholar] [CrossRef]
- Silva; Rosa-Santos, T.M.; de Castro França, S.; Kottapalli, P.; Kottapalli, K.R.; Zingaretti, S.M. Microtranscriptome analysis of sugarcane cultivars in response to aluminum stress. PLoS ONE 2019, 14, e0217806. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.; Arenhart, R.; Margis-Pinheiro, M.; Margis, R. Aluminum triggers broad changes in microRNA expression in rice roots. Genet. Mol. Res. 2011, 10, 2817–2832. [Google Scholar] [CrossRef]
- Wu, L.; Yu, J.; Shen, Q.; Huang, L.; Wu, D.; Zhang, G. Identification of microRNAs in response to aluminum stress in the roots of Tibetan wild barley and cultivated barley. BMC Genom. 2018, 19, 560. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Yang, C.Y.; Ma, Q.B.; Li, X.P.; Dong, W.W.; Nian, H. Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol. 2012, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yang, Z.; Tian, Z.; Gui, Q.; Dong, R.; Chen, C. Genome-wide analysis and identification of microRNAs in Medicago truncatula under aluminum stress. Front. Plant Sci. 2023, 14, 1137764. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Liu, Z.-W.; Wu, Z.-J.; Li, H.; Wang, W.-L.; Cui, X.; Zhuang, J. Genome-wide identification and expression analysis of GRAS family transcription factors in tea plant (Camellia sinensis). Sci. Rep. 2018, 8, 3949. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, X.; Huang, X.; Liu, J.H. Overexpression of a stress-responsive MYB transcription factor of Poncirus trifoliata confers enhanced dehydration tolerance and increases polyamine biosynthesis. Plant Physiol. Biochem. 2014, 78, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wei, W.; Pang, X.; Wang, X.; Zhang, H.; Dong, B.; Xing, Y.; Li, X.; Wang, M. Comparative transcriptome profiling of a desert evergreen shrub, Ammopiptanthus mongolicus, in response to drought and cold stresses. BMC Genom. 2014, 15, 671. [Google Scholar] [CrossRef]
- Wang, C.; Hao, X.; Wang, Y.; Maoz, I.; Zhou, W.; Zhou, Z.; Kai, G. Identification of WRKY transcription factors involved in regulating the biosynthesis of the anti-cancer drug camptothecin in Ophiorrhiza pumila. Hortic. Res. 2022, 9, uhac099. [Google Scholar] [CrossRef] [PubMed]
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.K.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type transcription factor, Aba-Inducible Bhlh-Type Transcription Factor/Ja-Associated Myc2-Like1, acts as a repressor to negatively regulate jasmonate signaling in Arabidopsis. Plant Cell 2013, 25, 1641–1656. [Google Scholar] [CrossRef]
- Allam, G.; Sakariyahu, S.K.; McDowell, T.; Pitambar, T.A.; Papadopoulos, Y.; Bernards, M.A.; Hannoufa, A. miR156 Is a Negative Regulator of Aluminum Response in Medicago sativa. Plants 2025, 14, 958. [Google Scholar] [CrossRef]
- Badhan, A.; Jin, L.; Wang, Y.; Han, S.; Kowalczys, K.; Brown, D.C.W.; Ayala, C.J.; Latoszek-Green, M.; Miki, B.; Tsang, A.; et al. Expression of a fungal ferulic acid esterase in alfalfa modifies cell wall digestibility. Biotechnol. Biofuels 2014, 7, 1–15. [Google Scholar] [CrossRef]
- Arshad, M.; Feyissa, B.A.; Amyot, L.; Aung, B.; Hannoufa, A. MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Sci. 2017, 258, 122–136. [Google Scholar] [CrossRef]
