Functional and Regulatory Effects of Factor V Leiden and Factor V rs6028 in Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Expression Vector and Mutagenesis
2.3. Transient Transfection and Harvesting of mRNA and Protein Lysates
2.4. Quantitative Measurement of F5 mRNA
2.5. Measurement of FV Protein and FV Coagulant Activity
2.6. Detection of Apoptosis
2.7. In Silico Analyses
2.8. Statistical Analyses
3. Results and Discussion
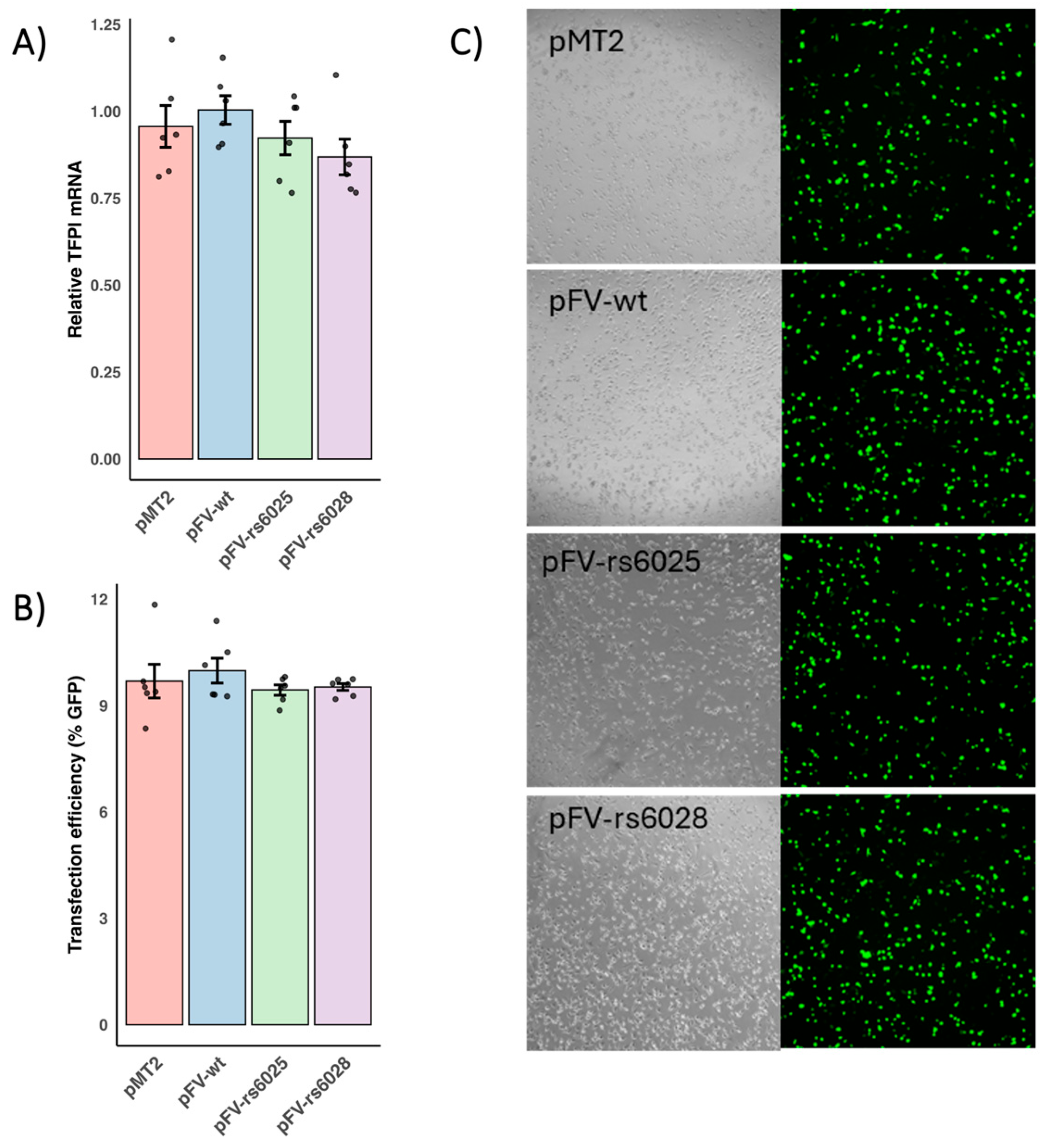
3.1. Functional Effects of F5 Variants on FV Production, Secretion, and Coagulant Activity
3.2. Functional Effects of F5 Variants on Apoptosis
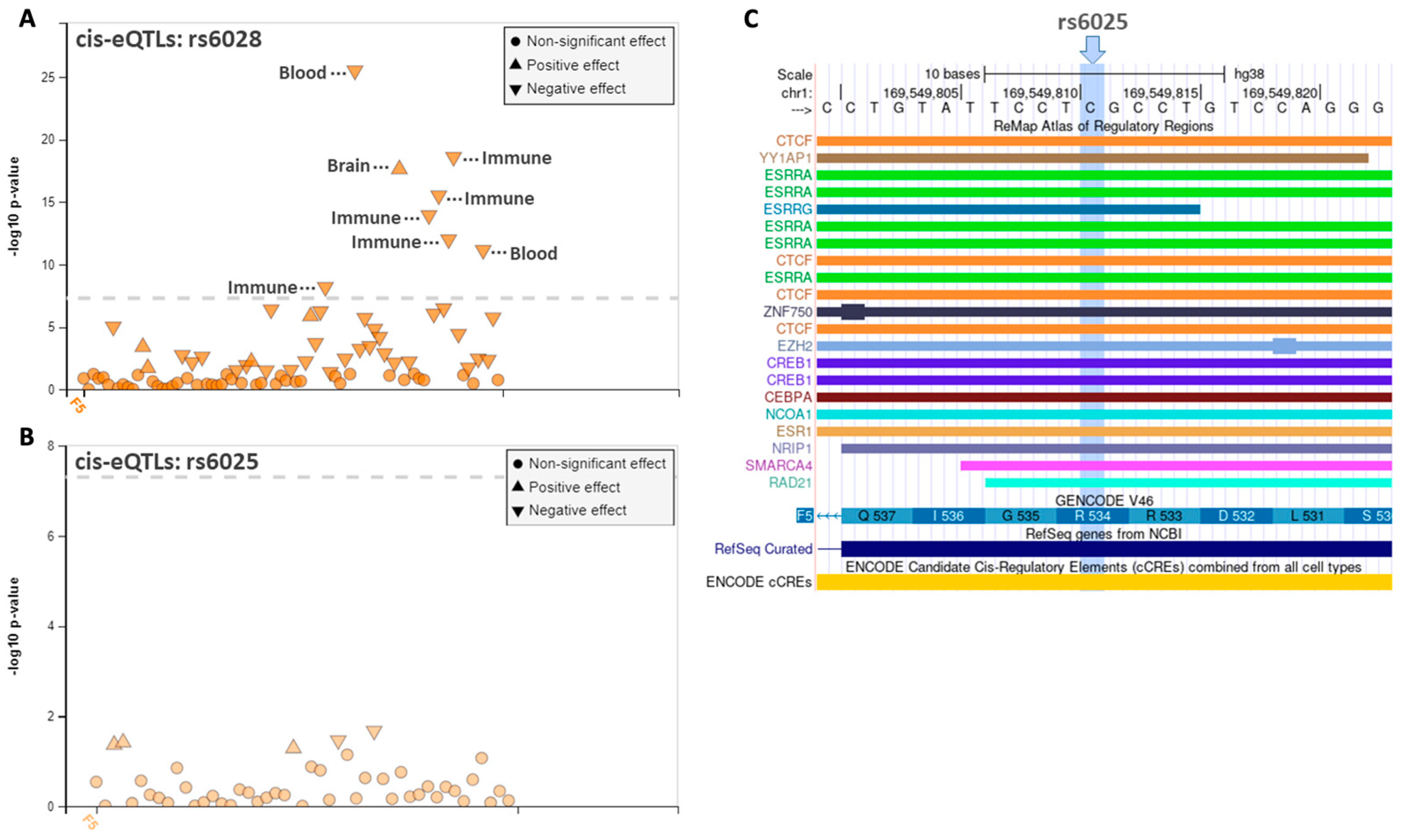
3.3. Regulatory Annotation Analysis of the F5 Variants
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Activated protein C |
| eQTL | Expression quantitative trait locus |
| F5 | Coagulation factor V gene |
| F5 rs6025 | FV Leiden |
| FV | Coagulation factor V |
| FVa | Activated coagulation factor V |
| GTEx | Gene–tissue expression |
| LD | Linkage disequilibrium |
| PMM1 | Phosphomannomutase 1 |
| pFV-rs6025 | pMT2-FV-rs6025 |
| pFV-rs6028 | pMT2-FV-rs6028 |
| qPCR | Quantitative RT-PCR |
| RQ | Relative mRNA quantification |
| SNPs | Single nucleotide polymorphisms |
| wt | Wild type |
| ΔΔCT | Comparative CT method |
| ESR1 | Estrogen receptor 1 |
| CTCF | CCCTC-binding factor |
| YY1AP1 | YY1 Associated protein 1 |
| ESRRA | Estrogen-related receptor alpha |
| ESRRG | Estrogen-related receptor gamma |
| ZNF750 | Zinc finger protein 750 |
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit |
| CREB1 | CAMP responsive element binding protein 1 |
| CEBPA | CCAAT enhancer binding protein alpha |
| NCOA1 | Nuclear receptor coactivator 1 |
| NRIP1 | Nuclear receptor interacting protein 1 |
| SMARCA4 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 4 |
| RAD21 | RAD21 cohesin complex component |
| NCBI | The national center for biotechnology information |
Appendix A
References
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Soff, G. Thrombosis and Hemostasis in Cancer; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Hisada, Y.; Mackman, N. Tissue factor and cancer: Regulation, tumor growth, and metastasis. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2019. [Google Scholar]
