The Dynamic Remodeling of Plant Cell Wall in Response to Heat Stress
Abstract
1. Introduction
2. Structural and Mechanical Properties of Plant Cell Walls
2.1. Structural Properties of the Plant Cell Wall
2.2. Mechanical Properties of Plant Cell Wall
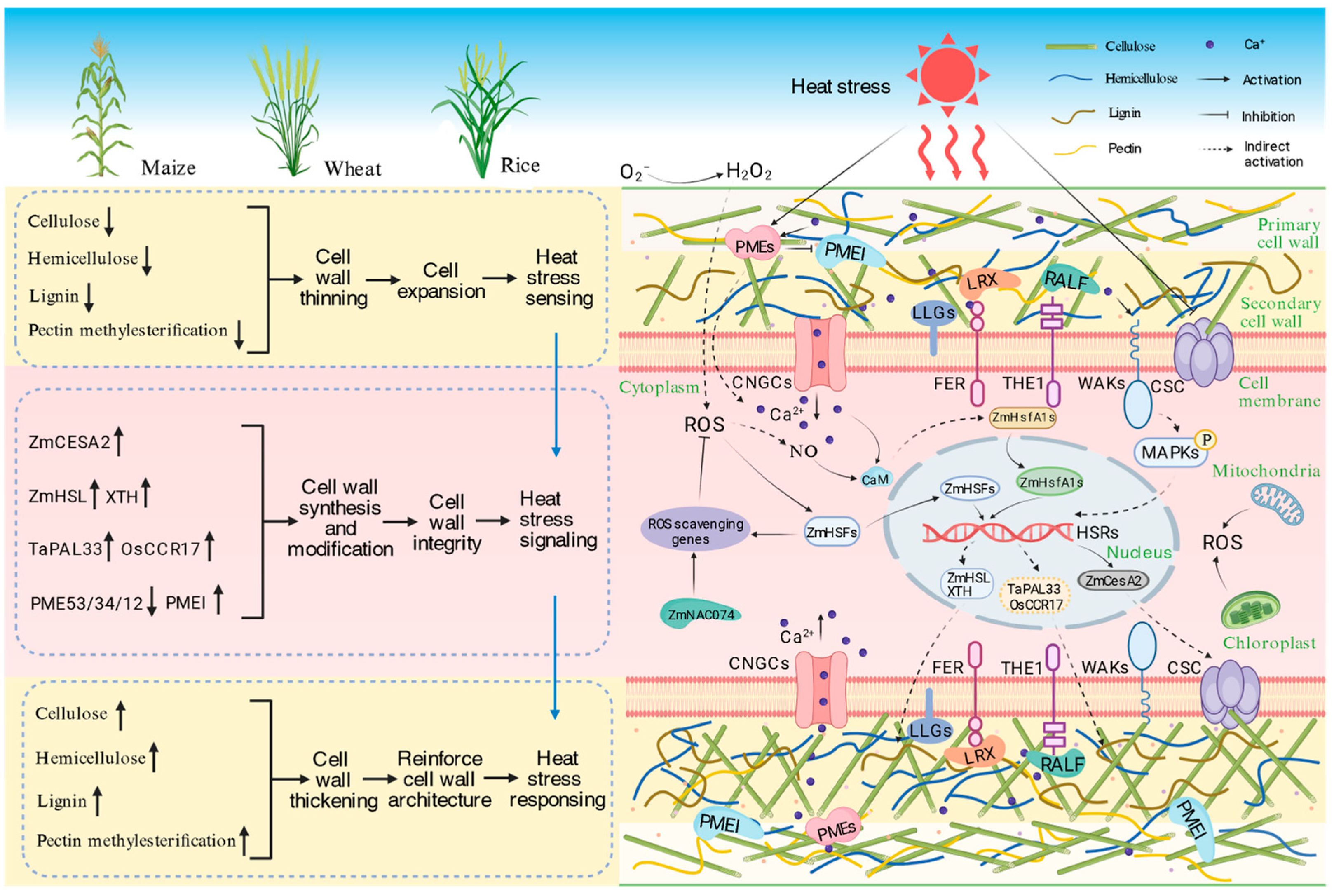
3. Cell Wall Expansion Mediates Heat Stress Sensing in Plants
4. Cell Wall Thickening as an Adaptive Response to Heat Stress
5. The Regulation of Heat Stress Response by Maintaining Cell Wall Integrity
5.1. The Role of Cell Wall Integrity in Signaling Heat Stress
5.2. Perception of the Change in Cell Wall Integrity
5.3. Molecular Mechanisms Underlying the Maintenance of Cell Wall Integrity Under Heat Stress
5.3.1. Transcriptional Regulation
5.3.2. Cross-Regulation of Hormone Signaling
6. Conclusions and Perspective
6.1. Which Components of the Plant Cell Wall Are Crucial for Responding to Heat Stress, and How Do the Mechanisms Differ Among Various Plant Species?
