Genetic Transformation of the Model Quorum Sensing Bacterium Vibrio campbellii by Electroporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids Used in This Study
2.2. Media and Culture Conditions
2.3. Electrocompetent Cell Preparation and Transformation by Electroporation
2.4. Transformation by Conjugation
2.5. Determination of Natural Antibiotic Resistance Levels and Plasmid Function
2.6. Extracellular Proteomics
3. Results
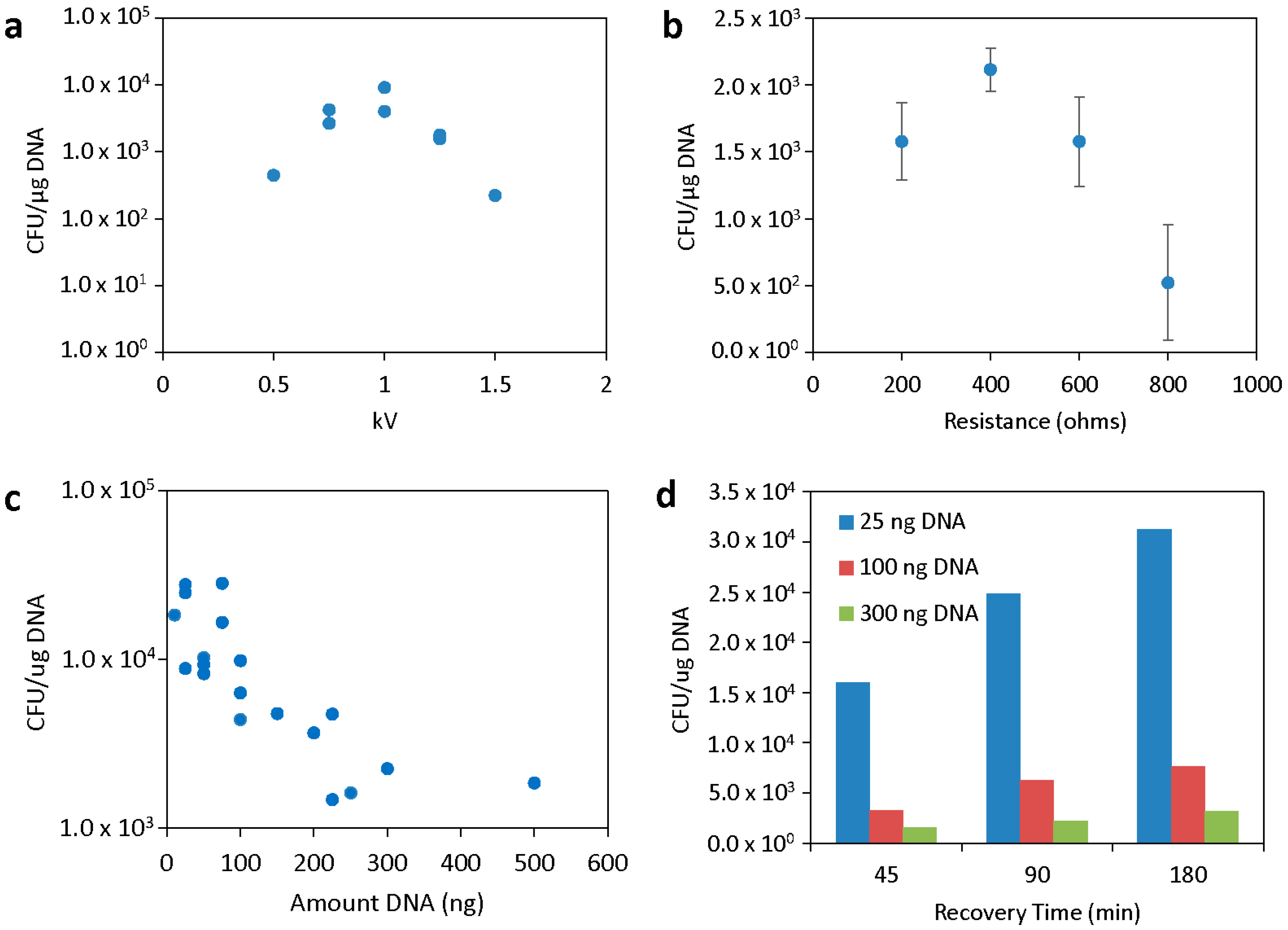
3.1. Electroporation Parameter Testing
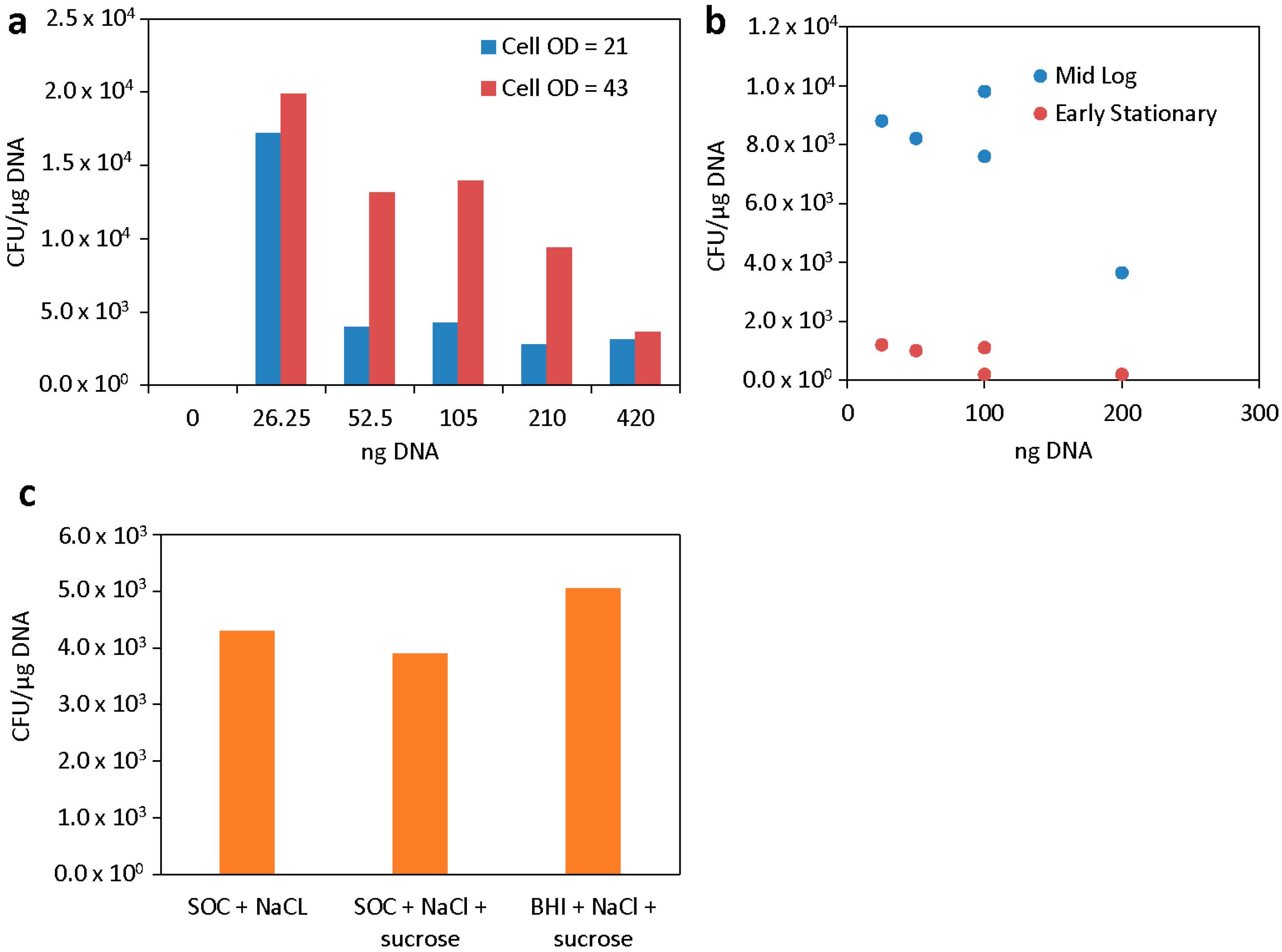
3.2. Effects of Cell Preparation and Media on Transformation Efficiency
3.3. Electroporation of Different Plasmids
3.4. Electroporation of Other V. campbellii and V. harveyi Strains
3.5. Possible Extracellular Nucleases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed]
- Ball, A.S.; Chaparian, R.R.; van Kessel, J.C. Quorum sensing gene regulation by LuxR/HapR master regulators in vibrios. J. Bacteriol. 2017, 199, e00105-17. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef]
- Defoirdt, T.; Verstraete, W.; Bossier, P. Luminescence, virulence and quorum sensing signal production by pathogenic Vibrio campbellii and Vibrio harveyi isolates. J. Appl. Microbiol. 2008, 104, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, B.; Mostaghim, A.; Rubin, R.; Glaser, E.; Mittraparp-arthorn, P.; Thompson, J.; Vuddhakul, V.; Vora, G. Vibrio campbellii hmgA-mediated pyomelanization impairs quorum sensing, virulence, and cellular fitness. Front. Microbiol. 2013, 4, 379. [Google Scholar] [CrossRef]
- Wang, Z.; O’Shaughnessy, T.J.; Soto, C.M.; Rahbar, A.M.; Robertson, K.L.; Lebedev, N.; Vora, G.J. Function and regulation of Vibrio campbellii proteorhodopsin: Acquired phototrophy in a classical organoheterotroph. PLoS ONE 2012, 7, e38749. [Google Scholar] [CrossRef]
- Wang, Z.; Robertson, K.L.; Liu, C.; Liu, J.L.; Johnson, B.J.; Leary, D.H.; Compton, J.R.; Vuddhakul, V.; Legler, P.M.; Vora, G.J. A novel Vibrio β-glucosidase (LamN) that hydrolyzes the algal storage polysaccharide laminarin. FEMS Microbiol. Ecol. 2015, 91, fiv087. [Google Scholar] [CrossRef][Green Version]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef]
