Unraveling the Transcriptomic Profiles of Large and Small Donkey Follicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Follicles and Hormones
2.3. Transcriptome Sequencing
2.3.1. RNA Extraction
2.3.2. Library Preparation for Transcriptome Sequencing
2.3.3. Data Analysis and Quality Control
2.3.4. Gene Expression Analysis
2.3.5. GO and KEGG Enrichment Analysis of DEGs
2.3.6. RNA-Seq Validation and Functional Gene Screening
2.4. Intracellular Validation Assays
2.4.1. Construction of EMCN, SYT12 Overexpression, and Interference Lentiviral Vectors
2.4.2. Detection of Carrier Titer
2.4.3. RT-qPCR Determination of Relative mRNA Expression
2.5. Data Analytics
3. Results
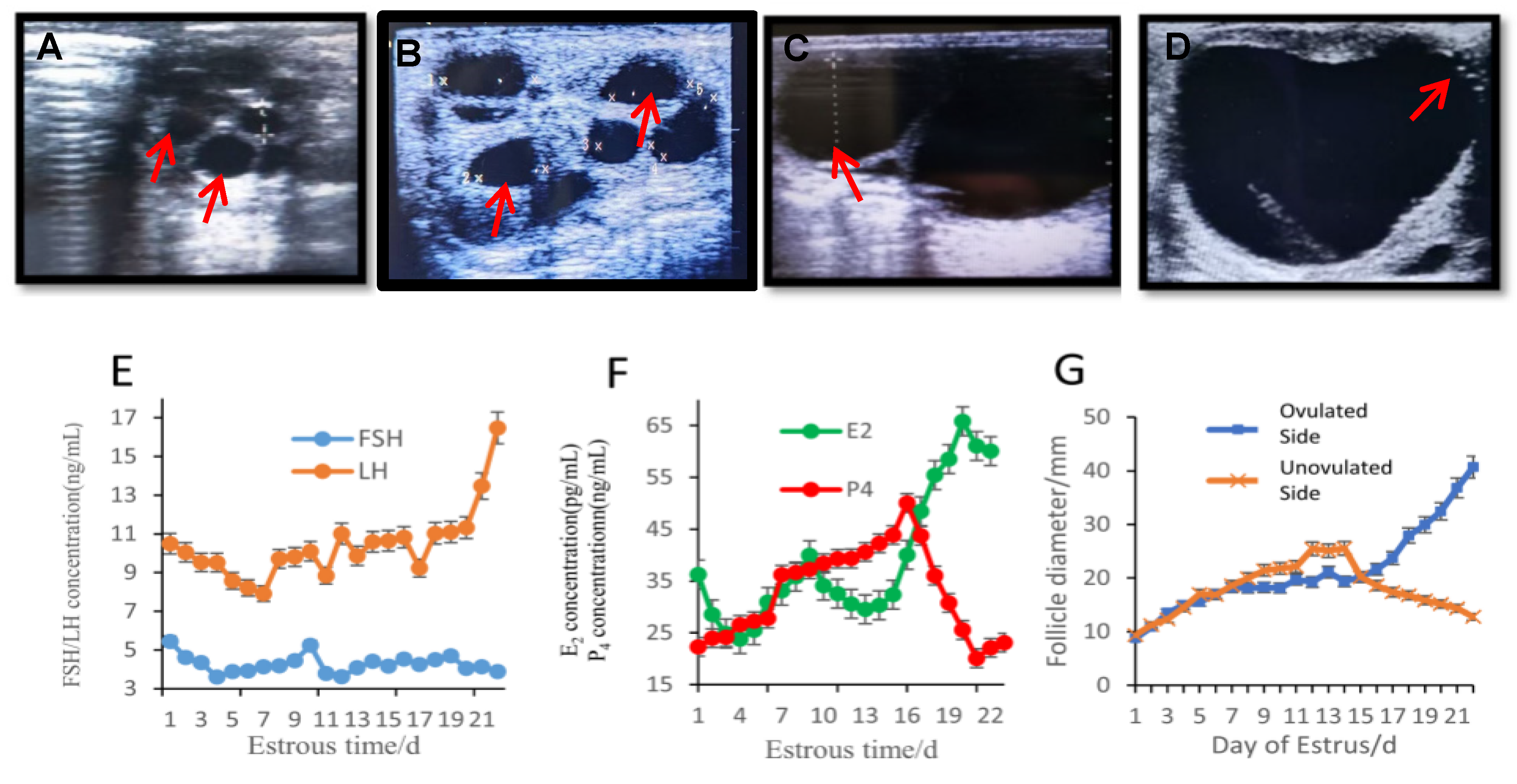
3.1. Follicle Development and Hormonal Changes
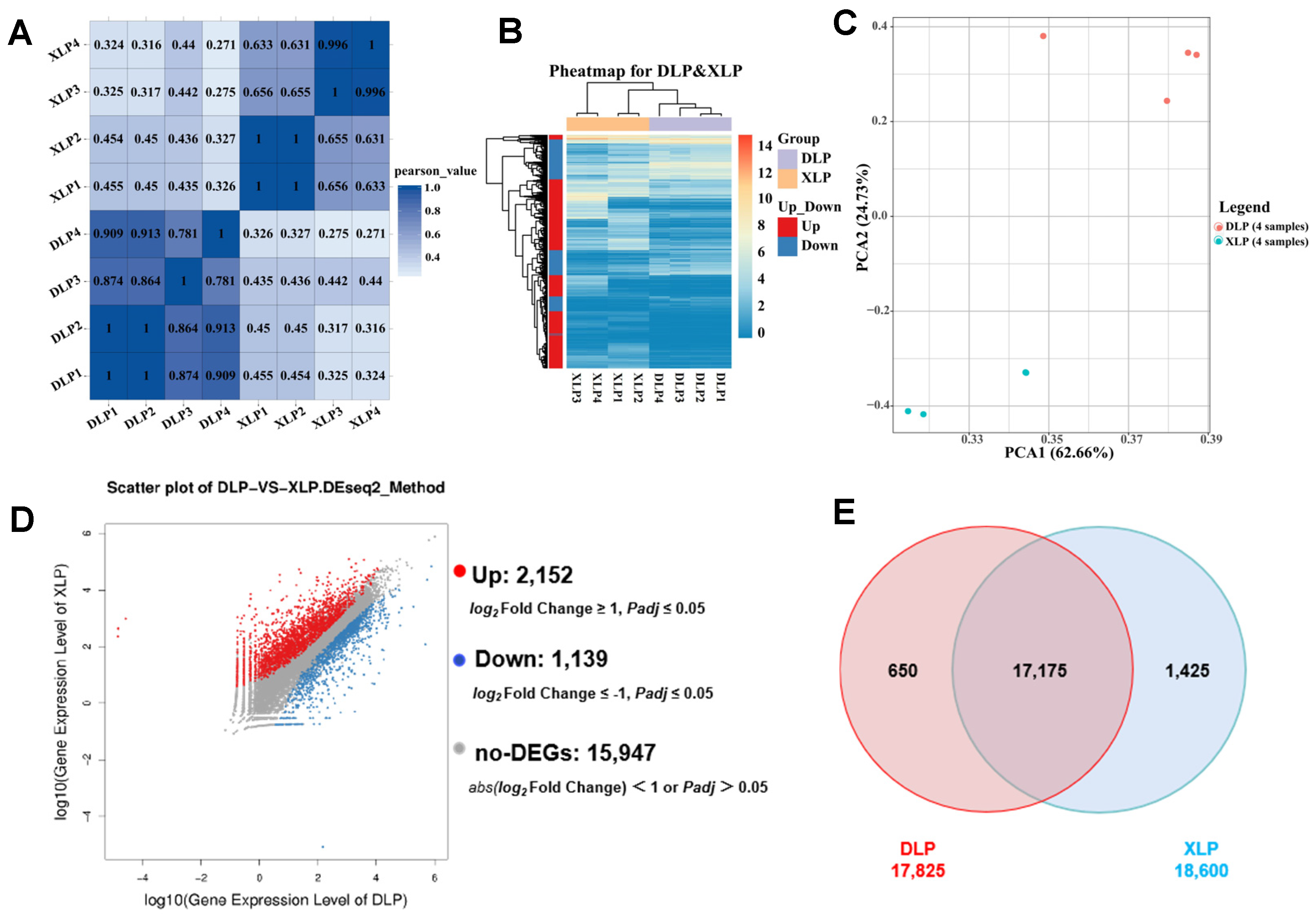
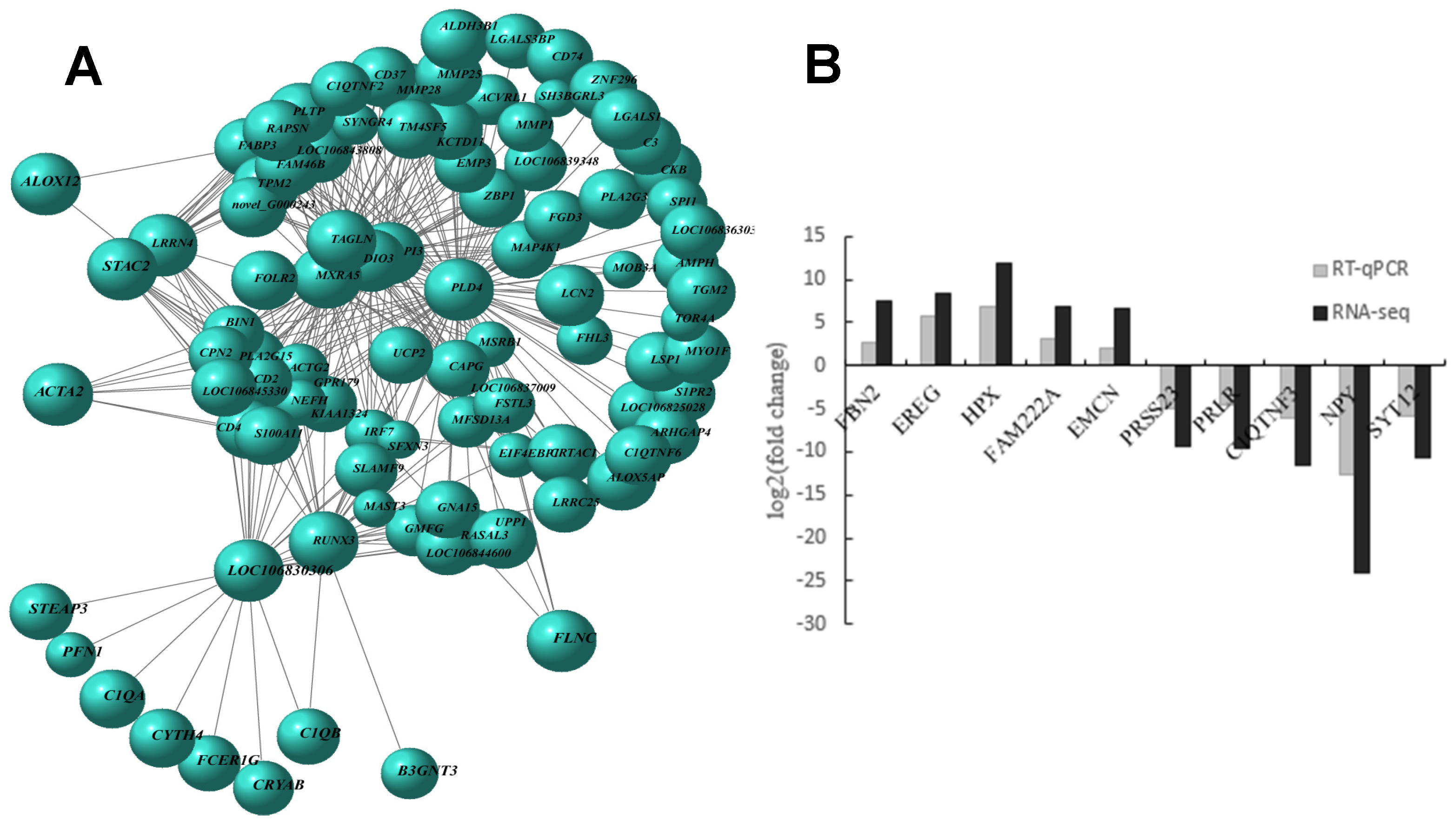
3.2. GC Gene Expression Differed Significantly Between Large and Small Follicles
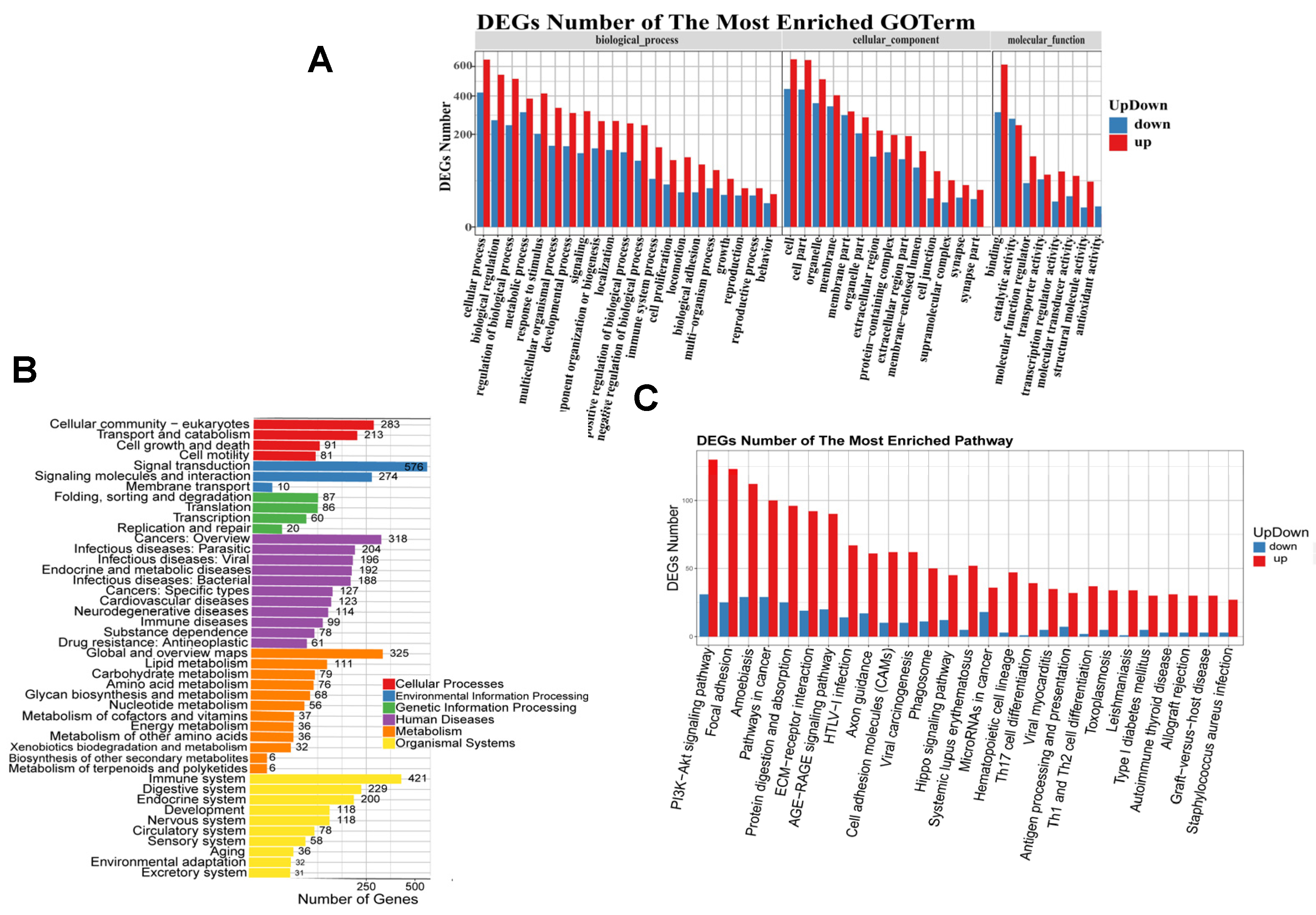
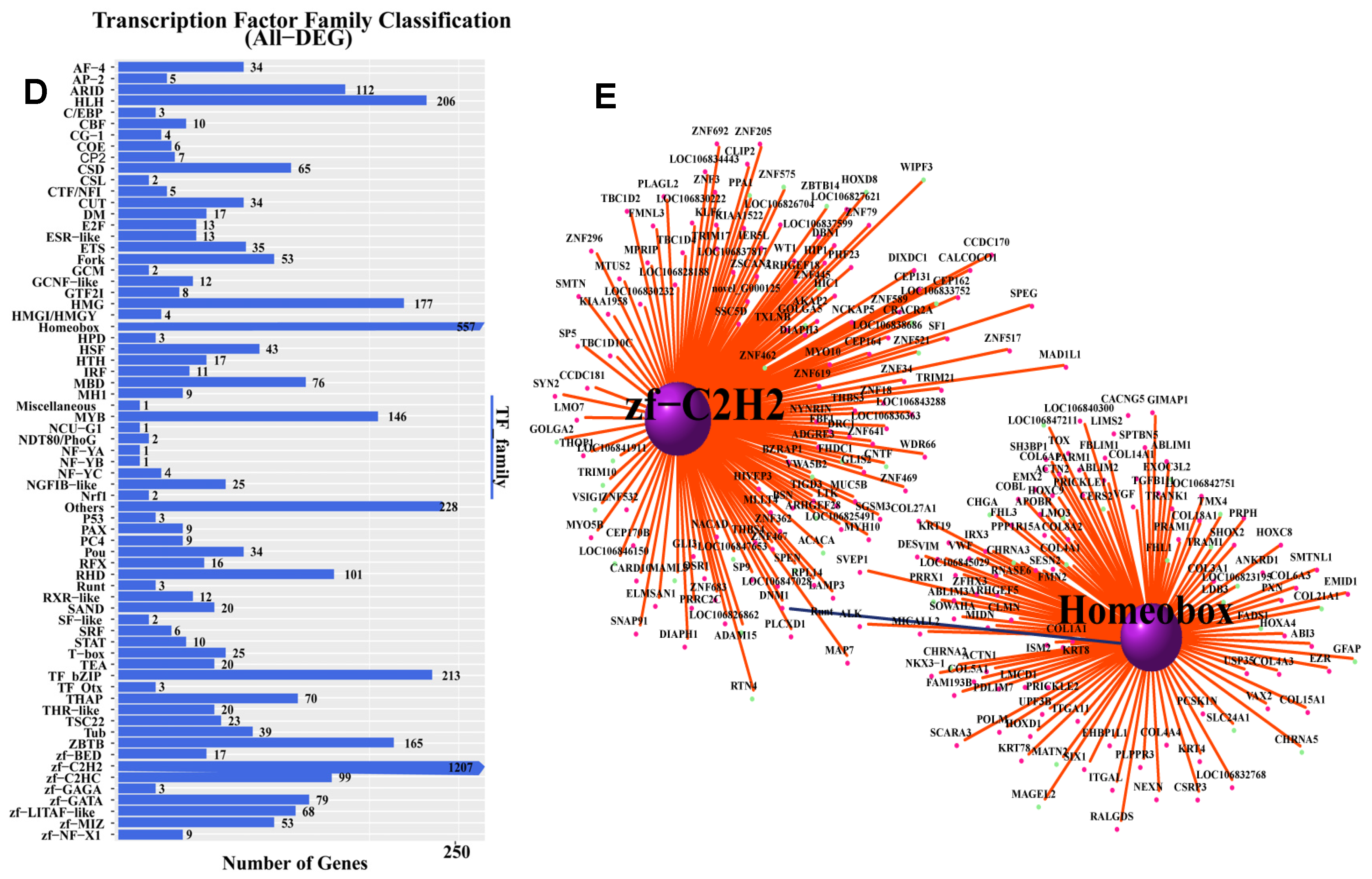
3.3. DEG Gene Ontology (GO) Functional Enrichment and KEGG Pathway Annotation
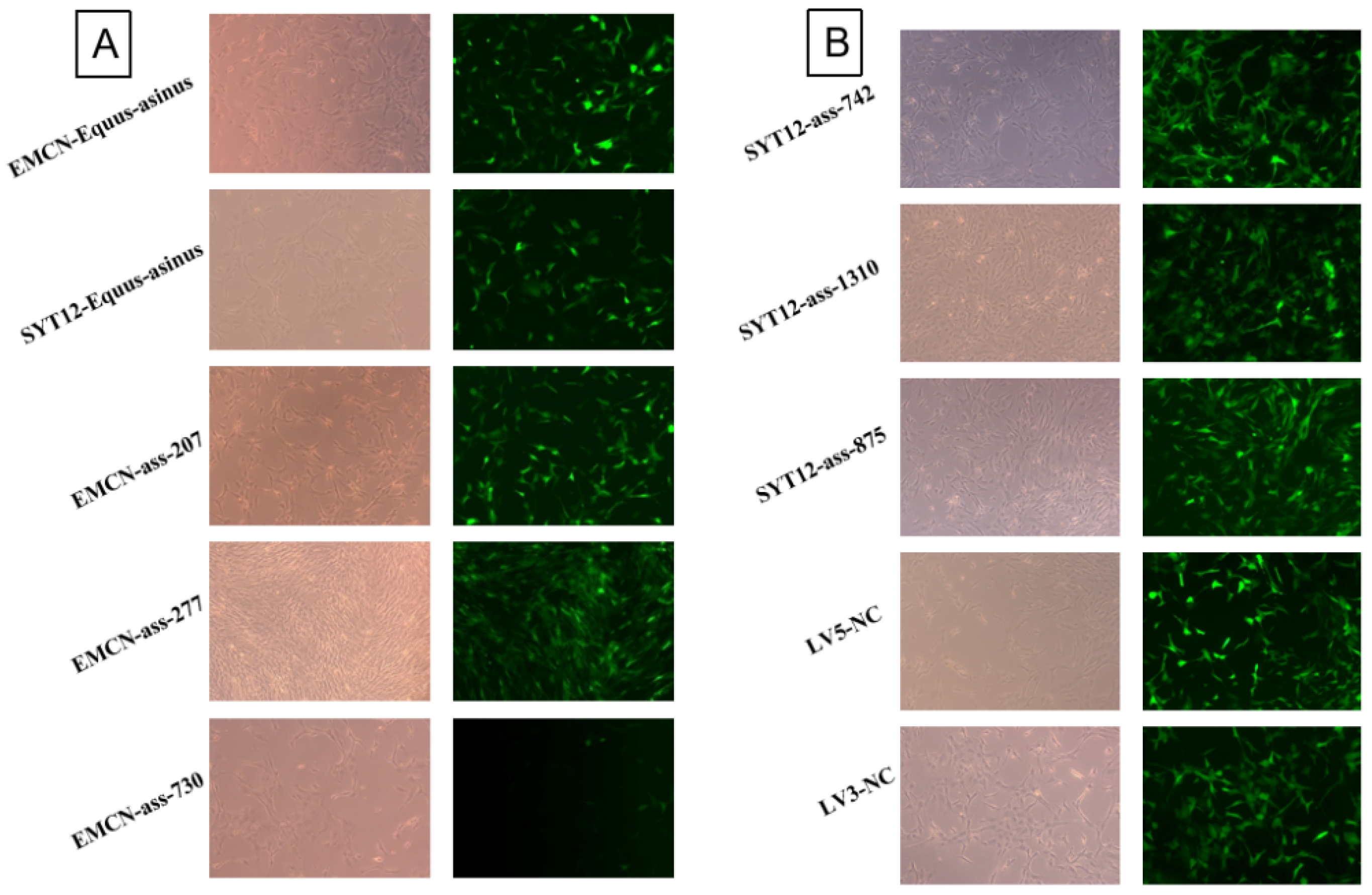
3.4. Screening of DEGs EMCN, and SYT12 and Construction of Viral Vectors
3.5. Lentiviral Vector Effectively Infects GCs