- Cramer, T.J.; Griffin, J.H.; Gale, A.J. Factor V is an anticoagulant cofactor for activated protein C during inactivation of factor Va. Pathophysiol. Haemost. Thromb. 2010, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lind, S.M.; Sletten, M.; Hellenes, M.; Mathelier, A.; Tekpli, X.; Tinholt, M.; Iversen, N. Coagulation factor V in breast cancer: A p53 regulated tumor suppressor and predictive marker for treatment response to chemotherapy. J. Thromb. Haemost. 2024, 22, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Tinholt, M.; Garred, Ø.; Borgen, E.; Beraki, E.; Schlichting, E.; Kristensen, V.; Sahlberg, K.; Iversen, N. Subtype-specific clinical and prognostic relevance of tumor-expressed F5 and regulatory F5 variants in breast cancer: The CoCaV study. J. Thromb. Haemost. 2018, 16, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Mast, A.E.; Ruf, W. Regulation of coagulation by tissue factor pathway inhibitor: Implications for hemophilia therapy. J. Thromb. Haemost. 2022, 20, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Albagoush, S.A.; Koya, S.; Chakraborty, R.K.; Schmidt, A.E. Factor V Leiden Mutation, in StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Pabinger, I.; Ay, C.; Dunkler, D.; Thaler, J.; Reitter, E.M.; Marosi, C.; Zielinski, C.; Mannhalter, C. Factor V Leiden mutation increases the risk for venous thromboembolism in cancer patients–results from the Vienna Cancer And Thrombosis Study (CATS). J. Thromb. Haemost. 2015, 13, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Vossen, C.Y.; Hoffmeister, M.; Chang-Claude, J.C.; Rosendaal, F.R.; Brenner, H. Clotting Factor Gene Polymorphisms and Colorectal Cancer Risk. J. Clin. Oncol. 2011, 29, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Paspatis, G.A.; Sfyridaki, A.; Papanikolaou, N.; Triantafyllou, K.; Livadiotaki, A.; Kapsoritakis, A.; Lydataki, N. Resistance to activated protein C, factor V leiden and the prothrombin G20210A variant in patients with colorectal cancer. Pathophysiol. Haemost. Thromb. 2002, 32, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Battistelli, S.; Stefanoni, M.; Genovese, A.; Vittoria, A.; Cappelli, R.; Roviello, F. Prevalence of factor V Leiden and prothrombin G20210A in patients with gastric cancer. World J. Gastroenterol. WJG 2006, 12, 4179. [Google Scholar] [CrossRef] [PubMed]