- Seghal Kiran, G.; Priyadharshini, S.; Dobson, A.D.W.; Gnanamani, E.; Selvin, J. Degradation intermediates of polyhydroxy butyrate inhibits phenotypic expression of virulence factors and biofilm formation in luminescent Vibrio sp. PUGSK8. NPJ Biofilms Microbiomes 2016, 2, 16002. [Google Scholar] [CrossRef]
- Yang, Q.; Pande, G.S.J.; Wang, Z.; Lin, B.; Rubin, R.A.; Vora, G.J.; Defoirdt, T. Indole signalling and (micro)algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ. Microbiol. 2017, 19, 1987–2004. [Google Scholar] [CrossRef]
- Austin, B. Vibrios as causal agents of zoonoses. Vet. Microbiol. 2010, 140, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Zhang, X.H. Vibrio harveyi: A significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 2006, 43, 119–124. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Z.; Malanoski, A.P.; O’Grady, E.A.; Wimpee, C.F.; Vuddhakul, V.; Alves, N., Jr.; Thompson, F.L.; Gomez-Gil, B.; Vora, G.J. Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA-1116 and HY01 as Vibrio campbellii. Environ. Microbiol. Rep. 2010, 2, 81–89. [Google Scholar] [CrossRef]
- Bassler, B.L.; Wright, M.; Showalter, R.E.; Silverman, M.R. Intercellular signalling in Vibrio harveyi: Sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993, 9, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, H.; Nakano, T.; Tamura, S.; Arai, T. Genetic Transformation of Vibrio parahaemolyticus, Vibrio alginolyticus, and Vibrio cholerae non O-1 with plasmid DNA by electroporation. Microbiol. Immunol. 1990, 34, 703–708. [Google Scholar] [CrossRef]
- Wang, H.; Griffiths, M.W. Mg2+-free buffer elevates transformation efficiency of Vibrio parahaemolyticus by electroporation. Lett. Appl. Microbiol. 2009, 48, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, I.; Okunishi, I.; Homma, M.; Imae, Y. Removal of the periplasmic DNase before electroporation enhances efficiency of transformation in the marine bacterium Vibrio alginolyticus. Microbiology 1994, 140, 2355–2361. [Google Scholar] [CrossRef][Green Version]
- Lee, H.H.; Ostrov, N.; Wong, B.G.; Gold, M.A.; Khalil, A.; Church, G.M. Vibrio natriegens, a new genomic powerhouse. bioRxiv 2016. [Google Scholar] [CrossRef]