3.6. EMCN Positively Modulates FOXO3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polidori, P.; Pucciarelli, S.; Ariani, A.; Polzonetti, V.; Vincenzetti, S. A comparison of the carcass and meat quality of Martina Franca donkey foals aged 8 or 12 months. Meat Sci. 2015, 106, 6–10. [Google Scholar] [CrossRef]
- Camillo, F.; Rota, A.; Biagini, L.; Tesi, M.; Fanelli, D.; Panzani, D. The Current Situation and Trend of Donkey Industry in Europe. J. Equine Vet. Sci. 2018, 65, 44–49. [Google Scholar] [CrossRef]
- Vidament, M.; Vincent, P.; Martin, F.-X.; Magistrini, M.; Blesbois, E. Differences in ability of jennies and mares to conceive with cooled and frozen semen containing glycerol or not. Anim. Reprod. Sci. 2009, 112, 22–35. [Google Scholar] [CrossRef]
- Stewart, F.; Allen, W.R. Biological functions and receptor binding activities of equine chorionic gonadotrophins. J. Reprod. Fert. 1981, 62, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.D.O.; Bittencourt, R.F.; Feliciano, M.A.R.; Santana, A.L.A.; Silva, M.A.D.A.; Felix, M.D.; Santana, L.R.; Barbosa, L.P. Ubdose of human chorionic gonadotropin applied at the Hou Hai acupoint on follicular dynamics and luteal development in donkeys. Anim. Reprod. 2020, 17, 554–563. [Google Scholar] [CrossRef]
- Quaresma, M.; Payan-Carreira, R. Characterization of the estrous cycle of Asinina de Miranda jennies (Equus asinus). Theriogenology 2015, 83, 606–624. [Google Scholar] [CrossRef]
