Transcriptomic Approach in Understanding Fabry Nephropathy: A Review of the Literature and Proof-of-Concept
Abstract
1. Introduction
1.1. Transcriptomic Studies in Chronic Kidney Disease
1.2. Transcriptomic Studies in Fabry Disease
2. Methods
2.1. Study Population
2.2. Sample Collection and RNA Sequencing
2.3. Bioinformatic and Statistical Analysis
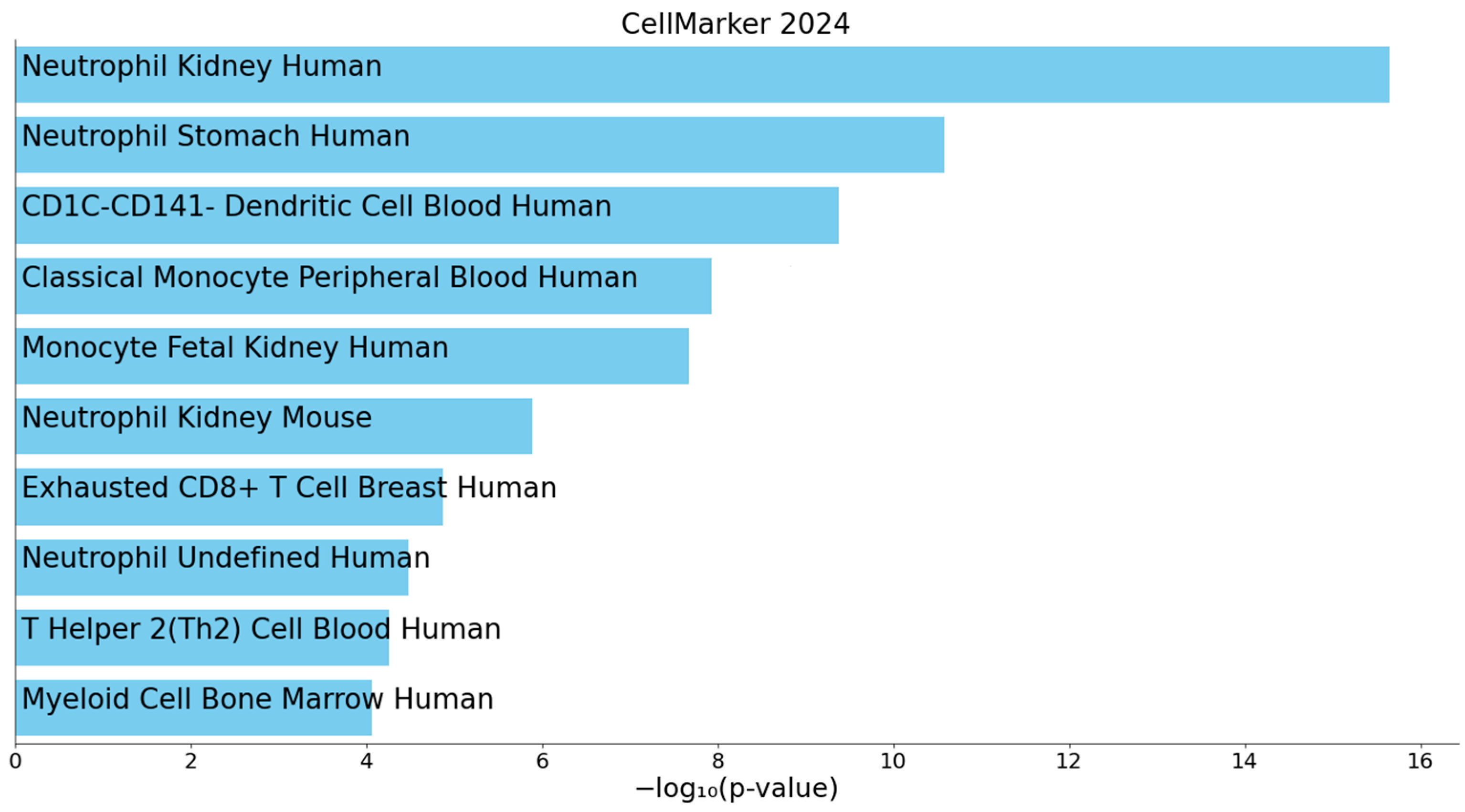
3. Results
3.1. Study Population Characteristics
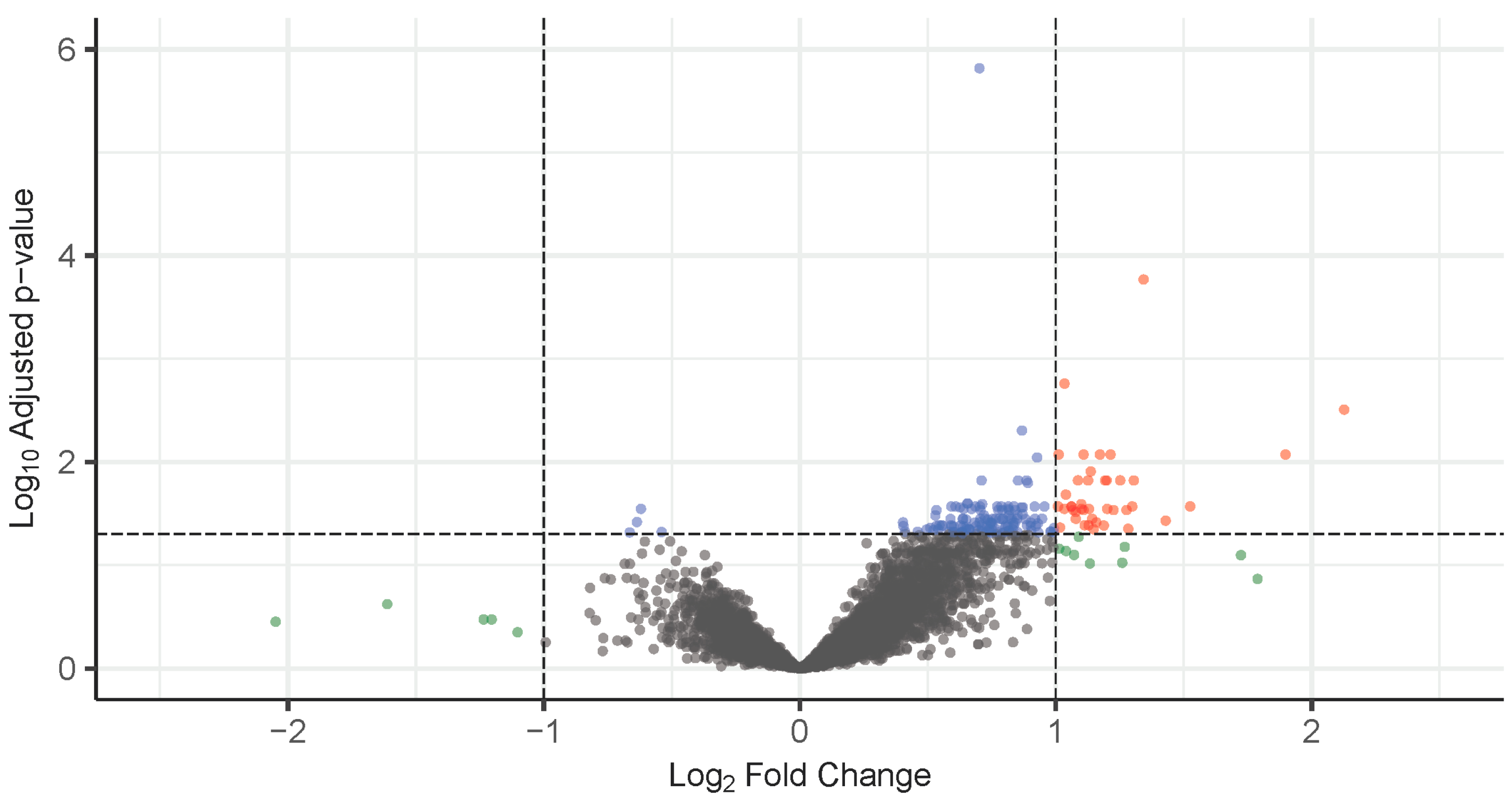
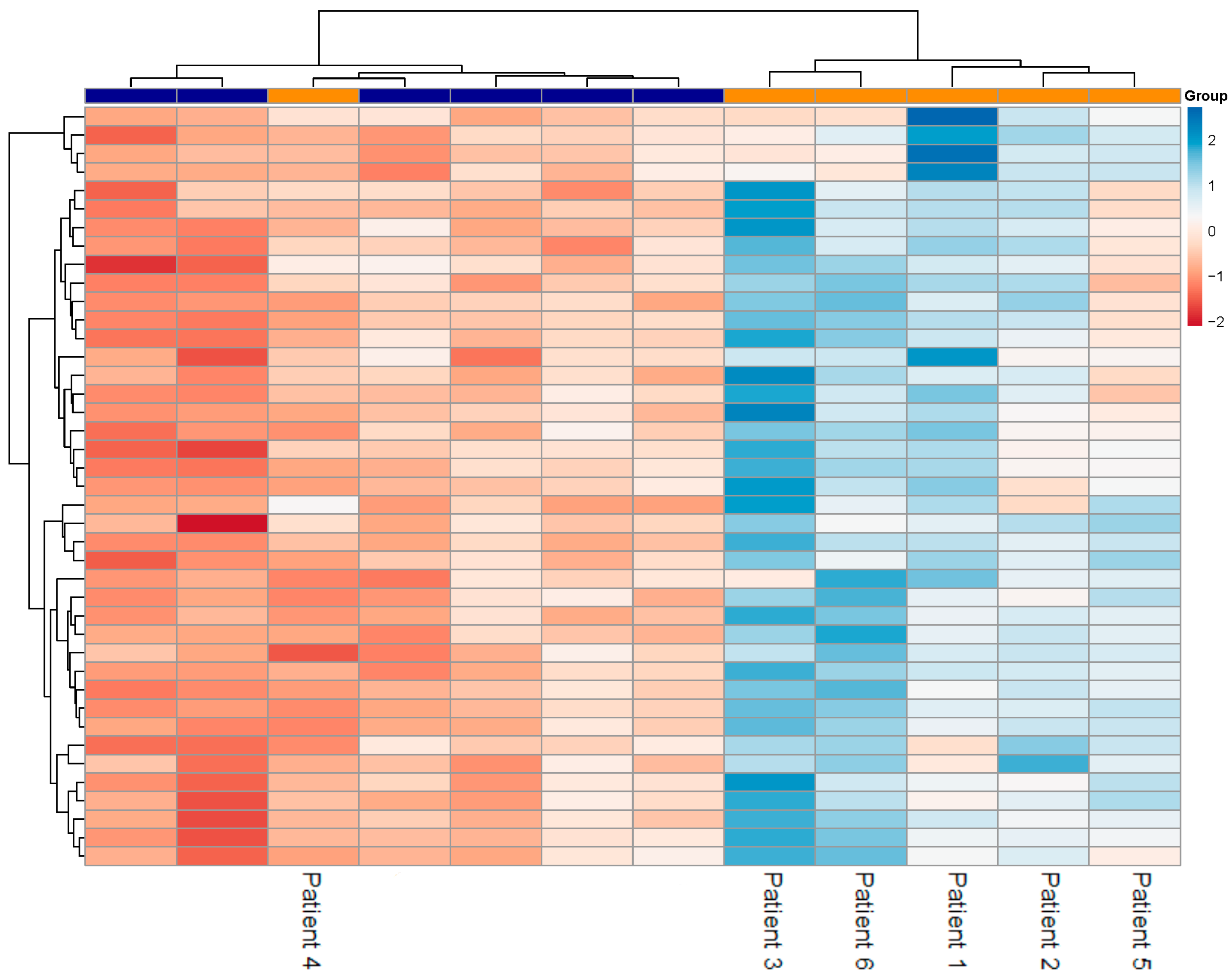
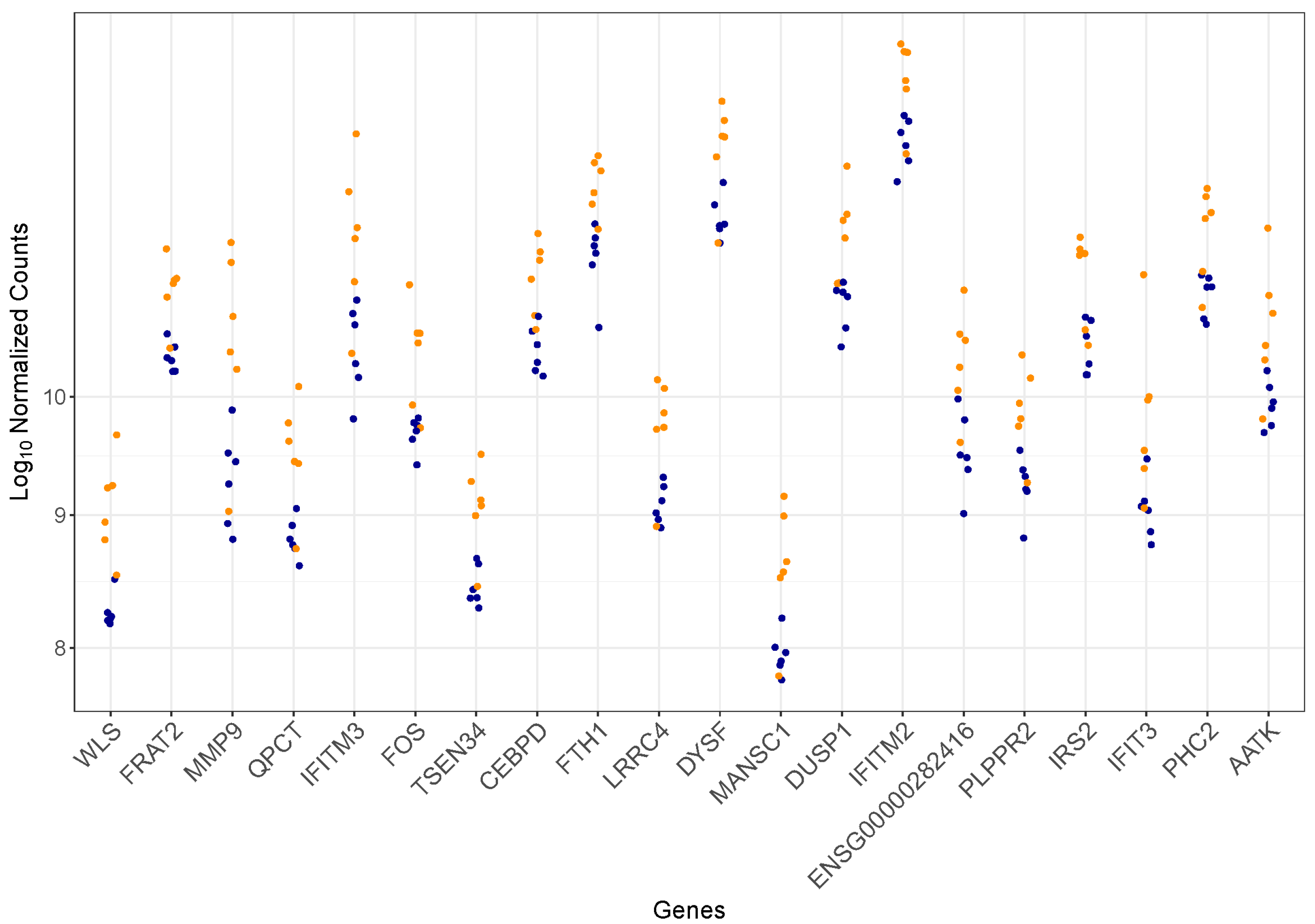
3.2. Differentially Expressed Genes
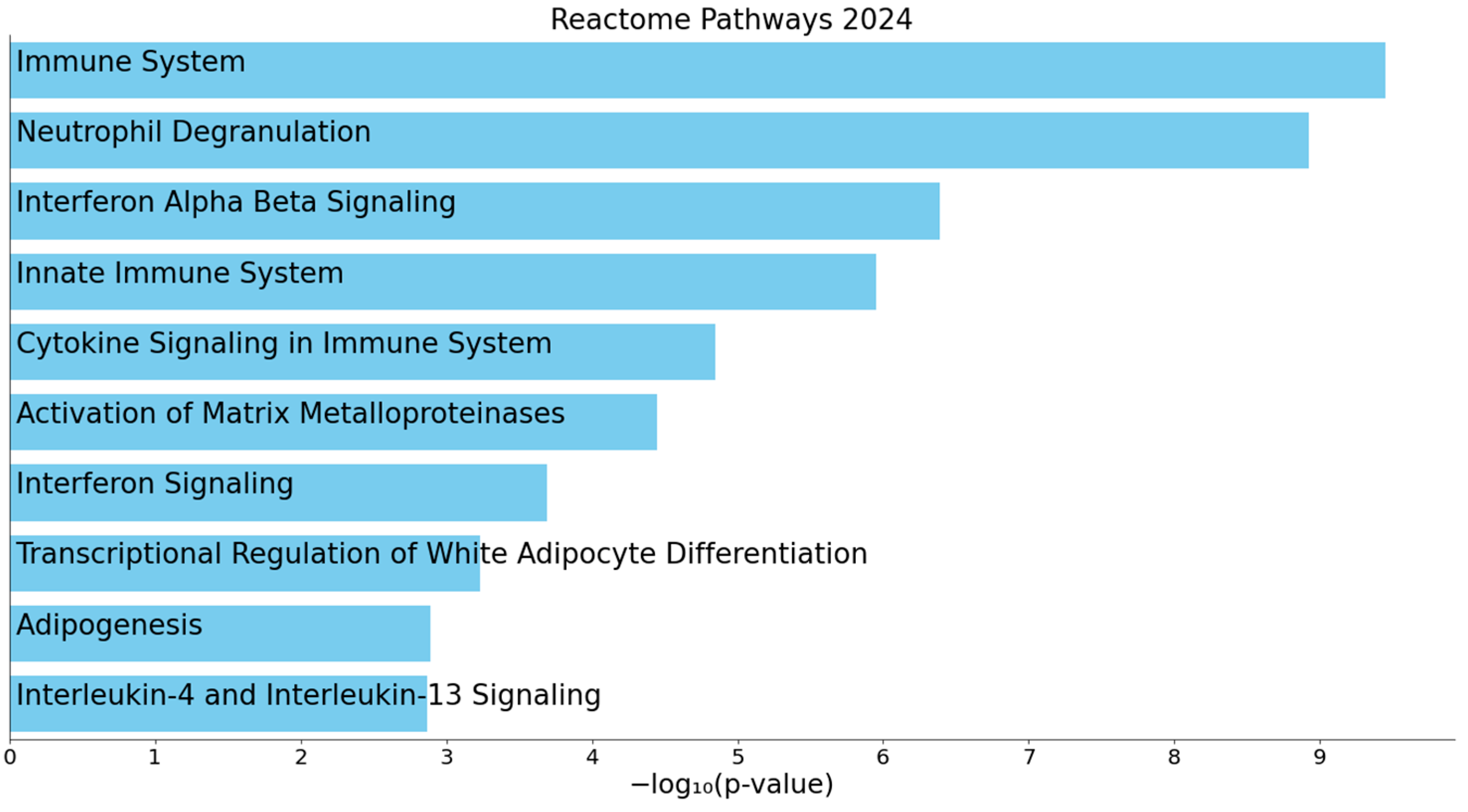
3.3. Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| DEGs | differentially expressed genes |
| DKD | diabetic kidney disease |
| ECM | extracellular matrix |
| eGFR | estimated glomerular filtration rate |
| EMT | epithelial–mesenchymal transition |
| ERT | enzyme replacement therapy |
| FD | Fabry disease |
| FDR | false discovery rate |
| FN | Fabry nephropathy |
| Gb3 | globotriaosylceramide |
| Lyso-Gb3 | globotriaosylsphingosine |
| MMPs | matrix metalloproteinases |
| TGF-β | transforming growth factor β |
| TLR4 | Toll-like receptor 4 |
References
- Germain, D.P. Fabry Disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef]
- Tøndel, C.; Bostad, L.; Hirth, A.; Svarstad, E. Renal Biopsy Findings in Children and Adolescents with Fabry Disease and Minimal Albuminuria. Am. J. Kidney Dis. 2008, 51, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Cianciaruso, B.; Cizmarik, M.; Germain, D.P.; Mignani, R.; Oliveira, J.P.; Villalobos, J.; Vujkovac, B.; Waldek, S.; Wanner, C.; et al. End-Stage Renal Disease in Patients with Fabry Disease: Natural History Data from the Fabry Registry. Nephrol. Dial. Transplant. 2010, 25, 769–775. [Google Scholar] [CrossRef]
- Schiffmann, R.; Warnock, D.G.; Banikazemi, M.; Bultas, J.; Linthorst, G.E.; Packman, S.; Sorensen, S.A.; Wilcox, W.R.; Desnick, R.J. Fabry Disease: Progression of Nephropathy, and Prevalence of Cardiac and Cerebrovascular Events before Enzyme Replacement Therapy. Nephrol. Dial. Transplant. 2009, 24, 2102. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Waldek, S.; Banikazemi, M.; Bushinsky, D.A.; Charrow, J.; Desnick, R.J.; Lee, P.; Loew, T.; Vedder, A.C.; Abichandani, R.; et al. Sustained, Long-Term Renal Stabilization after 54 Months of Agalsidase β Therapy in Patients with Fabry Disease. J. Am. Soc. Nephrol. 2007, 18, 1547–1557. [Google Scholar] [CrossRef]
- Verovnik, F.; Benko, D.; Vujkovac, B.; Linthorst, G.E. Remarkable Variability in Renal Disease in a Large Slovenian Family with Fabry Disease. Eur. J. Hum. Genet. 2004, 12, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Munaut, C.; Bergijk, E.C.; Baelde, J.J.; Noel, A.; Foidart, J.M.; Bruijn, J.A. A Molecular Biologic Study of Extracellular Matrix Components during the Development of Glomerulosclerosis in Murine Chronic Graft-versus-Host Disease. Lab. Investig. 1992, 67, 580–587. [Google Scholar] [PubMed]