6.2. What Are the Sensing Mechanisms of Plant Cell Wall Integrity Under Heat Stress?
6.3. How Does the Plant Cell Wall Balance Growth and Stress Resistance Under Heat Stress?
6.4. Genetic Engineering of Cell Walls to Augment Plant Heat Stress Adaptation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Rather, S.A.; Wani, S.H.; Elrys, A.S.; Bilal, M.; Huang, Q.; Dar, Z.A.; Elashtokhy, M.M.A.; Soaud, N.; Koul, M.; et al. Heat stress-mediated constraints in maize (Zea mays) production: Challenges and solutions. Front. Plant Sci. 2022, 13, 879366. [Google Scholar] [CrossRef]
- Khan, A.H.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature stress in crops: Male sterility, yield loss and potential remedy approaches. Plant Biotechnol. J. 2023, 21, 680–697. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Ruan, M.; Zhao, H.; Wen, Y.; Chen, H.; He, F.; Hou, X.; Song, X.; Jiang, H.; Ruan, Y.L.; Wu, L. The complex transcriptional regulation of heat stress response in maize. Stress Biol. 2024, 4, 24. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Xuan, H. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult. 2018, 132, 413–424. [Google Scholar] [CrossRef]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Pegoraro, C.; Mertz, L.M.; Da Maia, L.C.; Rombaldi, C.V.; De Oliveira, A.C. Importance of heat shock proteins in maize. J. Crop Sci. Biotechnol. 2011, 14, 85–95. [Google Scholar] [CrossRef]
- Yang, S.; Gao, S.; Lu, H.; Zhan, Y.; Zeng, F. Formation of plant cell walls and their role in abiotic stress responses. J. Plant Physiol. 2023, 59, 1251–1264. [Google Scholar]
- Wang, M.; Liu, J.; Zhao, C. Molecular mechanisms of cell wall integrity in plants under salt stress. Biotechnol. Bull. 2023, 39, 18–27. [Google Scholar]
- Li, Z.; Li, Z.; Ji, Y.; Wang, C.; Wang, S.; Shi, Y.; Le, J.; Zhang, M. The heat shock factor 20-HSF4-cellulose synthase A2 module regulates heat stress tolerance in maize. Plant Cell 2024, 36, 2652–2667. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, S.; Ding, Y. Research progress on mechanisms of plant response to high temperature stress. J. Plant Physiol. 2023, 59, 759–772. [Google Scholar]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fernie, A.R.; Persson, S. Transition of primary to secondary cell wall synthesis. J. Sci. Bull. 2016, 61, 838–846. [Google Scholar] [CrossRef]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.; Ohtani, M. NAC-MYB-based transcriptional regulation of secondary cell wall biosynthesis in land plants. Front. Plant Sci. 2015, 6, 288. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Haas, K.T.; Peaucelle, A. From monocots to dicots: The multifold aspect of cell wall expansion. J. Exp. Bot. 2021, 72, 1511–1513. [Google Scholar] [CrossRef] [PubMed]
- Meents, M.J.; Watanabe, Y.; Samuels, A.L. The cell biology of secondary cell wall biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Li, F.; Zhang, D.; Liu, X.; Wang, H.; Xu, Z.; Chu, C.; Zhou, Y. Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat. Plants 2017, 3, 17017. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Doblin, M.S.; Pettolino, F.; Bacic, A. Plant cell walls: The skeleton of the plant world. Funct. Plant Biol. 2010, 37, 357–381. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Gidley, M.J.; Bacic, A.; Doblin, M.S. Cell wall biomechanics: A tractable challenge in manipulating plant cell walls ‘fit for purpose’! Curr. Opin. Biotechnol. 2018, 49, 163–171. [Google Scholar] [CrossRef]