- Li, N.; Yang, F.; Yu, J.; Yang, W.; Wu, S.; Ma, J.; Liu, B.; Zhang, R.; Zhou, X.; Losinno, L.; et al. Characteristics of follicular dynamics and reproductive hormone profiles during oestrous cycles of jennies over an entire year. Reprod. Domest. Anim. 2021, 56, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, D.; Khallili, K. Relationship between follicle growth and circulating gonadotrophin levels during postnatal development of sheep. Anim. Reprod. Sci. 2008, 16, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, J. Progress of ovarian granulosa cells in poly-cystic ovary syndrome. Chin. J. Prac. Gynecol. Obstet. 2019, 35, 134–136. [Google Scholar]
- Li, D.; You, Y.; Bi, F.-F.; Zhang, T.-N.; Jiao, J.; Wang, T.-R.; Zhou, Y.-M.; Shen, Z.-Q.; Wang, X.-X.; Yang, Q. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 2018, 155, 85–92. [Google Scholar] [CrossRef]
- Wu, H.-C.; Zhang, J.-W.; Sun, Z.-G.; Xiang, S.; Qiao, Y.; Lian, F. Effects of Electroacupuncture on Expression of PI3K/Akt/Foxo3a in Granulosa Cells from Women with Shen (Kidney) Deficiency Syndrome Undergoing in vitro Fertilization-Embryo Transfer. Chin. J. Integr. Med. 2019, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Brazil, D.P.; Yang, Z.Z.; Hemmings, B.A. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 2004, 29, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Calis, J.J.A.; Rosenberg, B.R. Characterizing immune repertoires by high throughput sequencing: Strategies and applications. Trends Immunol. 