- Gao, R.; Gruber, M.Y.; Amyot, L.; Hannoufa, A. SPL13 regulates shoot branching and flowering time in Medicago sativa. Plant Mol. Biol. 2018, 96, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef]
- Froger, A.; Hall, J.E. Transformation of plasmid DNA into E. coli using the heat shock method. JoVE (J. Vis. Exp.) 2007, 6, e253. [Google Scholar]
- Höfgen, R.; Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef] [PubMed]
- Boukari, N.; Jelali, N.; Renaud, J.B.; Youssef, R.B.; Abdelly, C.; Hannoufa, A. Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of Alfalfa. Environ. Exp. Bot. 2019, 167, 103820. [Google Scholar] [CrossRef]
- Tistama, R.; Widyastuti, U.; Sopandie, D.; Yokota, A.; Akashi, K. Physiological and Biochemical Responses to Aluminum Stress in the Root of a Biodiesel Plant Jatropha curcas L. HAYATI J. Biosci. 2012, 19, 37–43. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool For High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 July 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef]
- Bjornson, M.; Pimprikar, P.; Nürnberger, T.; Zipfel, C. The transcriptional landscape of Arabidopsis thaliana pattern-triggered immunity. Nat. Plants 2021, 7, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2. Wiley Interdisciplinary Reviews. Computational Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 3, pp. 180–185. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenfuhrer, J. TopGo: Enrichment analysis for gene ontology. R. Package Version 2010, 2, 2010. [Google Scholar]
- Bailey, T.L.; Grant, C.E. SEA: Simple enrichment analysis of motifs. BioRxiv 2021. [Google Scholar] [CrossRef]
- Grant, C.E.; Bailey, T.L.; Noble, W.S. FIMO: Scanning for occurrences of a given motif. Bioinformatics 2011, 27, 1017–1018. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Alexander, T.W.; Reuter, T.; McAllister, T.A. Qualitative and quantitative polymerase chain reaction assays for an alfalfa (Medicago sativa)-specific reference gene to use in monitoring transgenic cultivars. J. Agric. Food Chem. 2007, 55, 2918–2922. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Nemchinov, L.G.; Shao, J.; Grinstead, S.; Postnikova, O.A. Transcription Factors in Alfalfa (Medicago sativa L.): Genome-Wide Identification and a Web Resource Center AlfalfaTFDB. In The Alfalfa Genome; Springer International Publishing: Cham, Switzerland, 2021; pp. 111–127. [Google Scholar]
- Huynh-Thu, V.A.; Irrthum, A.; Wehenkel, L.; Geurts, P. Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 2010, 5, e12776. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Wang, Y.; Renaud, J.; Tian, G.; Kambhampati, S.; Saatian, B.; Nguyen, V.; Hannoufa, A.; Marsolais, F.; et al. Cytosolic acetyl-CoA promotes histone acetylation predominantly at H3K27 in Arabidopsis. Nat. Plants 2017, 3, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nussbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.W.; Helt, G.A.; Blanchard, S.G., Jr.; Raja, A.; Loraine, A.E. The Integrated Genome Browser: Free software for distribution and exploration of genome-scale datasets. Bioinformatics 2009, 25, 2730–2731. [Google Scholar] [CrossRef] [PubMed]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. Available online: https://www.R-project.org (accessed on 10 July 2024).