- Rudnicki, M.; Perco, P.; D’haene, B.; Leierer, J.; Heinzel, A.; Mühlberger, I.; Schweibert, N.; Sunzenauer, J.; Regele, H.; Kronbichler, A.; et al. Renal MicroRNA- and RNA-Profiles in Progressive Chronic Kidney Disease. Eur. J. Clin. Investig. 2016, 46, 213–226. [Google Scholar] [CrossRef]
- Scherer, A.; Günther, O.P.; Balshaw, R.F.; Hollander, Z.; Wilson-Mcmanus, J.; Ng, R.; McMaster, W.R.; McManus, B.M.; Keown, P.A. Alteration of Human Blood Cell Transcriptome in Uremia. BMC Med. Genom. 2013, 6, 23. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Zhou, Y. Identification of Hub Genes in Diabetic Kidney Disease via Multiple-Microarray Analysis. Ann. Transl. Med. 2020, 8, 997. [Google Scholar] [CrossRef]
- Sur, S.; Nguyen, M.; Boada, P.; Sigdel, T.K.; Sollinger, H.; Sarwal, M.M. FcER1: A Novel Molecule Implicated in the Progression of Human Diabetic Kidney Disease. Front. Immunol. 2021, 12, 769972. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Lee, C.H.; Moon, M.H.; Hong, G.R.; Cheon, C.K.; Lee, J.S. Unravelling the Mechanism of Action of Enzyme Replacement Therapy in Fabry Disease. J. Hum. Genet. 2016, 61, 143–149. [Google Scholar] [CrossRef]
- Delaleu, N.; Marti, H.P.; Strauss, P.; Sekulic, M.; Osman, T.; Tøndel, C.; Skrunes, R.; Leh, S.; Svarstad, E.; Nowak, A.; et al. Systems Analyses of the Fabry Kidney Transcriptome and Its Response to Enzyme Replacement Therapy Identified and Cross-Validated Enzyme Replacement Therapy-Resistant Targets Amenable to Drug Repurposing. Kidney Int. 2023, 104, 803–819. [Google Scholar] [CrossRef]