2014, 35, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Zhang, Q.; Wang, L.; Zeng, W. Effects of different sera on in vitro culture of donkey follicular granulosa cells. J. Shihezi Univ. 2021, 39, 440–445. [Google Scholar]
- Kanai, Y.; Shimizu, H. Plasma concentrations of LH, progesterone and oestradiol during the oestrous cycle in swamp buffaloes (Bubalus bubalis). Reproduction 1984, 70, 507–510. [Google Scholar] [CrossRef]
- Contri, A.; Robbe, D.; Gloria, A.; De Amicis, I.; Veronesi, M.; Carluccio, A. Effect of the season on some aspects of the estrous cycle in Martina Franca donkey. Theriogenology 2014, 81, 657–661. [Google Scholar] [CrossRef]
- Vandeplassche, G.M.; Wesson, J.; Ginther, O.J.T. Behavioral, follicular and gonadotropin changes during the estrous cycle in donkeys. Theriogenology 1981, 16, 239–249. [Google Scholar] [CrossRef]
- Gastal, E.L. Recent advances and new concepts on follicle and endocrine dynamics during the equine periovulatory period. Anim. Reprod. 2018, 6, 144–158. [Google Scholar]
- Dadarwal, D.; Tandon, S.N.; Purohit, G.N.; Pareek, P.K. Ultrasonographic evaluation of uterine involution and postpartum follicular dynamics in French Jennies (Equus asinus). Theriogenology 2004, 62, 257–264. [Google Scholar] [CrossRef]
