Evaluating the Diagnostic Utility of dd-cfDNA in Renal Allograft Surveillance: A Single-Center Perspective
Abstract
1. Introduction
2. Materials and Methods
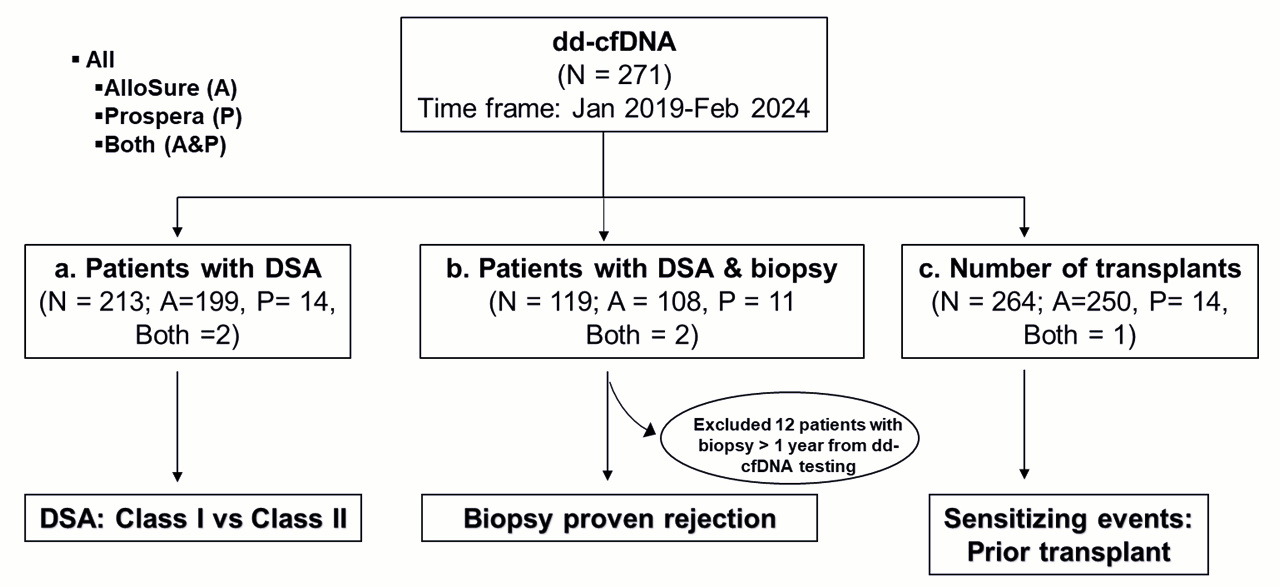
2.1. Data Collection and Division into Subgroups
2.2. Demographic Description
2.3. HLA Antibody Screening and Histocompatibility Testing
2.4. Donor-Derived Cell-Free DNA (dd-cfDNA) Assay
2.5. Biopsy
2.6. Statistical Analysis
2.7. Evaluation of Diagnostic Test Utility
3. Results
3.1. Study Demographic
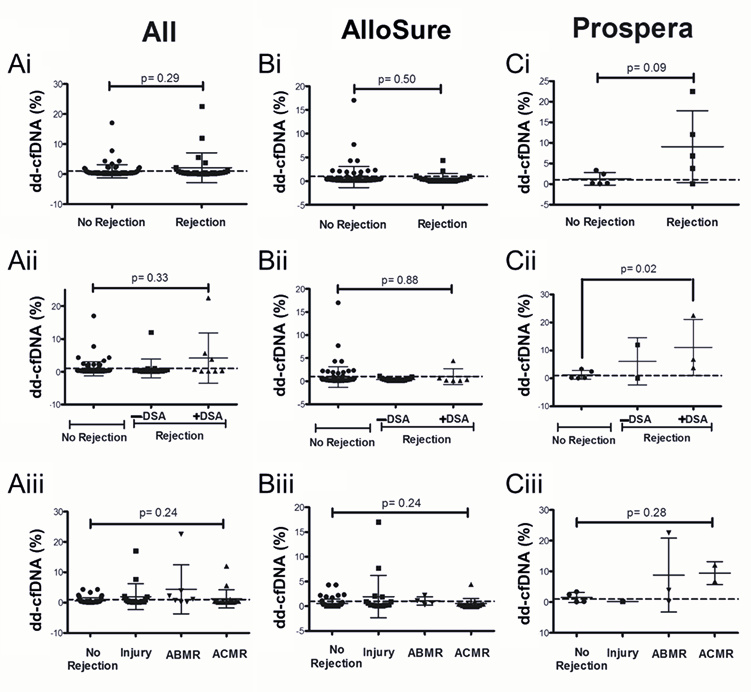
3.2. Dd-cfDNA Is Elevated in Patients with DSA
3.3. Dd-cfDNA Levels Show Limited Correlation with Biopsy-Proven Rejection
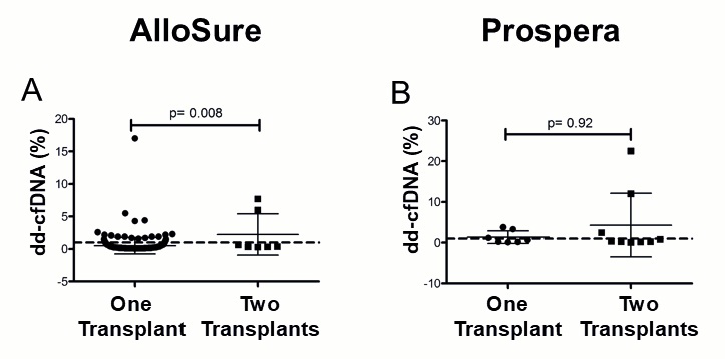
3.4. Multi-Organ Transplants Elevate dd-cfDNA in a Vendor-Specific Manner
3.5. Diagnostic Utility Varies Between dd-cfDNA Vendors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOT | Multi-organ transplantation |
| Dd-cfDNA | Donor-derived cell-free DNA |
| DSA | Donor-specific antibody |
| NGS | Next-generation sequencing |
| ABMR | Antibody mediated rejection |
| ACMR | Acute cellular rejection |
| AUC | Area under the curve |
| SNPs | Single nucleotide polymorphisms |
References
- Oellerich, M.; Budde, K.; Osmanodja, B.; Bornemann-Kolatzki, K.; Beck, J.; Schütz, E.; Walson, P.D. Donor-derived cell-free DNA as a diagnostic tool in transplantation. Front. Genet. 2022, 13, 1031894. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Gröne, H.-J.; Friede, T. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Am. J. Transplant. 2019, 19, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Brunet, M.; Shipkova, M.; Van Gelder, T.; Wieland, E.; Sommerer, C.; Budde, K.; Haufroid, V.; Christians, U.; López-Hoyos, M.; Barten, M.J. Barcelona consensus on biomarker-based immunosuppressive drugs management in solid organ transplantation. Ther. Drug Monit. 2016, 38, S1–S20. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.A.; Budde, K.; Jilma, B.; Doberer, K.; Boehmig, G.A. Emerging drugs for antibody-mediated rejection after kidney transplantation: A focus on phase II & III trials. Expert Opin. Emerg. Drugs 2022, 27, 151–167. [Google Scholar]
- Ghonge, N.P.; Goyal, N.; Vohra, S.; Chowdhury, V. Renal transplant evaluation: Multimodality imaging of post-transplant complications. Br. J. Radiol. 2021, 94, 20201253. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, A.; Calderon-Zapata, A.; Gomez-Montero, A.; Lozano-Suarez, N.; Giron-Luque, F. The value of protocol biopsy in kidney transplantation on monitoring transplant outcomes: A systematic review and meta-analysis. Transplant. Proc. 2024, 56, 1231–1240. [Google Scholar] [CrossRef]