- Snanoudj, S.; Derambure, C.; Zhang, C.; Hai Yen, N.T.; Lesueur, C.; Coutant, S.; Abily-Donval, L.; Marret, S.; Yang, H.; Mardinoglu, A.; et al. Genome-Wide Expression Analysis in a Fabry Disease Human Podocyte Cell Line. Heliyon 2024, 10, e34357. [Google Scholar] [CrossRef]
- Wanner, C.; Arad, M.; Baron, R.; Burlina, A.; Elliott, P.M.; Feldt-Rasmussen, U.; Fomin, V.V.; Germain, D.P.; Hughes, D.A.; Jovanovic, A.; et al. European Expert Consensus Statement on Therapeutic Goals in Fabry Disease. Mol. Genet. Metab. 2018, 124, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Levstek, T.; Bahčič, E.; Vujkovac, B.; Cokan Vujkovac, A.; Tesovnik, T.; Remec, Ž.I.; Čuk, V.; Trebušak Podkrajšek, K. Telomere Length, Oxidative Stress, and Kidney Damage Biomarkers in Fabry Nephropathy. Cells 2025, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90. [Google Scholar] [CrossRef]
- Granata, S.; Dalla Gassa, A.; Bellin, G.; Lupo, A.; Zaza, G. Transcriptomics: A Step behind the Comprehension of the Polygenic Influence on Oxidative Stress, Immune Deregulation, and Mitochondrial Dysfunction in Chronic Kidney Disease. Biomed. Res. Int. 2016, 2016, 9290857. [Google Scholar] [CrossRef] [PubMed]
- Wodarz, A.; Nusse, R. Mechanisms of Wnt Signaling in Development. Annu. Rev. Cell Dev. Biol. 1998, 14, 59–88. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Dai, C.; Li, Y.; Zeng, G.; Monga, S.P.; Liu, Y. Wnt/ β-Catenin Signaling Promotes Renal Interstitial Fibrosis. J. Am. Soc. Nephrol. 2009, 20, 765–776. [Google Scholar] [CrossRef]