- Feyissa, B.A.; Amyot, L.; Nasrollahi, V.; Papadopoulos, Y.; Kohalmi, S.E.; Hannoufa, A. Involvement of the miR156/SPL module in flooding response in Medicago sativa. Sci. Rep. 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Austin, R.S.; Amyot, L.; Hannoufa, A. Comparative transcriptome investigation of global gene expression changes caused by miR156 overexpression in Medicago sativa. BMC Genom. 2016, 17, 658. [Google Scholar] [CrossRef]
- Ma, L.; Liu, X.; Liu, W.; Wen, H.; Zhang, Y.; Pang, Y.; Wang, X. Characterization of Squamosa-Promoter Binding Protein-Box Family Genes Reveals the Critical Role of MsSPL20 in Alfalfa Flowering Time Regulation. Front. Plant Sci. 2022, 12, 775690. [Google Scholar] [CrossRef]
- Iki, T.; Yoshikawa, M.; Nishikiori, M.; Jaudal, M.C.; Matsumoto-Yokoyama, E.; Mitsuhara, I.; Meshi, T.; Ishikawa, M. In Vitro Assembly of Plant RNA-Induced Silencing Complexes Facilitated by Molecular Chaperone HSP90. Mol. Cell 2010, 39, 282–291. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Gruber, M.Y.; Wall, K.; Hannoufa, A. An insight into microRNA156 role in salinity stress responses of alfalfa. Front. Plant Sci. 2017, 8, 356. [Google Scholar] [CrossRef]
- Wei, P.; Demulder, M.; David, P.; Eekhout, T.; Yoshiyama, K.O.; Nguyen, L.; Vercauteren, I.; Eeckhout, D.; Galle, M.; de Jaeger, G.; et al. Arabidopsis casein kinase 2 triggers stem cell exhaustion under Al toxicity and phosphate deficiency through activating the DNA damage response pathway. Plant Cell 2021, 33, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Szurman-Zubrzycka, M.; Chwiałkowska, K.; Niemira, M.; Kwaśniewski, M.; Nawrot, M.; Gajecka, M.; Larsen, P.B.; Szarejko, I. Aluminum or low pH–which is the bigger enemy of barley? transcriptome analysis of barley root meristem under Al and low pH stress. Front. Genet. 2021, 12, 675260. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-M.; Wang, Y.-Y.; Zhou, Y.-F.; Meng, X.; Huang, Z.-R.; Chen, L.-S.; Yang, L.-T. Analysis of interacting proteins of aluminum toxicity response factor ALS3 and CAD in Citrus. Int. J. Mol. Sci. 2019, 20, 4846. [Google Scholar] [CrossRef]
- Agrahari, R.K.; Kobayashi, Y.; Enomoto, T.; Miyachi, T.; Sakuma, M.; Fujita, M.; Ogata, T.; Fujita, Y.; Iuchi, S.; Kobayashi, M. STOP1-regulated SMALL AUXIN UP RNA55 (SAUR55) is involved in proton/malate co-secretion for Al tolerance in Arabidopsis. Plant Direct 2024, 8, e557. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Yang, C.; Cheng, Y.; Han, Z.; Cai, Z.; Nian, H.; Ma, Q. GsMAS1 encoding a MADS-box transcription factor enhances the tolerance to aluminum stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2004. [Google Scholar] [CrossRef]
- Jin, J.F.; Zhu, H.H.; He, Q.Y.; Li, P.F.; Fan, W.; Xu, J.M.; Yang, J.L.; Chen, W.W. The tomato transcription factor SlNAC063 is required for aluminum tolerance by regulating SlAAE3-1 expression. Front. Plant Sci. 2022, 13, 826954. [Google Scholar] [CrossRef]
- Lu, Z.; Qiu, W.; Jin, K.; Yu, M.; Han, X.; He, X.; Wu, L.; Wu, C.; Zhuo, R. Identification and analysis of bZIP family genes in Sedum plumbizincicola and their potential roles in response to cadmium stress. Front. Plant Sci. 2022, 13, 859386. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Wang, X.; Zhang, X.; Cheng, Y.; Cai, Z.; Nian, H.; Ma, Q. GmWRKY21, a soybean WRKY transcription factor gene, enhances the tolerance to aluminum stress in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 833326. [Google Scholar] [CrossRef]
- Huang, D.; Gong, Z.; Chen, X.; Wang, H.; Tan, R.; Mao, Y. Transcriptomic responses to aluminum stress in tea plant leaves. Sci. Rep. 2021, 11, 5800. [Google Scholar] [CrossRef] [PubMed]