- Whitlam, J.B.; Ling, L.; Skene, A.; Kanellis, J.; Ierino, F.L.; Slater, H.R.; Bruno, D.L.; Power, D.A. Diagnostic application of kidney allograft-derived absolute cell-free DNA levels during transplant dysfunction. Am. J. Transplant. 2019, 19, 1037–1049. [Google Scholar] [CrossRef]
- Chopra, B.; Sureshkumar, K.K. Emerging role of cell-free DNA in kidney transplantation. World J. Exp. Med. 2021, 11, 55. [Google Scholar] [CrossRef]
- Oellerich, M.; Sherwood, K.; Keown, P.; Schütz, E.; Beck, J.; Stegbauer, J.; Rump, L.C.; Walson, P.D. Liquid biopsies: Donor-derived cell-free DNA for the detection of kidney allograft injury. Nat. Rev. Nephrol. 2021, 17, 591–603. [Google Scholar] [CrossRef]
- Jiménez-Coll, V.; El kaaoui El band, J.; Llorente, S.; González-López, R.; Fernández-González, M.; Martínez-Banaclocha, H.; Galián, J.A.; Botella, C.; Moya-Quiles, M.R.; Minguela, A. All that glitters in cfDNA analysis is not gold or its utility is completely established due to graft damage: A critical review in the field of transplantation. Diagnostics 2023, 13, 1982. [Google Scholar] [CrossRef]
- Edwards, R.L.; Menteer, J.; Lestz, R.M.; Baxter-Lowe, L.A. Cell-free DNA as a solid-organ transplant biomarker: Technologies and approaches. Biomark. Med. 2022, 16, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Grskovic, M.; Hiller, D.J.; Eubank, L.A.; Sninsky, J.J.; Christopherson, C.; Collins, J.P.; Thompson, K.; Song, M.; Wang, Y.S.; Ross, D. Validation of a clinical-grade assay to measure donor-derived cell-free DNA in solid organ transplant recipients. J. Mol. Diagn. 2016, 18, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.M.; Nocon, A.; Held, S.A.; Kobilay, M.; Skowasch, D.; Bronkhorst, A.J.; Ungerer, V.; Fredebohm, J.; Diehl, F.; Holdenrieder, S. Pre-analytical evaluation of Streck Cell-Free DNA blood collection tubes for liquid profiling in oncology. Diagnostics 2023, 13, 1288. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; Farber, J.L. The monitoring of donor-derived cell-free DNA in kidney transplantation. Transplantation 2021, 105, 509–516. [Google Scholar] [CrossRef]
- Paul, R.S.; Almokayad, I.; Collins, A.; Raj, D.; Jagadeesan, M. Donor-derived cell-free DNA: Advancing a novel assay to new heights in renal transplantation. Transplant. Direct 2021, 7, e664. [Google Scholar] [CrossRef] [PubMed]
- Lum, E.L.; Nieves-Borrero, K.; Homkrailas, P.; Lee, S.; Danovitch, G.; Bunnapradist, S. Single center experience comparing two clinically available donor derived cell free DNA tests and review of literature. Transplant. Rep. 2021, 6, 100079. [Google Scholar] [CrossRef]
- Reddy, T.; Esmail, A.; Chang, J.C.; Ghobrial, R.M.; Abdelrahim, M. Utility of cell-free DNA detection in transplant oncology. Cancers 2022, 14, 743. [Google Scholar] [CrossRef]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Am. J. Transplant. 2019, 19, 1663–1670. [Google Scholar] [CrossRef]