- Dai, C.; Stolz, D.B.; Kiss, L.P.; Monga, S.P.; Holzman, L.B.; Liu, Y. Wnt/β-Catenin Signaling Promotes Podocyte Dysfunction and Albuminuria. J. Am. Soc. Nephrol. 2009, 20, 1997–2008. [Google Scholar] [CrossRef]
- Kato, H.; Gruenwald, A.; Suh, J.H.; Miner, J.H.; Barisoni-Thomas, L.; Taketo, M.M.; Faul, C.; Millar, S.E.; Holzman, L.B.; Susztak, K. Wnt/β-Catenin Pathway in Podocytes Integrates Cell Adhesion, Differentiation, and Survival. J. Biol. Chem. 2011, 286, 26003–26015. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; He, X.; Cheng, R.; Zhang, B.; Zhang, R.R.; Chen, Y.; Takahashi, Y.; Murray, A.R.; Lee, K.; Gao, G.; et al. Implication of Dysregulation of the Canonical Wingless-Type MMTV Integration Site (WNT) Pathway in Diabetic Nephropathy. Diabetologia 2012, 55, 255–266. [Google Scholar] [CrossRef]
- Stamenkovic, I. Extracellular Matrix Remodelling: The Role of Matrix Metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef]
- Tan, R.J.; Liu, Y. Matrix Metalloproteinases in Kidney Homeostasis and Diseases. Am. J. Physiol.-Ren. Physiol. 2012, 302, 1351–1361. [Google Scholar] [CrossRef]
- Tan, R.J.; Liu, Y. Matrix Metalloproteinases in Kidney Homeostasis and Diseases: An Update. Am. J. Physiol.-Ren. Physiol. 2024, 327, F967–F984. [Google Scholar] [CrossRef]
- Li, S.Y.; Huang, P.H.; Yang, A.H.; Tarng, D.C.; Yang, W.C.; Lin, C.C.; Chen, J.W.; Schmid-Schönbein, G.; Lin, S.J. Matrix Metalloproteinase-9 Deficiency Attenuates Diabetic Nephropathy by Modulation of Podocyte Functions and Dedifferentiation. Kidney Int. 2014, 86, 358–369. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Jung, N.; Park, J.W.; Park, H.Y.; Jung, S.C. Epithelial–Mesenchymal Transition in Kidney Tubular Epithelial Cells Induced by Globotriaosylsphingosine and Globotriaosylceramide. PLoS ONE 2015, 10, e0136442. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Yoo, T.H. TGF-β Inhibitors for Therapeutic Management of Kidney Fibrosis. Pharmaceuticals 2022, 15, 1485. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Lichtnekert, J.; Allam, R. Interferon-α and -β in Kidney Inflammation. Kidney Int. 2010, 77, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, G.; Anders, H.J. Neutrophils, Dendritic Cells, Toll-Like Receptors, and Interferon-α in Lupus Nephritis. Semin. Nephrol. 2015, 35, 410–426. [Google Scholar] [CrossRef]
- De Francesco, P.N.; Mucci, J.M.; Ceci, R.; Fossati, C.A.; Rozenfeld, P.A. Fabry Disease Peripheral Blood Immune Cells Release Inflammatory Cytokines: Role of Globotriaosylceramide. Mol. Genet. Metab. 2013, 109, 93–99. [Google Scholar] [CrossRef]
- Lin, M.; Yiu, W.H.; Wu, H.J.; Chan, L.Y.Y.; Leung, J.C.K.; Au, W.S.; Chan, K.W.; Lai, K.N.; Tang, S.C.W. Toll-Like Receptor 4 Promotes Tubular Inflammation in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2011, 23, 86. [Google Scholar] [CrossRef]
- Rozenfeld, P.; Feriozzi, S. Contribution of Inflammatory Pathways to Fabry Disease Pathogenesis. Mol. Genet. Metab. 2017, 122, 19–27. [Google Scholar] [CrossRef]