- Ishida, K.; Noutoshi, Y. The function of the plant cell wall in plant–microbe interactions. Plant Physiol. Biochem. 2022, 192, 273–284. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The regulation of plant cell wall organisation under salt stress. Front. Plant Sci. 2023, 14, 1118313. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Yu, H.; Jiang, W.; Sun, N.; Liu, X.; Liu, X.; Zhang, X.; Wang, Y.; Gu, X. RNA-Seq-based transcriptome profiling of early nitrogen deficiency response in cucumber seedlings provides new insight into the putative nitrogen regulatory network. Plant Cell Physiol. 2015, 56, 455–467. [Google Scholar] [CrossRef]
- Landi, S.; Esposito, S. Nitrate Uptake Affects Cell Wall Synthesis and Modeling. Front. Plant Sci. 2017, 8, 1376. [Google Scholar] [CrossRef]
- Fujita, M.; Himmelspach, R.; Hocart, C.H.; Williamson, R.E.; Mansfield, S.D.; Wasteneys, G.O. Cortical microtubules optimize cell-wall crystallinity to drive unidirectional growth in Arabidopsis. Plant J. 2011, 66, 915–928. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Characterization of the temperature activation of pectin methylesterase in green beans and tomatoes. J. Agric. Food Chem. 2006, 54, 204–211. [Google Scholar] [CrossRef]
- Wu, H.C.; Jinn, T.L. Heat shock-triggered Ca2+ mobilization accompanied by pectin methylesterase activity and cytosolic Ca2+ oscillation are crucial for plant thermotolerance. Plant Signal. Behav. 2010, 5, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Sun, G.; Yu, Q.; Gao, T.; Zhu, Q.; Wang, R.; Huang, S.; Han, Z.; Cervone, F.; Yin, H.; et al. A plant mechanism of hijacking pathogen virulence factors to trigger innate immunity. Science 2024, 383, 732–739. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Guillou, E.; Dumazert, L.; Caër, C.; Beigbeder, A.; Ouagne, P.; Le Saout, G.; Beaugrand, J.; Bourmaud, A.; Le Moigne, N. In-situ monitoring of changes in ultrastructure and mechanical properties of flax cell walls during controlled heat treatment. Carbohydr. Polym. 2023, 321, 121253. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Qin, W.; Zhou, Z.; Wu, A.M.; Deng, W.; Li, Z.; Shan, W.; Chen, J.Y.; Kuang, J.F.; Lu, W.J. Banana MaNAC1 activates secondary cell wall cellulose biosynthesis to enhance chilling resistance in fruit. Plant Biotechnol. J. 2024, 22, 413–426. [Google Scholar] [CrossRef]
- Han, X.; Zhao, Y.; Chen, Y.; Xu, J.; Jiang, C.; Wang, X.; Zhuo, R.; Lu, M.Z.; Zhang, J. Lignin biosynthesis and accumulation in response to abiotic stresses in woody plants. For. Res. 2022, 2, 9. [Google Scholar] [CrossRef]
- Ishida, K.; Yokoyama, R. Reconsidering the function of the xyloglucan endotransglucosylase/hydrolase family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, J.; Chen, Y.; Wei, Y.; Du, H.; Sun, J.; Lv, D.; Wen, H.; He, H.; Wang, G.; et al. Mapping and identification of CsSh5.1, a gene encoding a xyloglucan galactosyltransferase required for hypocotyl elongation in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2021, 134, 979–991. [Google Scholar] [CrossRef]
- De Caroli, M.; Manno, E.; Piro, G.; Lenucci, M.S. Ride to cell wall: Arabidopsis XTH11, XTH29 and XTH33 exhibit different secretion pathways and responses to heat and drought stress. Plant J. 2021, 107, 448–466. [Google Scholar] [CrossRef]