- Klevanskaa, K.; Bier, N.; Stingl, K.; Strauch, E.; Hertwig, S. PVv3, a new shuttle vector for gene expression in Vibrio vulnificus. Appl. Environ. Microbiol. 2014, 80, 1477–1481. [Google Scholar] [CrossRef]
- Weinstock, M.T.; Hesek, E.D.; Wilson, C.M.; Gibson, D.G. Vibrio natriegens as a fast-growing host for molecular biology. Nat. Methods 2016, 13, 849–851. [Google Scholar] [CrossRef]
- Newland, J.W.; Green, B.A.; Foulds, J.; Holmes, R.K. Cloning of extracellular DNase and construction of a DNase-negative strain of Vibrio cholerae. Infect. Immun. 1985, 47, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Focareta, T.; Manning, P.A. Distinguishing between the extracellular DNases of Vibrio cholerae and development of a transformation system. Mol. Microbiol. 1991, 5, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K.; Millikan, D.S.; Adin, D.M.; Bose, J.L.; Stabb, E.V. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 2006, 72, 802–810. [Google Scholar] [CrossRef]
- van Kessel, J.C.; Rutherford, S.T.; Cong, J.P.; Quinodoz, S.; Healy, J.; Bassler, B.L. Quorum sensing regulates the osmotic stress response in Vibrio harveyi. J. Bacteriol. 2015, 197, 73–80. [Google Scholar] [CrossRef]
- Silva-Rocha, R.; Martínez-García, E.; Calles, B.; Chavarría, M.; Arce-Rodríguez, A.; de las Heras, A.; Páez-Espino, A.D.; Durante-Rodríguez, G.; Kim, J.; Nikel, P.I.; et al. The Standard European Vector Architecture (SEVA): A coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 2013, 41, D666–D675. [Google Scholar] [CrossRef]
- Delavat, F.; Bidault, A.; Pichereau, V.; Paillard, C. Rapid and efficient protocol to introduce exogenous DNA in Vibrio harveyi and Pseudoalteromonas sp. J. Microbiol. Methods 2018, 154, 1–5. [Google Scholar] [CrossRef]
- Stukenberg, D.; Hensel, T.; Hoff, J.; Daniel, B.; Inckemann, R.; Tedeschi, J.N.; Nousch, F.; Fritz, G. The Marburg Collection: A Golden Gate Assembly Framework for Synthetic Biology Applications in Vibrio natriegens. ACS Synth. Biol. 2021, 10, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, A.S.M.; Lapinska, E.; Rajarajan, N.; Dobretsov, S.; Upstill-Goddard, R.; Burgess, J.G. Secretion of DNases by marine bacteria: A culture based and bioinformatics approach. Front. Microbiol. 2019, 10, 969. [Google Scholar] [CrossRef]