- Jordan, S.C.; Bunnapradist, S.; Bromberg, J.S.; Langone, A.J.; Hiller, D.; Yee, J.P.; Sninsky, J.J.; Woodward, R.N.; Matas, A.J. Donor-derived cell-free DNA identifies antibody-mediated rejection in donor specific antibody positive kidney transplant recipients. Transplant. Direct 2018, 4, e379. [Google Scholar] [CrossRef]
- Altuğ, Y.; Liang, N.; Ram, R.; Ravi, H.; Ahmed, E.; Brevnov, M.; Swenerton, R.K.; Zimmermann, B.; Malhotra, M.; Demko, Z.P. Analytical validation of a single-nucleotide polymorphism-based donor-derived cell-free DNA assay for detecting rejection in kidney transplant patients. Transplantation 2019, 103, 2657–2665. [Google Scholar] [CrossRef]
- Jang, M.K.; Tunc, I.; Berry, G.J.; Marboe, C.; Kong, H.; Keller, M.B.; Shah, P.D.; Timofte, I.; Brown, A.W.; Ponor, I.L. Donor-derived cell-free DNA accurately detects acute rejection in lung transplant patients, a multicenter cohort study. J. Heart Lung Transplant. 2021, 40, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.A.; Doberer, K.; Tillgren, A.; Viard, T.; Haindl, S.; Krivanec, S.; Reindl-Schwaighofer, R.; Eder, M.; Eskandary, F.; Casas, S. Diagnostic value of donor-derived cell-free DNA to predict antibody-mediated rejection in donor-specific antibody-positive renal allograft recipients. Transpl. Int. 2021, 34, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A. Cell-free DNA and active rejection in kidney allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Frischknecht, L.; Deng, Y.; Wehmeier, C.; de Rougemont, O.; Villard, J.; Ferrari-Lacraz, S.; Golshayan, D.; Gannage, M.; Binet, I.; Wirthmueller, U. The impact of pre-transplant donor specific antibodies on the outcome of kidney transplantation–Data from the Swiss transplant cohort study. Front. Immunol. 2022, 13, 1005790. [Google Scholar] [CrossRef]
- DeVos, J.M.; Gaber, A.O.; Knight, R.J.; Land, G.A.; Suki, W.N.; Gaber, L.W.; Patel, S.J. Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int. 2012, 82, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.; Mounsey, K.; Hughes, P.; Cullen, K.; Smith, M.W.; Daga, S.; Carter, C.; Clark, B.; Baker, R. Isolated pre-existing HLA-DP donor-specific antibodies are associated with poorer outcomes in renal transplantation. Kidney Int. Rep. 2022, 7, 2251–2263. [Google Scholar] [CrossRef]
- Xiao, H.; Gao, F.; Pang, Q.; Xia, Q.; Zeng, X.; Peng, J.; Fan, L.; Liu, J.; Wang, Z.; Li, H. Diagnostic accuracy of donor-derived cell-free DNA in renal-allograft rejection: A meta-analysis. Transplantation 2021, 105, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.K.; Archila, F.A.; Constantin, T.; Prins, S.A.; Liberto, J.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.P. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef]
