Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Revolution in Brazilian Agribusiness
Abstract
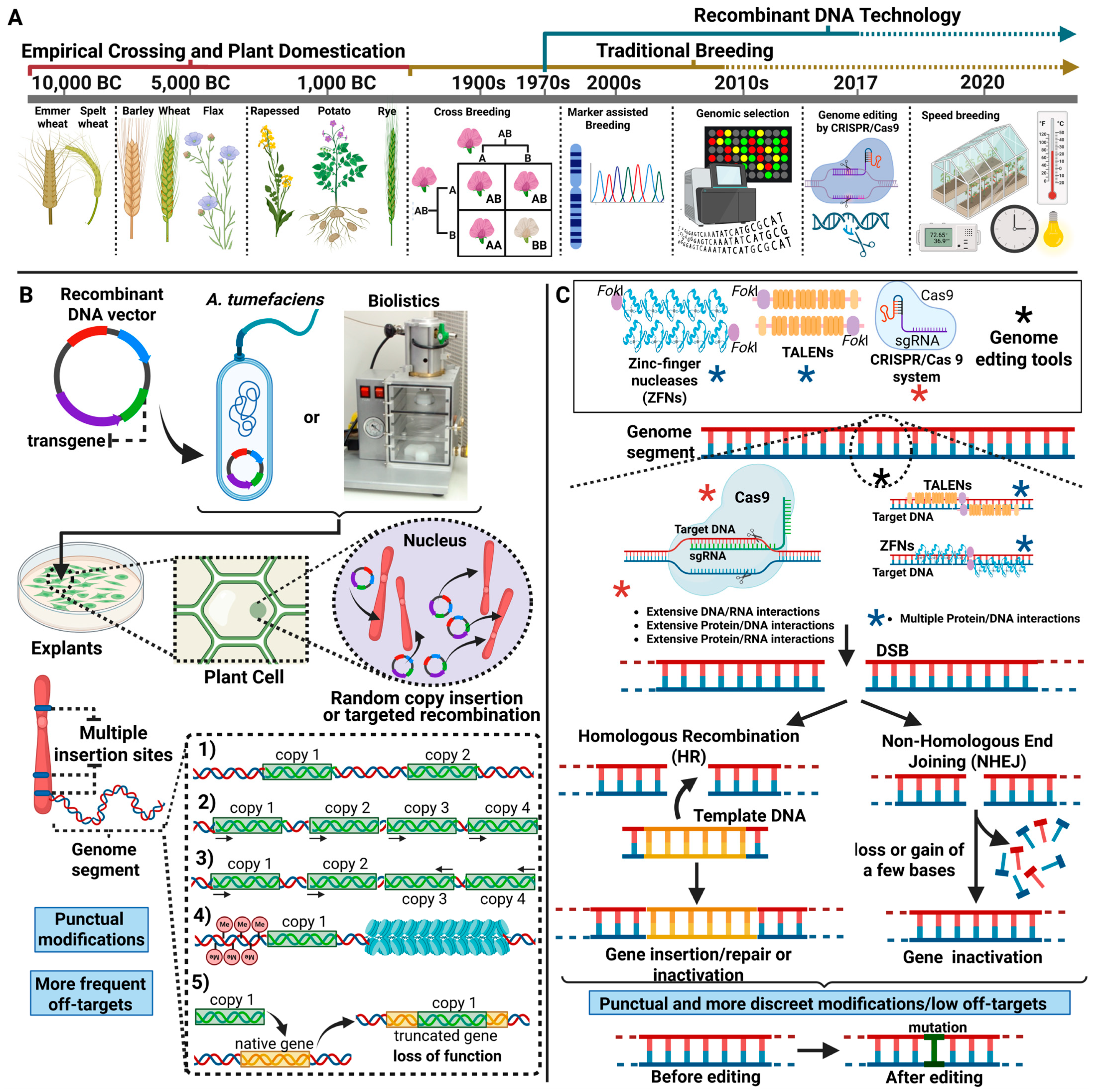
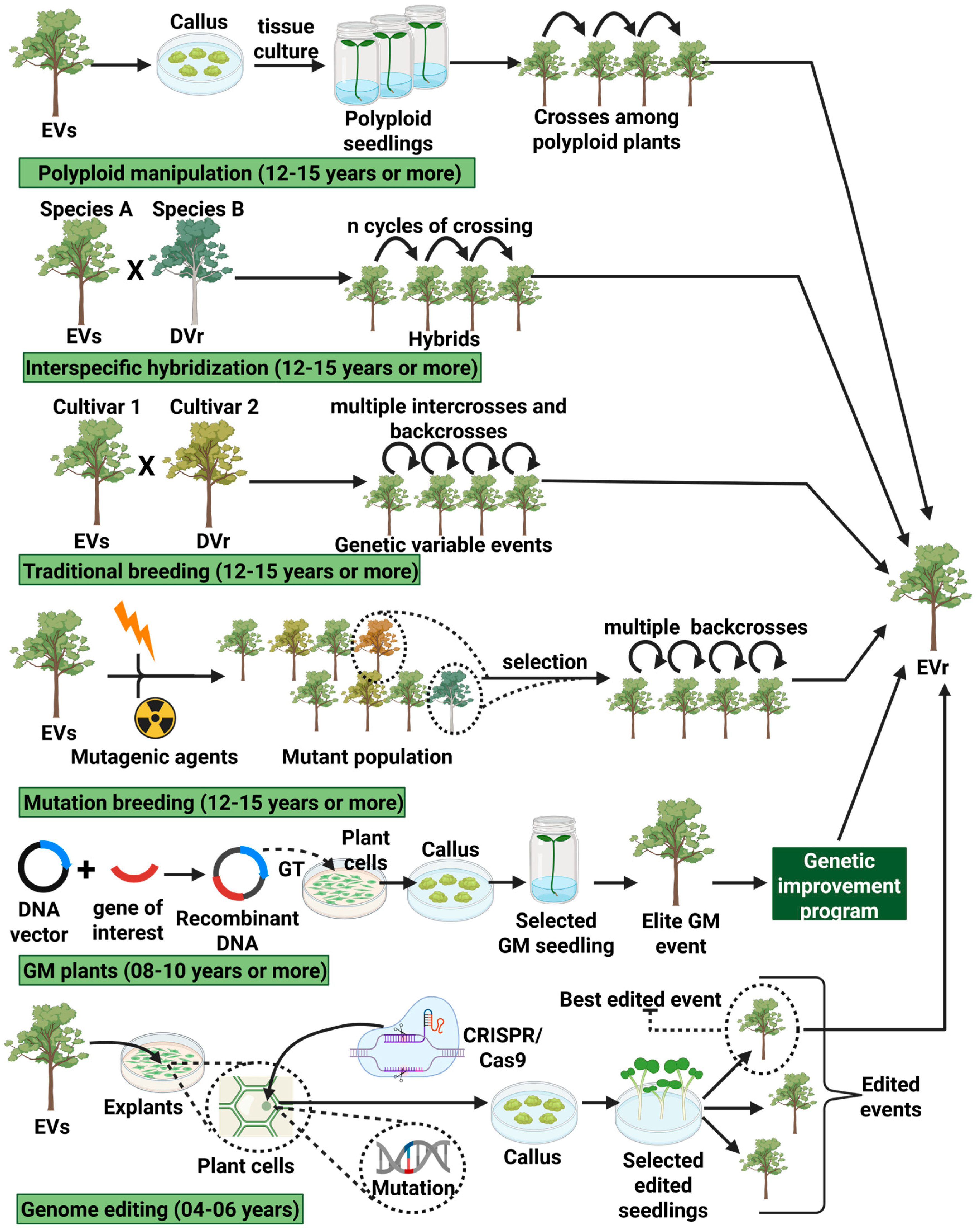
1. Introduction
- The high complexity and size of plant eukaryotic genomes, notably presenting extensions of the order of a few hundred million bases (Megabase) to a few billion bases (Gigabase) [15].
- The abundance of extensive non-coding genomic regions full of repetitive sequences [16].
- The presence of extensive regions of heterochromatin associated with hypercondensed DNA, where genes often present low levels of expression [19].