- Blokesch, M.; Schoolnik, G.K. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J. Bacteriol. 2008, 190, 7232–7240. [Google Scholar] [CrossRef]
- Dalia, T.N.; Yoon, S.H.; Galli, E.; Barre, F.-X.; Waters, C.M.; Dalia, A.B. Enhancing multiplex genome editing by natural transformation (MuGENT) via inacitivation of ssDNA exonucleases. Nucleic Acids Res. 2017, 45, 7527–7537. [Google Scholar] [CrossRef]
- Lee, H.H.; Ostrov, N.; Gold, M.A.; Church, G.M. Recombineering in Vibrio natriegens. bioRxiv 2017. [Google Scholar] [CrossRef]
- Rattanama, P.; Srinitiwarawong, K.; Thompson, J.R.; Pomwised, R.; Supamattaya, K.; Vuddhakul, V. Shrimp pathogenicity, hemolysis, and the presence of hemolysin and TTSS genes in Vibrio harveyi isolated from Thailand. Dis. Aquat. Organ. 2009, 86, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Haygood, M.G.; Holt, P.D.; Butler, A. Aerobactin production by a planktonic marine Vibrio sp. Limnol. Oceanogr. 1993, 38, 1091–1097. [Google Scholar] [CrossRef]
- Amaral, G.R.S.; de O Silva, B.S.; Santos, E.O.; Dias, G.M.; Lopes, R.M.; Edwards, R.A.; Thompson, C.C.; Thompson, F.L. Genome sequence of the bacterioplanktonic, mixotrophic Vibrio campbellii strain PEL22A, isolated in the Abrolhos Bank. J. Bacteriol. 2012, 194, 2759–2760. [Google Scholar] [CrossRef]
- Espinoza-Valles, I.; Vora, G.J.; Lin, B.; Leekitcharoenphon, P.; González-Castillo, A.; Ussery, D.; Høj, L.; Gomez-Gil, B. Unique and conserved genome regions in Vibrio harveyi and related species in comparison with the shrimp pathogen Vibrio harveyi CAIM 1792. Microbiology 2015, 161, 1762–1779. [Google Scholar] [CrossRef]
- Baumann, P.; Baumann, L.; Mandel, M. Taxonomy of marine bacteria: The genus Beneckea. J. Bacteriol. 1971, 107, 268–294. [Google Scholar] [CrossRef]
- Colston, S.M.; Ellis, G.A.; Kim, S.; Wijesekera, H.W.; Leary, D.H.; Lin, B.; Kirkup, B.C.; Hervey, W.J., IV; Vora, G.J. Complete genome sequences of two bioluminescent Vibrio campbellii strains isolated from biofouling communities in the Bay of Bengal. Genome Announc. 2018, 6, e00422-18. [Google Scholar] [CrossRef]