- Magalhaes, H.B.; Canisso, I.F.; Dell-Aqua, J.A. The Temporal Associations of B-Mode and Power-Doppler Ultrasonography, and Ovarian Steroid Changes of the Periovulatory Follicle and Corpus Luteum During Luteogenesis and Luteolysis in Jennies. J. Equine Vet. Sci. 2023, 122, 104224. [Google Scholar] [CrossRef]
- Quaresma, M.; Sara, R.; Nóvoa, M.; Camillo, F.; Payan-Carreira, R. Follicular dynamics during the non-reproductive season in Miranda jennies. Reprod. Domest. Anim. 2022, 57, 616–624. [Google Scholar] [CrossRef]
- Ginther, O.; Woods, B.; Meira, C.; Beg, M.; Bergfelt, D.R. Hormonal mechanism of follicle deviation as indicated by major versus minor follicular waves during the transition into the anovulatory season in mares. Reproduction 2003, 126, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Donadeu, F.; Pedersen, H. Follicle development in mares. Reprod. Domest. Anim. 2008, 43, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Regassa, A.; Rings, F.; Hoelker, M.; Cinar, U.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Transcriptome dynamics and molecular cross-talk between bovine oocyte and its companion cumulus cells. BMC Genom. 2011, 12, 57–74. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185–195. [Google Scholar] [CrossRef]
- Matsuzawa, M.; Arai, C.; Nomura, Y.; Murata, T.; Yamakoshi, Y.; Oida, S.; Hanada, N.; Nakamura, Y. Periostin of human periodontal ligament fibroblasts promotes migration of human mesenchymal stem cell through the v3 integrin/FAK/PI3K/Akt pathway. J. Biol. Chem. 2015, 50, 855–863. [Google Scholar]