- Anders, H.J.; Banas, B.; Schlöndorff, D. Signaling Danger: Toll-Like Receptors and Their Potential Roles in Kidney Disease. J. Am. Soc. Nephrol. 2004, 15, 854–867. [Google Scholar] [CrossRef]
- Wagner, H. Endogenous TLR Ligands and Autoimmunity. Adv. Immunol. 2006, 91, 159–173. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef]
- Fogo, A.B.; Bostad, L.; Svarstad, E.; Cook, W.J.; Moll, S.; Barbey, F.; Geldenhuys, L.; West, M.; Ferluga, D.; Vujkovac, B.; et al. Scoring System for Renal Pathology in Fabry Disease: Report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol. Dial. Transplant. 2010, 25, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Wagenhäuser, L.; Rickert, V.; Sommer, C.; Wanner, C.; Nordbeck, P.; Rost, S.; Üçeyler, N. X-Chromosomal Inactivation Patterns in Women with Fabry Disease. Mol. Genet. Genom. Med. 2022, 10, e2029. [Google Scholar] [CrossRef] [PubMed]
- Alroy, J.; Sabnis, S.; Kopp, J.B. Renal Pathology in Fabry Disease. J. Am. Soc. Nephrol. 2002, 13, S134–S138. [Google Scholar] [CrossRef] [PubMed]
- Wanner, N.; Hartleben, B.; Herbach, N.; Goedel, M.; Stickel, N.; Zeiser, R.; Walz, G.; Moeller, M.J.; Grahammer, F.; Huber, T.B. Unraveling the Role of Podocyte Turnover in Glomerular Aging and Injury. J. Am. Soc. Nephrol. 2014, 25, 707–716. [Google Scholar] [CrossRef]
- Harkness, A.; Ring, L.; Augustine, D.X.; Oxborough, D.; Robinson, S.; Sharma, V. Normal Reference Intervals for Cardiac Dimensions and Function for Use in Echocardiographic Practice: A Guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, X1. [Google Scholar] [CrossRef]