- Cardon, G.H.; Höhmann, S.; Nettesheim, K.; Saedler, H.; Huijser, P. Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: A novel gene involved in the floral transition. Plant J. 1997, 12, 367–377. [Google Scholar] [CrossRef]
- Birkenbihl, R.P.; Jach, G.; Saedler, H.; Huijser, P. Functional dissection of the plant-specific SBP-domain: Overlap of the DNA-binding and nuclear localization domains. J. Mol. Biol. 2005, 352, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Baluska, F.; Matsumoto, H. Aluminum stress signaling in plants. Plant. Signal. Behav. 2009, 4, 592–597. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X. The Regulatory Effect of Salicylic Acid on the Physiology of Mentha under Aluminum Stress and Its Alleviation of DNA Damage. Open Access Libr. J. 2025, 12, 1–11. [Google Scholar] [CrossRef]
- Yan, J.; Zhu, W.; Wu, D.; Chen, X.; Yang, S.; Xue, Y.; Liu, Y. Mechanisms of Aluminum Toxicity Impacting Root Growth in Shatian Pomelo. Int. J. Mol. Sci. 2024, 25, 13454. [Google Scholar] [CrossRef]
- Chen, X. Small RNAs in development-insights from plants. Curr. Opin. Genet. Dev. 2012, 22, 361–367. [Google Scholar] [CrossRef]
- Sunkar, R.; Li, Y.F.; Jagadeeswaran, G. Functions of microRNAs in plant stress responses. Trends Plant Sci. 2012, 17, 196–203. [Google Scholar] [CrossRef]
- Bi, H.; Fei, Q.; Li, R.; Liu, B.; Xia, R.; Char, S.N.; Meyers, B.C.; Yang, B. Disruption of miRNA sequences by TALENs and CRISPR/Cas9 induces varied lengths of miRNA production. Plant Biotechnol. J. 2020, 18, 1526–1536. [Google Scholar] [CrossRef]
- Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.A.; Yu, D.Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, M.; Zhao, Y.; He, X.; Ding, C.; Wang, S.; Feng, Y.; Song, X.; Li, P.; Wang, B. Identification of salt tolerance-related microRNAs and their targets in maize (Zea mays L.) using high-throughput sequencing and degradome analysis. Front. Plant Sci. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Fu, R.; Xie, Y.; Chen, Q.; Wang, Y.; Li, Z.; Song, X.; Li, P.; Wang, B. Regulatory Mechanism of Maize (Zea mays L.) miR164 in Salt Stress Response. Russ. J. Genet. 2020, 56, 835–842. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Hiltz, D.; Norrie, J.; Prithiviraj, B. Ascophyllum nodosum extract mitigates salinity stress in Arabidopsis thaliana by modulating the expression of miRNA involved in stress tolerance and nutrient acquisition. PLoS ONE 2018, 13, e0206221. [Google Scholar] [CrossRef]
- Yue, E.; Tao, H.; Xu, J. Genome-wide analysis of microRNA156 and its targets, the genes encoding SQUAMOSA promoter-binding protein-like (SPL) transcription factors, in the grass family Poaceae. J. Zhejiang University. B Sci. 2021, 22, 366–382. [Google Scholar] [CrossRef]
- Long, J.M.; Liu, C.Y.; Feng, M.Q.; Liu, Y.; Wu, X.M.; Guo, W.W. Corrigendum: MiR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J. Exp. Bot. 2018, 69, 4141. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. MiR156/SPL10 modulates lateral root development, branching and leaf morphology in arabidopsis by silencing AGAMOUS-LIKE 79. Front. Plant Sci. 2018, 8, 2226. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. Corrigendum: Mir156/spl10 modulates lateral root development, branching and leaf morphology in arabidopsis by silencing AGAMOUS-LIKE 79. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Jiang, H.; Wang, H.; Chen, K.; Duan, J.; Feng, S.; Wu, G. Regulation of cadmium tolerance and accumulation by miR156 in Arabidopsis. Chemosphere 2020, 242, 125168. [Google Scholar] [CrossRef] [PubMed]