- Hu, X.; Cui, Y.; Lu, X.; Song, W.; Lei, L.; Zhu, J.; Lai, J.; E, L.; Zhao, H. Maize WI5 encodes an endo-1,4-β-xylanase required for secondary cell wall synthesis and water transport in xylem. J. Integr. Plant Biol. 2020, 62, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Zheng, H.; Zhang, J.; Ren, X.; Zong, X.; Zou, J.; Wang, L. Function analysis of a Maize endo-1,4-β-xylanase gene ZmHSL in response to high-temperature stress. Int. J. Mol. Sci. 2024, 25, 8834. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant Cell Wall Loosening by Expansins. Annu. Rev. Cell Dev. Biol. 2024, 40, 329–352. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.; Li, C.; Lei, C.; Kong, C.; Yang, Y.; Gong, M. A comprehensive expression analysis of the expansin gene family in potato (Solanum tuberosum) discloses stress-responsive expansin-like B genes for drought and heat tolerances. PLoS ONE 2019, 14, e0219837. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; He, F.; Wang, L.; Zhang, T.; Liu, D.; Xu, Y.; Li, F.; Feng, X. A Study on the Functional Identification of Overexpressing Winter Wheat Expansin Gene TaEXPA7-B in Rice under Salt Stress. Int. J. Mol. Sci. 2024, 25, 7707. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, H.C.; Wang, Y.D.; Liu, C.H.; Lin, C.C.; Luo, D.L.; Jinn, T.L. Pectin methylesterase34 contributes to heat tolerance through its role in promoting stomatal movement. Plant Physiol. 2017, 174, 748–763. [Google Scholar] [CrossRef]
- Wu, H.C.; Yu, S.Y.; Vivek, S.; Wang, Y.D.; Jinn, T.L. ABA-mediated regulation of PME12 influences stomatal density, pore aperture, and heat stress response in Arabidopsis thaliana. Planta 2025, 261, 29. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, Z.; Xu, L.; Galli, M.; Gallavotti, A.; Dooner, H.K.; Chuck, G. Necrotic upper tips1 mimics heat and drought stress and encodes a protoxylem–specific transcription factor in maize. Proc. Natl. Acad. Sci. USA 2020, 117, 20908–20919. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Z.; Wu, X.; Xia, J.; Wang, L.; Wang, Z.; Zhang, Y. Deciphering High-Temperature-Induced Lignin Biosynthesis in Wheat through Comprehensive Transcriptome Analysis. Plants 2024, 13, 1832. [Google Scholar] [CrossRef]
- Wang, S.Y. Physiological Basis of Rice Coping with High Temperature Stress and Functional Study of Key Genes of Phenylpropane Metabolic Pathway. Master’s thesis, Yangtze University, Jingzhou, China, 2023. Master’s Thesis, Yangtze University, Jingzhou, China, 2023. [Google Scholar]
- Yang, W.; Yao, D.; Duan, H.; Zhang, J.; Cai, Y.; Lan, C.; Zhao, B.; Mei, Y.; Zheng, Y.; Yang, E.; et al. VAMP726 from maize and Arabidopsis confers pollen resistance to heat and UV radiation by influencing lignin content of sporopollenin. Plant Commun. 2023, 4, 100682. [Google Scholar] [CrossRef]
- De la Rubia, A.G.; Largo-Gosens, A.; Yusta, R.; Sepúlveda-Orellana, P.; Riveros, A.; Centeno, M.L.; Sanhueza, D.; Meneses, C.; Saez-Aguayo, S.; García-Angulo, P. A novel pectin methylesterase inhibitor, PMEI3, in common bean suggests a key role of pectin methylesterification in Pseudomonas resistance. J. Exp. Bot. 2024, 75, 364–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Yu, S.Y.; Wang, Y.D.; Jinn, T.L. Guard cell-specific pectin METHYLESTERASE53 is required for abscisic acid-mediated stomatal function and heat response in Arabidopsis. Front. Plant Sci. 2022, 13, 836151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Yeh, F.J.; Yvon, R.; Simpson, K.; Jordan, S.; Chambers, J.; Wu, H.-M.; Cheung, A.Y. Extracellular pectin–RALF phase separation mediates FERONIA global signaling function. Cell 2024, 187, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Herger, A.; Dünser, K.; Kleine-Vehn, J.; Ringli, C. Leucine–rich repeat extensin proteins and their role in cell wall sensing. Curr. Biol. 2019, 29, 851–858. [Google Scholar] [CrossRef]