- Pihusch, R.; Danzl, G.; Scholz, M.; Harich, D.; Pihusch, M.; Lohse, P.; Hiller, E. Impact of thrombophilic gene mutations on thrombosis risk in patients with gastrointestinal carcinoma. Cancer 2002, 94, 3120–3126. [Google Scholar] [CrossRef] [PubMed]
- Tormene, D.; Beltramello, P.; Perlati, M.; Brandolin, B.; Barbar, S.; De Toffoli, G.; Simioni, P. The risk of cancer progression in women with gynecological malignancies and thrombophilic polymorphisms: A pilot case-control study. Clin. Appl. Thromb./Hemost. 2009, 15, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Vairaktaris, E.; Yapijakis, C.; Wiltfang, J.; Ries, J.; Vylliotis, A.; Derka, S.; Vasiliou, S.; Neukam, F.W. Are factor V and prothrombin mutations associated with increased risk of oral cancer? Anticancer. Res. 2005, 25, 2561–2565. [Google Scholar] [PubMed]
- Tinholt, M.; Viken, M.K.; Dahm, A.E.; Vollan, H.K.M.; Sahlberg, K.K.; Garred, Ø.; Børresen-Dale, A.-L.; Jacobsen, A.F.; Kristensen, V.; Bukholm, I. Increased coagulation activity and genetic polymorphisms in the F5, F10 and EPCR genes are associated with breast cancer: A case-control study. Bmc Cancer 2014, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.; Sauna, Z.E.; Ambudkar, S.V.; Gottesman, M.M.; Kimchi-Sarfaty, C. Silent (synonymous) SNPs: Should we care about them? Single Nucleotide Polymorph. Methods Protoc. 2009, 578, 23–39. [Google Scholar] [CrossRef]
- Hammal, F.; De Langen, P.; Bergon, A.; Lopez, F.; Ballester, B. ReMap 2022: A database of Human, Mouse, Drosophila and Arabidopsis regulatory regions from an integrative analysis of DNA-binding sequencing experiments. Nucleic Acids Res. 2022, 50, D316–D325. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Kwong, A.; Boughton, A.P.; Wang, M.; VandeHaar, P.; Boehnke, M.; Abecasis, G.; Kang, H.M. FIVEx: An interactive eQTL browser across public datasets. Bioinformatics 2022, 38, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Rennert, H.; DeSimone, R.A. Molecular testing for factor V Leiden and prothrombin gene mutations in inherited thrombophilia. In Transfusion Medicine and Hemostasis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 903–906. [Google Scholar]