- Kuang, L.; Yu, J.; Shen, Q.; Fu, L.; Wu, L. Identification of micrornas responding to aluminium, cadmium and salt stresses in barley roots. Plants 2021, 10, 2754. [Google Scholar] [CrossRef]
- Liu, W.; Ji, X.; Cao, H.; Huo, C.; He, L.; Peng, X.; Yang, Y.; Yang, F.; Xiong, S. Comparative Transcriptome Analysis Reveals the Effect of miR156a Overexpression on Mineral Nutrient Homeostasis in Nicotiana tabacum. Plants 2023, 12, 1739. [Google Scholar] [CrossRef]
- Singh, C.K.; Singh, D.; Taunk, J.; Chaudhary, P.; Tomar, R.S.S.; Chandra, S.; Singh, D.; Pal, M.; Konjengbam, N.S.; Singh, M.P. Comparative inter-and intraspecies transcriptomics revealed key differential pathways associated with aluminium stress tolerance in lentil. Front. Plant Sci. 2021, 12, 693630. [Google Scholar] [CrossRef]
- You, J.; Zhang, H.; Liu, N.; Gao, L.; Kong, L.; Yang, Z.; Gustafson, P. Transcriptomic responses to aluminum stress in soybean roots. Genome 2011, 54, 923–933. [Google Scholar] [CrossRef]
- Eticha, D.; Zahn, M.; Bremer, M.; Yang, Z.; Rangel, A.F.; Rao, I.M.; Horst, W.J. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. 2010, 105, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Duressa, D.; Soliman, K.; Chen, D. Identification of aluminum responsive genes in Al-tolerant soybean line PI 416937. Int. J. Plant Genom. 2010, 2010, 164862. [Google Scholar] [CrossRef]
- Ye, J.; Wang, X.; Hu, T.; Zhang, F.; Wang, B.; Li, C.; Yang, T.; Li, H.; Lu, Y.; Giovannoni, J.J. An InDel in the promoter of Al-Activated Malate Transporter9 selected during tomato domestication determines fruit malate contents and aluminum tolerance. Plant Cell 2017, 29, 2249–2268. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, S.; Feng, J.; Chen, H.; Qi, X.; Wang, H.; Deng, Y. Identification of aluminum-activated malate transporters (ALMT) family genes in hydrangea and functional characterization of HmALMT5/9/11 under aluminum stress. PeerJ 2022, 10, e13620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, L.; Wang, H.; Xiao, J. Characterization of the heavy-metal-associated isoprenylated plant protein (HIPP) gene family from Triticeae species. Int. J. Mol. Sci. 2020, 21, 6191. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Wang, J.; Su, C.; Cui, Z.; Huang, L.; Gu, S.; Jiang, S.; Feng, J.; Xu, H.; Zhang, W.; Jiang, L. Transcriptomics and metabolomics reveal tolerance new mechanism of rice roots to Al stress. Front. Genet. 2023, 13, 1063984. [Google Scholar] [CrossRef]
- Liu, E.; MacMillan, C.P.; Shafee, T.; Ma, Y.; Ratcliffe, J.; Van de Meene, A.; Bacic, A.; Humphries, J.; Johnson, K.L. Fasciclin-like arabinogalactan-protein 16 (FLA16) is required for stem development in Arabidopsis. Front. Plant Sci. 2020, 11, 615392. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Wang, L.; Zhong, H.; Xu, X.; Cheng, Y.; Nian, H.; Liu, W.; Chen, P.; Zhang, A.; et al. GmABR1 encoding an ERF transcription factor enhances the tolerance to aluminum stress in Arabidopsis thaliana. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, R.; Shu, K.; Lv, W.; Wang, S.; Wang, C. Aluminum stress signaling, response, and adaptive mechanisms in plants. Plant. Signal. Behav. 2022, 17, 130. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, Y.; Huang, J.; Liu, Y.S.; Yang, X.Z.; Jing, H.K.; Shen, R.F.; Zhu, X.F. The MYB transcription factor MYB103 acts upstream of Trichome Birefringence-Like27 in regulating aluminum sensitivity by modulating the O-acetylation level of cell wall xyloglucan in Arabidopsis thaliana. Plant J. 2022, 111, 529–545. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.-P.; Yang, L.