- Kitasaka, H.; Kawai, T.; Hoque, S.A.M.; Umehara, T.; Fujita, Y.; Shimada, M. Inductions of granulosa cell luteinization and cumulus expansion are dependent on the fibronectin-integrin pathway during ovulation process in mice. PLoS ONE 2018, 13, e0192458. [Google Scholar] [CrossRef]
- Cottom, J.; Salvador, L.M.; Maizels, E.T.; Reierstad, S.; Park, Y.; Carr, D.W.; Davare, M.A.; Hell, J.W.; Palmer, S.S.; Dent, P.; et al. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J. Biol. Chem. 2003, 278, 7167–7179. [Google Scholar] [CrossRef]
- Jia, Y.; Mo, S.-J.; Feng, Q.-Q.; Zhan, M.-L.; OuYang, L.-S.; Chen, J.-C.; Ma, Y.-X.; Wu, J.-J.; Lei, W.-L. EPO-dependent activation of PI3K/Akt/Foxo3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson disease. J. Mol. Neurosci. 2014, 53, 117–124. [Google Scholar] [CrossRef]
- Adhikari, D.; Adhikari, D.; Liu, K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009, 30, 438–464. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.-B.; Jiang, Z.-Z.; Hu, M.-W.; Lin, F.; Zhang, Q.-H.; Luo, Y.-B.; Hou, Y.; Zhao, Y.; Fan, H.-Y.; et al. Specific disruption of Tsc1 in ovarian granulosa cells promotes ovulation and causes progressive accumulation of corpora lutea. PLoS ONE 2013, 8, e54052. [Google Scholar] [CrossRef]