| FD-PN (n = 6) | Control Group (n = 6) | p-Value | |
|---|---|---|---|
| Male (%) | 3 (50) | 3 (50) | >0.999 |
| Age (years) | 56.2 (47.5–59.4) | 56.6 (48.4–60.1) | 0.818 |
| eGFR (mL/min/1.73 m2) | 58.0 (47.0–87.8) | 94.5 (93.3–96.5) | 0.199 |
| S-Cr (μmol/L) | 109.0 (78.8–141.5) | 69.5 (62.5–78.0) | 0.078 |
| UPCR (g/mol) | 9.2 (8.7–52.8) | 7.2 (6.5–7.3) | 0.052 |
| UACR (g/mol) | 1.1 (1.0–508.6) | 0.6 (0.4–0.8) | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breznik, N.; Levstek, T.; Vujkovac, B.; Cokan Vujkovac, A.; Trebušak Podkrajšek, K. Transcriptomic Approach in Understanding Fabry Nephropathy: A Review of the Literature and Proof-of-Concept. Genes 2025, 16, 601. https://doi.org/10.3390/genes16050601
Breznik N, Levstek T, Vujkovac B, Cokan Vujkovac A, Trebušak Podkrajšek K. Transcriptomic Approach in Understanding Fabry Nephropathy: A Review of the Literature and Proof-of-Concept. Genes. 2025; 16(5):601. https://doi.org/10.3390/genes16050601
Chicago/Turabian StyleBreznik, Nika, Tina Levstek, Bojan Vujkovac, Andreja Cokan Vujkovac, and Katarina Trebušak Podkrajšek. 2025. "Transcriptomic Approach in Understanding Fabry Nephropathy: A Review of the Literature and Proof-of-Concept" Genes 16, no. 5: 601. https://doi.org/10.3390/genes16050601
APA StyleBreznik, N., Levstek, T., Vujkovac, B., Cokan Vujkovac, A., & Trebušak Podkrajšek, K. (2025). Transcriptomic Approach in Understanding Fabry Nephropathy: A Review of the Literature and Proof-of-Concept. Genes, 16(5), 601. https://doi.org/10.3390/genes16050601