-T.; Lai, N.-W.; Ye, X.; Yang, Y.; Chen, L.-S. Root adaptive responses to aluminum-treatment revealed by RNA-Seq in two Citrus species with different aluminum-tolerance. Front. Plant Sci. 2017, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, R.; Yu, Y. GmMYB183, a R2R3-MYB Transcription Factor in Tamba Black Soybean (Glycine max. cv. Tamba), Conferred Aluminum Tolerance in Arabidopsis and Soybean. Biomolecules 2024, 14, 724. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, Z.Q.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.Q.; Li, G.X.; Wu, Y.R.; Yang, J.L.; Ma, J.F. Transcription factor WRKY 22 promotes aluminum tolerance via activation of Os FRDL 4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef]
- Li, C.X.; Yan, J.Y.; Ren, J.Y.; Sun, L.; Xu, C.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Biol. 2020, 62, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, H.; Chen, L.; Lin, J.; Wang, Z.; Pan, J.; Yang, F.; Ni, X.; Wang, Y.; Wang, Y.; et al. Multifaceted roles of WRKY transcription factors in abiotic stress and flavonoid biosynthesis. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Z.; Zhou, X. WRKY Transcription Factors in Response to Metal Stress in Plants: A Review. Int. J. Mol. Sci. 2024, 25, 10952. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Zhao, Q.; Gao, Y.; Xiang, Y.; Chai, J.; Li, Y.; Hou, X. Study on molecular response of alfalfa to low temperature stress based on transcriptomic analysis. BMC Plant Biol. 2024, 24, 1244. [Google Scholar] [CrossRef]
- Moreno-Alvarado, M.; García-Morales, S.; Trejo-Téllez, L.I.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Aluminum enhances growth and sugar concentration, alters macronutrient status and regulates the expression of NAC transcription factors in rice. Front. Plant Sci. 2017, 8, 73. [Google Scholar] [CrossRef]
- Liu, J.; Magalhaes, J.V.; Shaff, J.; Kochian, L.V. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tian, Y. STOP1 and STOP1-like proteins, key transcription factors to cope with acid soil syndrome. Front. Plant Sci. 2023, 14, 1200139. [Google Scholar] [CrossRef] [PubMed]
- Barros, V.A.; Chandnani, R.; De Sousa, S.M.; Maciel, L.S.; Tokizawa, M.; Guimaraes, C.T.; Magalhaes, J.V.; Kochian, L.V. Root adaptation via common genetic factors conditioning tolerance to multiple stresses for crops cultivated on acidic tropical soils. Front. Plant Sci. 2020, 11, 565339. [Google Scholar] [CrossRef]
- An, Y.-M.; Song, L.-L.; Liu, Y.-R.; Shu, Y.-J.; Guo, C.-H. De novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, H.; Wang, S.; Yu, D.; Wei, Y. Physiological and Transcriptomic Analysis Reveals That Melatonin Alleviates Aluminum Toxicity in Alfalfa (Medicago sativa L.). Int. J. Mol. Sci. 2023, 24, 17221. [Google Scholar] [CrossRef]
- Zhou, P.; Su, L.; Lv, A.; Wang, S.; Huang, B.; An, Y. Gene expression analysis of alfalfa seedlings response to acid-aluminum. Int. J. Genom. 2016, 2016, 2095195. [Google Scholar] [CrossRef]
- Wang, P.; Hsu, C.C.; Du, Y.; Zhu, P.; Zhao, C.; Fu, X.; Zhang, C.; Paez, J.S.; Macho, A.P.; Andy Tao, W.; et al. Mapping proteome-wide targets of protein kinases in plant stress responses. Proc. Natl. Acad. Sci. USA 2020, 117, 3270–3280. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, Q.; Zhu, X.; Wang, B.; Wei, B.; Wei, X. Identification of Alfalfa SPL gene family and expression analysis under biotic and abiotic stresses. Sci. Rep. 2023, 13, 84. [Google Scholar] [CrossRef]