- Takeuchi, A.; Koga, K.; Satake, E.; Makabe, T.; Taguchi, A.; Miyashita, M.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; et al. Endometriosis Triggers Excessive Activation of Primordial Follicles via PI3K-PTEN-Akt-Foxo3 Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 5547–5554. [Google Scholar] [CrossRef]
- John, G.B.; Shirley, L.J.; Gallardo, T.D.; Castrillon, D.H. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction 2007, 133, 855–863. [Google Scholar] [CrossRef] [PubMed]
- John, G.B.; Gallardo, T.D.; Shirley, L.J.; Castrillon, D.H. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev. Biol. 2008, 321, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Benskey, M.J.; Manfredsson, F.P. Lentivirus Production and Purification. Methods Mol. Biol. 2016, 1382, 107–114. [Google Scholar] [PubMed]
- Leblanc, M.E.; Saez-Torres, K.L.; Cano, I.; Hu, Z.; Saint-Geniez, M.; Ng, Y.; D’Amore, P.A. Glycocalyx regulation of vascular endothelial growth factor receptor 2 activity. FASEB J. 2019, 33, 9362–9373. [Google Scholar] [CrossRef]
- Van Blerkom, J. Epigenetic influences on oocyte developmental competence: Perifollicular vascularity and intrafollicular oxygen. J. Assist. Reprod. Gen. 1998, 15, 226–234. [Google Scholar] [CrossRef]