- Du, J.; Anderson, C.T.; Xiao, C.W. Dynamics of pectic homogalacturonan in cellular morphogenesis and adhesion, wall integrity sensing and plant development. Nat. Plants 2022, 8, 332–340. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Zou, K.; Wang, Y.; Ma, Y.; Meng, D.; Luo, H.; Qu, J.; Li, F.; Xuan, Y.; et al. The resistance of maize to Ustilago maydis infection is correlated with the degree of methyl esterification of pectin in the cell wall. Int. J. Mol. Sci. 2023, 24, 14737. [Google Scholar] [CrossRef]
- Kamel, H.; Geitmann, A. Strength in numbers: An isoform variety of homogalacturonan modifying enzymes may contribute to pollen tube fitness. Plant Physiol. 2023, 194, 67–80. [Google Scholar] [CrossRef]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef]
- Lin, W.; Tang, W.; Pan, X.; Huang, A.; Gao, X.; Anderson, C.T.; Yang, Z. Arabidopsis pavement cell morphogenesis requires FERONIA binding to pectin for activation of ROP GTPase signaling. Curr. Biol. 2022, 32, 497–507. [Google Scholar] [CrossRef]
- Tang, W.; Lin, W.; Zhou, X.; Guo, J.; Dang, X.; Li, B.; Lin, D.; Yang, Z. Mechano–transduction via the pectin–FERONIA complex activates ROP6 GTPase signaling in Arabidopsis pavement cell morphogenesis. Curr. Biol. 2022, 32, 508–517. [Google Scholar] [CrossRef]
- Zeng, R.; Li, Z.; Shi, Y.; Fu, D.; Yin, P.; Cheng, J.; Jiang, C.; Yang, S. Natural variation in a type-A response regulator confers maize chilling tolerance. Nat Commun. 2021, 12, 4713. [Google Scholar] [CrossRef]
- Ai, Y.; Wang, H.; Liu, P.; Yu, H.; Sun, M.; Zhang, R.; Tang, J.; Wang, Y.; Feng, S.; Peng, L. Insights into contrastive cellulose nanofibrils assembly and nanocrystals catalysis from dual regulations of plant cell walls. Sci. Bull. 2024, 69, 3815–3819. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Feng, S.; Wang, Y.; Wang, Y.; Fan, C.; Li, Y.; Liu, Z.; Schneider, R.; Xia, T.; et al. Three AtCesA6-like members enhance biomass production by distinctively promoting cell growth in Arabidopsis. Plant Biotechnol. J. 2018, 16, 976–988. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Liu, X.; Xu, T.; Zhang, Y.; Meng, H.; Xia, T. High temperature increased lignin contents of poplar (Populus spp.) stem via inducing the synthesis caffeate and coniferaldehyde. Front. Genet. 2022, 13, 1007513. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.R.; Belo, J.; Beeckman, T.; Barros, P.-M.; Oliveira, M.M. The Combined Effect of Heat and Osmotic Stress on Suberization of Arabidopsis Roots. Cells 2022, 11, 2341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, J.; Luo, W.; Niu, S.; Qu, L.; Li, J.; Chen, Y.; Li, G.; Yang, H.; Lu, D. Salicylic acid cooperates with lignin and sucrose signals to alleviate waxy maize leaf senescence under heat stress. Plant Cell Environ. 2025, 48, 4341–4355. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Xiang, J.; Teng, R.; Zhang, D.; Teng, N. A lily membrane–associated NAC transcription factor LlNAC014 is involved in thermotolerance via activation of the DREB2–HSFA3 module. J. Exp. Bot. 2023, 74, 945–963. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, C.; Jiang, R.; Wang, F.; Zhang, Z.; Zeng, J. A novel NAC transcription factor from Eucalyptus, egnac141, positively regulates lignin biosynthesis and increases lignin deposition. Front. Plant Sci. 2021, 12, 642090. [Google Scholar] [CrossRef]