- Keller, M.B.; Meda, R.; Fu, S.; Yu, K.; Jang, M.K.; Charya, A.; Berry, G.J.; Marboe, C.C.; Kong, H.; Luikart, H. Comparison of donor-derived cell-free DNA between single versus double lung transplant recipients. Am. J. Transplant. 2022, 22, 2451–2457. [Google Scholar] [CrossRef]
- Moeller, C.M.; Oren, D.; Fernandez Valledor, A.; Rubinstein, G.; Lotan, D.; Mehlman, Y.; Slomovich, S.; Rahman, S.; Lee, C.; Baranowska, J. Clinical Utility of Donor-Derived Cell-Free DNA in Heart Transplant Recipients with Multi-Organ Transplants. Clin. Transplant. 2024, 38, e15479. [Google Scholar] [CrossRef]
- Melancon, J.K.; Khalil, A.; Lerman, M.J. Donor-derived cell free DNA: Is it all the same? Kidney360 2020, 1, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Stites, E.; Kumar, D.; Olaitan, O.; John Swanson, S.; Leca, N.; Weir, M.; Bromberg, J.; Melancon, J.; Agha, I.; Fattah, H. High levels of dd-cfDNA identify patients with TCMR 1A and borderline allograft rejection at elevated risk of graft injury. Am. J. Transplant. 2020, 20, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Garg, N. Donor-Derived Cell-Free DNA: Is It All the Same? The Jury Is Still Out. Kidney360 2020, 1, 1038–1039. [Google Scholar] [CrossRef] [PubMed]


| Demographic DSA (n = 271) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Race (%) | Sex (%) | Age (Years) | Organ Type (%) | Donor Type (%) | |||||
| White/Caucasian | 61.5 | Male | 58 | Mean | 53.4 | Kidney | 92.7 | Living | 40.5 |
| Black/African American | 16.8 | Female | 41.6 | Minimum | 1.3 | Pancreas | 1.1 | Deceased | 59.5 |
| Hispanic/Latino | 1.9 | Other | 0.4 | Maximum | 78.4 | KP | 2.7 | ||
| Asian | 10.7 | KL | 1.5 | ||||||
| Middle Eastern | 0.4 | KLu | 0.7 | ||||||
| Indigenous American/ Alaskan Native | 1.9 | KH | 1.1 | ||||||
| Mixed | 5.7 | ||||||||
| Other | 1.1 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravamudhan, A.; Krug, K.; Stoffel, M.; Muluhngwi, P. Evaluating the Diagnostic Utility of dd-cfDNA in Renal Allograft Surveillance: A Single-Center Perspective. Genes 2025, 16, 724. https://doi.org/10.3390/genes16070724
Aravamudhan A, Krug K, Stoffel M, Muluhngwi P. Evaluating the Diagnostic Utility of dd-cfDNA in Renal Allograft Surveillance: A Single-Center Perspective. Genes. 2025; 16(7):724. https://doi.org/10.3390/genes16070724
Chicago/Turabian StyleAravamudhan, Aja, Kira Krug, Michelle Stoffel, and Penn Muluhngwi. 2025. "Evaluating the Diagnostic Utility of dd-cfDNA in Renal Allograft Surveillance: A Single-Center Perspective" Genes 16, no. 7: 724. https://doi.org/10.3390/genes16070724
APA StyleAravamudhan, A., Krug, K., Stoffel, M., & Muluhngwi, P. (2025). Evaluating the Diagnostic Utility of dd-cfDNA in Renal Allograft Surveillance: A Single-Center Perspective. Genes, 16(7), 724. https://doi.org/10.3390/genes16070724