- Liu, K.; Luo, J.; Shao, C.; Ren, Z.; Sun, S.; Zhu, Y.; Zhou, H.; Jiang, D.; Li, K.; Gu, W.; et al. Synaptotagmin 12 (SYT12) Gene Expression Promotes Cell Proliferation and Progression of Lung Adenocarcinoma and Involves the Phosphoinositide 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway. Med. Sci. Monit. 2020, 26, e920351. [Google Scholar] [CrossRef]
- Kaeser-Woo, Y.J.; Younts, T.J.; Yang, X.; Zhou, P.; Wu, D.; Castillo, P.E.; Südhof, T.C. Synaptotagmin-12 phosphorylation by cAMP-dependent protein kinase is essential for hippocampal mossy fiber LTP. J. Neurosci. 2013, 33, 9769–9780. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gao, Z.; Zhang, Q.; Guo, X.; Xia, W.; Gu, X.; Zeng, W. Unraveling the Transcriptomic Profiles of Large and Small Donkey Follicles. Genes 2025, 16, 602. https://doi.org/10.3390/genes16050602
Wang Y, Gao Z, Zhang Q, Guo X, Xia W, Gu X, Zeng W. Unraveling the Transcriptomic Profiles of Large and Small Donkey Follicles. Genes. 2025; 16(5):602. https://doi.org/10.3390/genes16050602
Chicago/Turabian StyleWang, Yanping, Zihao Gao, Qiang Zhang, Xuchuan Guo, Wei Xia, Xinli Gu, and Weibin Zeng. 2025. "Unraveling the Transcriptomic Profiles of Large and Small Donkey Follicles" Genes 16, no. 5: 602. https://doi.org/10.3390/genes16050602
APA StyleWang, Y., Gao, Z., Zhang, Q., Guo, X., Xia, W., Gu, X., & Zeng, W. (2025). Unraveling the Transcriptomic Profiles of Large and Small Donkey Follicles. Genes, 16(5), 602. https://doi.org/10.3390/genes16050602