- Guo, G.; Bai, F.; Liu, W.; Bi, C. Advances in research of the regulation of transcription factors of lignin biosynthesis. Sci. Agric. Sin. 2015, 48, 1277–1287. [Google Scholar]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Oelmüller, R.; Tseng, Y.H.; Gandhi, A. Signals and their perception for remodelling, adjustment and repair of the plant cell wall. Int. J. Mol. Sci. 2023, 24, 7417. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- He, N.Y.; Chen, L.S.; Sun, A.Z.; Zhao, Y.; Yin, S.N.; Guo, F.Q. A nitric oxide burst at the shoot apex triggers a heat–responsive pathway in Arabidopsis. Nat. Plants 2022, 8, 434–450. [Google Scholar] [CrossRef]
- Mittle, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Kirui, A.; Huang, S.; Wang, L.; Barnes, W.J.; Kiemle, S.N.; Zheng, Y.; Rui, Y.; Ruan, M.; Qi, S.; et al. Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell 2020, 32, 3576–3597. [Google Scholar] [CrossRef]
- Kirui, A.; Du, J.; Zhao, W.; Barnes, W.; Kang, X.; Anderson, C.T.; Xiao, C.; Wang, T. A pectin methyltransferase modulates polysaccharide dynamics and interactions in Arabidopsis primary cell walls: Evidence from solid–state NMR. Carbohydr. Polym. 2021, 270, 118370. [Google Scholar] [CrossRef]
- Wang, H.; Niu, H.; Liang, M.; Zhai, Y.; Huang, W.; Ding, Q.; Du, Y.; Lu, M. A wall–associated kinase gene CaWAKL20 from pepper negatively modulates plant thermotolerance by reducing the expression of ABA–responsive genes. Front. Plant Sci. 2019, 10, 591. [Google Scholar] [CrossRef]
- Brutus, A.; Sicilia, F.; Macone, A.; Cervone, F.; De Lorenzo, G. A domain swap approach reveals a role of the plant wall–associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA 2010, 107, 9452–9457. [Google Scholar] [CrossRef]
- Endler, A.; Kesten, C.; Schneider, R.; Zhang, Y.; Ivakov, A.; Froehlich, A.; Funke, N.; Persson, S. A mechanism for sustained cellulose synthesis during salt stress. Cell 2015, 162, 1353–1364. [Google Scholar] [CrossRef]
- Cao, J.; Qin, Z.; Cui, G.; Chen, Z.; Cheng, X.; Peng, H.; Yao, Y.; Hu, Z.; Guo, W.; Ni, Z.; et al. Natural variation of STKc–GSK3 kinase TaSG–D1 contributes to heat stress tolerance in Indian dwarf wheat. Nat. Commun. 2024, 15, 2097. [Google Scholar] [CrossRef] [PubMed]
- Fabri, J.H.T.M.; Rocha, M.C.; Fernandes, C.M.; Persinoti, G.F.; Ries, L.N.A.; da Cunha, A.F.; Goldman, G.H.; Del Poeta, M.; Malavazi, I. The heat shock transcription factor HsfA is essential for thermotolerance and regulates cell wall integrity in Aspergillus fumigatus. Front. Microbiol. 2021, 12, 656548. [Google Scholar] [CrossRef] [PubMed]
- Lien, P.T.K.; Viet, N.T.M.; Mizuno, T.; Suda, Y.; Irie, K. Pop2 phosphorylation at S39 contributes to the glucose repression of stress response genes, HSP12 and HSP26. PLoS ONE 2019, 14, e0215064. [Google Scholar] [CrossRef]
- Xiong, H.; He, H.; Chang, Y.; Miao, B.; Liu, Z.; Wang, Q.; Dong, F.; Xiong, L. Multiple roles of NAC transcription factors in plant development and stress responses. J. Integr. Plant Biol. 2025, 67, 510–538. [Google Scholar] [CrossRef]
- Xi, Y.; Ling, Q.; Zhou, Y.; Liu, X.; Qian, Y. ZmNAC074, a maize stress–responsive NAC transcription factor, confers heat stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2022, 13, 986628. [Google Scholar] [CrossRef]
- Rao, X.L.; Dixon, R.A. Brassinosteroid mediated cell wall remodeling in grasses under abiotic stress. Front. Plant Sci. 2017, 8, 806. [Google Scholar] [CrossRef]