- Johnson, F.H.; Shunk, I.V. An interesting new species of luminous bacteria. J. Bacteriol. 1936, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.W.; Weber, K.; Friedland, J.; Eberhhard, A.; Mitchell, G.W.; Gunsalus, A. Structurally distinct bacterial luciferases. Biochemistry 1969, 8, 4681–4689. [Google Scholar] [CrossRef]
- Pedersen, K.; Verdonck, L.; Austin, B.; Austin, D.A.; Blanch, A.R.; Grimont, P.A.D.; Jofre, J.; Koblavi, S.; Larsen, J.L.; Tiainen, T.; et al. Taxonomic evidence that Vibrio carchariae Grimes et al. 1985 is a junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int. J. Syst. Bacteriol. 1998, 48, 749–758. [Google Scholar] [CrossRef]


| Plasmid | Features | Size (bp) | Source |
|---|---|---|---|
| pVSV105 | pES213 ori, oriT, RK6, Cm | 5780 | Ref. [23] |
| pVSV102 | Constitutive GFP, pES213 ori, oriT, RK6, Kan | 6440 | Ref. [23] |
| pJV021 | IPTG-inducible GFP, p15A ori, oriT, Kan | 5017 | Ref. [24] |
| pJV298 | IPTG-inducible GFP, p15A ori, oriT, Kan, Cm | 5120 | Ref. [24] |
| pJV315 | IPTG-inducible GFP, p15A ori, oriT, Kan, Tet | 7124 | Ref. [24] |
| pSEVA237R | pBBR1 ori, Kan | 3816 | SEVA collection [25] |
| pBBR1-MCS:GFP | GFP production, pBBR1 ori, Kan | 5863 | Lab stocks |
| pSEVA351 | RSF101 ori, Cm | 5120 | SEVA collection [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschirhart, T.; Wang, Z.; Leary, D.H.; Vora, G.J. Genetic Transformation of the Model Quorum Sensing Bacterium Vibrio campbellii by Electroporation. Genes 2025, 16, 626. https://doi.org/10.3390/genes16060626
Tschirhart T, Wang Z, Leary DH, Vora GJ. Genetic Transformation of the Model Quorum Sensing Bacterium Vibrio campbellii by Electroporation. Genes. 2025; 16(6):626. https://doi.org/10.3390/genes16060626
Chicago/Turabian StyleTschirhart, Tanya, Zheng Wang, Dagmar H. Leary, and Gary J. Vora. 2025. "Genetic Transformation of the Model Quorum Sensing Bacterium Vibrio campbellii by Electroporation" Genes 16, no. 6: 626. https://doi.org/10.3390/genes16060626
APA StyleTschirhart, T., Wang, Z., Leary, D. H., & Vora, G. J. (2025). Genetic Transformation of the Model Quorum Sensing Bacterium Vibrio campbellii by Electroporation. Genes, 16(6), 626. https://doi.org/10.3390/genes16060626