- Ding, Z.J.; Xu, C.; Yan, J.Y.; Wang, Y.X.; Cui, M.Q.; Yuan, J.J.; Wang, Y.N.; Li, G.X.; Wu, J.X.; Wu, Y.R. The LRR receptor-like kinase ALR1 is a plant aluminum ion sensor. Cell Res. 2024, 34, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Y.; Zhu, X.; Zhang, N.; Ul-Ain, N.; Raza, A.; Haider, F.U.; Si, H. Harnessing the role of mitogen-activated protein kinases against abiotic stresses in plants. Front. Plant Sci. 2023, 14, 932923. [Google Scholar] [CrossRef] [PubMed]
- Daspute, A.A.; Sadhukhan, A.; Tokizawa, M.; Kobayashi, Y.; Panda, S.K.; Koyama, H. Transcriptional regulation of aluminum-tolerance genes in higher plants: Clarifying the underlying molecular mechanisms. Front. Plant Sci. 2017, 8, 1358. [Google Scholar] [CrossRef]
- Huang, C.F.; Ma, Y. Aluminum resistance in plants: A critical review focusing on STOP1. Plant Commun. 2025, 6, 101200. [Google Scholar] [CrossRef]
- Liu, C.; Hu, X.; Zang, L.; Liu, X.; Wei, Y.; Wang, X.; Jin, X.; Du, C.; Yu, Y.; He, W. Overexpression of ZmSTOP1-A Enhances Aluminum Tolerance in Arabidopsis by Stimulating Organic Acid Secretion and Reactive Oxygen Species Scavenging. Int. J. Mol. Sci. 2023, 24, 15669. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant 2021, 173, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [CrossRef]
- Mundade, R.; Ozer, H.G.; Wei, H.; Prabhu, L.; Lu, T. Role of ChIP-seq in the discovery of transcription factor binding sites, differential gene regulation mechanism, epigenetic marks and beyond. Cell Cycle 2014, 13, 2847–2852. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, H.; Zhang, M.; Deng, S.; Li, T.; Xing, S. Genome-wide identification and characterization of SPL family genes in Chenopodium quinoa. Genes 2022, 13, 1455. [Google Scholar] [CrossRef]
- Martin, R.C.; Vining, K.; Dombrowski, J.E. Genome-wide (ChIP-seq) identification of target genes regulated by BdbZIP10 during paraquat-induced oxidative stress. BMC Plant Biol. 2018, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, B.A.; Renaud, J.; Nasrollahi, V.; Kohalmi, S.E.; Hannoufa, A. Transcriptome-IPMS analysis reveals a tissue-dependent miR156/SPL13 regulatory mechanism in alfalfa drought tolerance. BMC Genom. 2020, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Allelign Ashagre, H.; Zaltzman, D.; Idan-Molakandov, A.; Romano, H.; Tzfadia, O.; Harpaz-Saad, S. FASCICLIN-LIKE 18 is a new player regulating root elongation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 645286. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by his Majesty the King in Right of Canada as represented by the Minister of Agriculture and Agri-Food Canada. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, G.; Sakariyahu, S.K.; Shan, B.; Aung, B.; McDowell, T.; Papadopoulos, Y.; Bernards, M.A.; Hannoufa, A. Aluminum Stress Response Is Regulated Through a miR156/SPL13 Module in Medicago sativa. Genes 2025, 16, 751. https://doi.org/10.3390/genes16070751
Allam G, Sakariyahu SK, Shan B, Aung B, McDowell T, Papadopoulos Y, Bernards MA, Hannoufa A. Aluminum Stress Response Is Regulated Through a miR156/SPL13 Module in Medicago sativa. Genes. 2025; 16(7):751. https://doi.org/10.3390/genes16070751
Chicago/Turabian StyleAllam, Gamalat, Solihu K. Sakariyahu, Binghui Shan, Banyar Aung, Tim McDowell, Yousef Papadopoulos, Mark A. Bernards, and Abdelali Hannoufa. 2025. "Aluminum Stress Response Is Regulated Through a miR156/SPL13 Module in Medicago sativa" Genes 16, no. 7: 751. https://doi.org/10.3390/genes16070751
APA StyleAllam, G., Sakariyahu, S. K., Shan, B., Aung, B., McDowell, T., Papadopoulos, Y., Bernards, M. A., & Hannoufa, A. (2025). Aluminum Stress Response Is Regulated Through a miR156/SPL13 Module in Medicago sativa. Genes, 16(7), 751. https://doi.org/10.3390/genes16070751