- Bacete, L.; Schulz, J.; Engelsdorf, T.; Bartosova, Z.; Vaahtera, L.; Yan, G.; Gerhold, J.M.; Tichá, T.; Øvstebø, C.; Gigli-Bisceglia, N.; et al. THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2022, 119, e2119258119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Yang, K.F.; Hu, X.; Jiang, H. Fine control of growth and thermotolerance in the plant response to heat stress. J. Integr. Agric. 2025, 24, 409–428. [Google Scholar] [CrossRef]
- Wan, J.X.; He, M.; Hou, Q.Q.; Zou, L.J.; Yang, Y.H.; Wei, Y.; Chen, X.W. Cell wall associated immunity in plants. Stress Biol. 2021, 18, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhang, G.; Yang, Q.; Lu, J.; Xia, T.; Peng, L.; Wang, Y. Cascading of engineered bioenergy plants and fungi sustainable for low–cost bioethanol and high–value biomaterials under green–like biomass processing. Renew. Sust. Energy Rev. 2021, 137, 110586. [Google Scholar] [CrossRef]
| Cell Wall Components | Associated Genes and Enzyme Proteins | Name of the Species | Regulatory Role Under Heat Stress | References |
|---|---|---|---|---|
| Cellulose | ZmCESA2 | Zea mays | Promotes cell wall thickening, positive regulation of HS | [11] |
| ZmHSF4 | Zea mays | Direct activation of ZmCesA2 expression, positive regulation of HS | [11] | |
| nut1 | Zea mays | Directly affects cellulose biosynthesis, positive regulation of HS | [48] | |
| RIC1 | Arabidopsis thaliana | Enhances cell wall extensibility, positive regulation of HS | [29] | |
| Hemicellulose | XTHs | Arabidopsis thaliana | Enhances cell wall reinforcement, positive regulation of HS | [40] |
| CsSh5.1 | Cucumis sativus | Encodes galactosyltransferase for xyloglucan, positive regulation of HS | [39] | |
| ZmHSL | Zea mays | Promotes cell wall thickening, positive regulation of HS | [42] | |
| Lignin | TaPAL33 | Triticum aestivum | Promotes lignin deposition, positive regulation of HS | [49] |
| OsCCR17 | Oryza sativa | Promotes lignin deposition, positive regulation of HS | [50] | |
| VAMP726 | Zea mays | Promotes the transport of lignin monomers, positive regulation of HS | [51] | |
| Pectin | PMEIs | Arabidopsis thaliana | Blocks PME activity, maintaining wall flexibility, positive regulation of HS | [52] |
| AtPME12/AtPME34/AtPME53 | Arabidopsis thaliana | Promotes pectin demethylesterification, negative regulation of HS | [46,47,53] | |
| RALFs | Arabidopsis thaliana | Pectin binding triggers phase separation of RALF-FER-LLG1 signaling modules, positive regulation of HS | [54] | |
| LRX | Arabidopsis thaliana | Perception of plant cell wall integrity through LRR domain–RALF peptide interactions, positive regulation of HS | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Li, W.; Feng, X.; Chen, J.; Hu, S.; Tan, Y.; Wu, L. The Dynamic Remodeling of Plant Cell Wall in Response to Heat Stress. Genes 2025, 16, 628. https://doi.org/10.3390/genes16060628
Lu C, Li W, Feng X, Chen J, Hu S, Tan Y, Wu L. The Dynamic Remodeling of Plant Cell Wall in Response to Heat Stress. Genes. 2025; 16(6):628. https://doi.org/10.3390/genes16060628
Chicago/Turabian StyleLu, Chengchen, Wenfei Li, Xiaomeng Feng, Jiarui Chen, Shijie Hu, Yirui Tan, and Leiming Wu. 2025. "The Dynamic Remodeling of Plant Cell Wall in Response to Heat Stress" Genes 16, no. 6: 628. https://doi.org/10.3390/genes16060628
APA StyleLu, C., Li, W., Feng, X., Chen, J., Hu, S., Tan, Y., & Wu, L. (2025). The Dynamic Remodeling of Plant Cell Wall in Response to Heat Stress. Genes, 16(6), 628. https://doi.org/10.3390/genes16060628