- Stein, R.A.; Chang, C.-Y.; Kazmin, D.A.; Way, J.; Schroeder, T.; Wergin, M.; Dewhirst, M.W.; McDonnell, D.P. Estrogen-related receptor α is critical for the growth of estrogen receptor–negative breast cancer. Cancer Res. 2008, 68, 8805–8812. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Catalá, C.F.; Gretton, S.; Vostrov, A.; Pugacheva, E.; Farrar, D.; Ito, Y.; Docquier, F.; Kita, G.-X.; Murrell, A.; Lobanenkov, V. A novel mechanism for CTCF in the epigenetic regulation of Bax in breast cancer cells. Neoplasia 2013, 15, 898–912, IN5–IN14. [Google Scholar] [CrossRef] [PubMed]
- Docquier, F.; Farrar, D.; D’Arcy, V.; Chernukhin, I.; Robinson, A.F.; Loukinov, D.; Vatolin, S.; Pack, S.; Mackay, A.; Harris, R.A. Heightened expression of CTCF in breast cancer cells is associated with resistance to apoptosis. Cancer Res. 2005, 65, 5112–5122. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lind, S.M.; Sletten, M.; Henriksson, C.E.; Tinholt, M.; Iversen, N. Functional and Regulatory Effects of Factor V Leiden and Factor V rs6028 in Breast Cancer. Genes 2025, 16, 735. https://doi.org/10.3390/genes16070735
Lind SM, Sletten M, Henriksson CE, Tinholt M, Iversen N. Functional and Regulatory Effects of Factor V Leiden and Factor V rs6028 in Breast Cancer. Genes. 2025; 16(7):735. https://doi.org/10.3390/genes16070735
Chicago/Turabian StyleLind, Sara Marie, Marit Sletten, Carola Elisabeth Henriksson, Mari Tinholt, and Nina Iversen. 2025. "Functional and Regulatory Effects of Factor V Leiden and Factor V rs6028 in Breast Cancer" Genes 16, no. 7: 735. https://doi.org/10.3390/genes16070735
APA StyleLind, S. M., Sletten, M., Henriksson, C. E., Tinholt, M., & Iversen, N. (2025). Functional and Regulatory Effects of Factor V Leiden and Factor V rs6028 in Breast Cancer. Genes, 16(7), 735. https://doi.org/10.3390/genes16070735