2. The Brazilian Biosafety Law and GMOs
3. Legal Regulation of Published Organizations in Brazil: Normative Resolution No. 16
4. The GMOs and Edited Organisms’ Regulatory System in Brazil
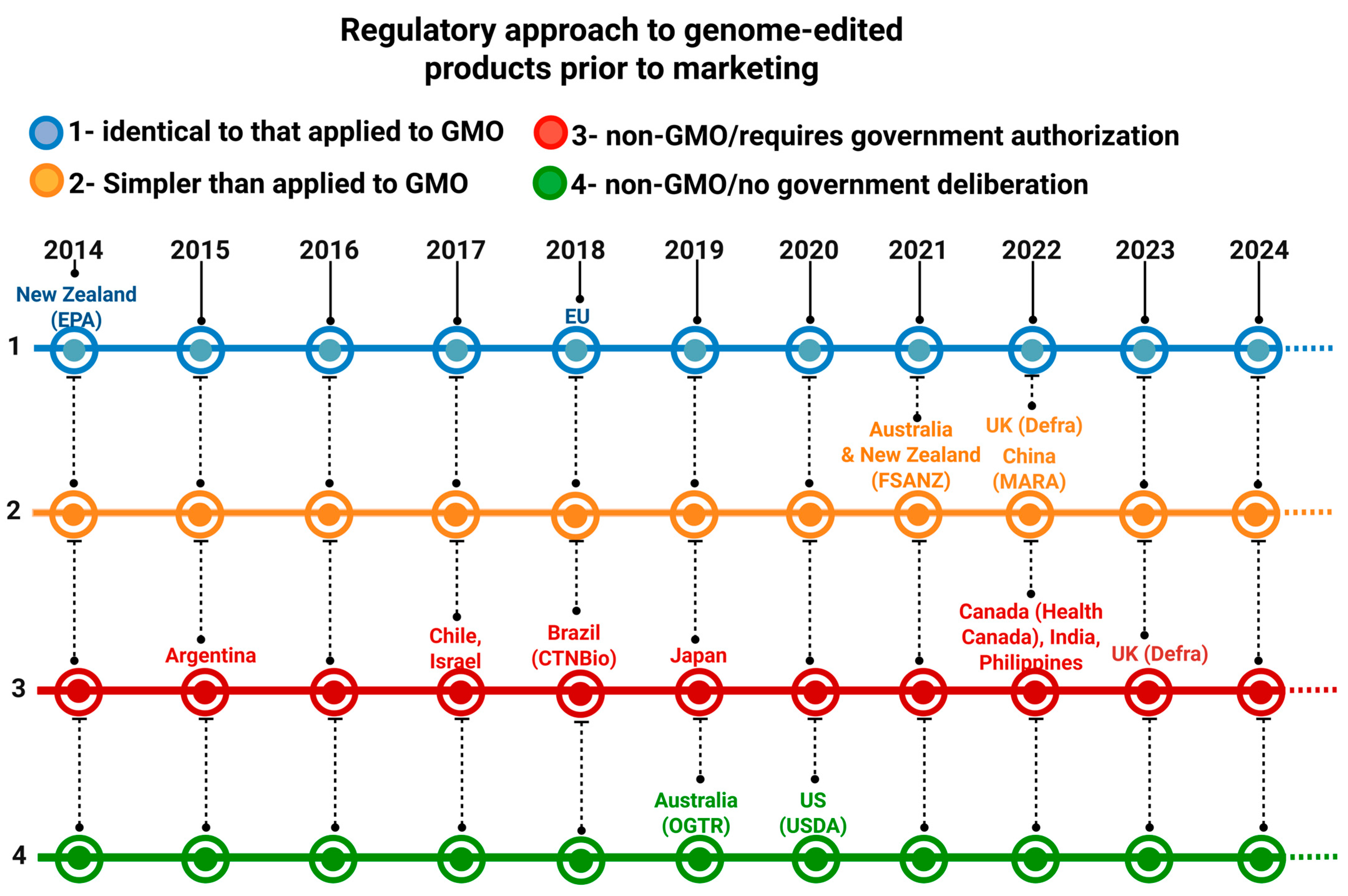
5. Effects of RN16 on Brazilian Agribusiness
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, A.; Hassan, A.U.; Khan, S.H.; Abbasi, A.; Hina, A.; Khan, M.T.; Abdelsalam, N.R. The Changing Landscape of Agriculture: Role of Precision Breeding in Developing Smart Crops. Funct. Integr. Genom. 2023, 23, 167. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Simmons, I.; Roberts, B. The Origins and Spread of Agriculture; Cambridge University Press: Cambridge, UK, 1998; pp. 13–26. [Google Scholar]
- Lamichhane, S.; Thapa, S. Advances from Conventional to Modern Plant Breeding Methodologies. Plant Breed. Biotechnol. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of Recombinant DNA Technology to Improve Life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Matsuo, M. Global Regulatory Trends of Genome Editing Technology in Agriculture and Food. Breed. Sci. 2024, 74, 3–10. [Google Scholar] [CrossRef]
- Obembe, O.O.; Popoola, J.O.; Leelavathi, S.; Reddy, S.V. Advances in Plant Molecular Farming. Biotechnol. Adv. 2011, 29, 210–222. [Google Scholar] [CrossRef]
- da Cunha, N.B.; Leite, M.L.; Rodrigues, J.C.M.; Carrijo, J.; Costa, F.F.; Dias, S.C.; Rech, E.L.; Vianna, G.R. Molecular Tools for Genome Editing in Plants: A Synthetic Overview. Veg. Res. 2025, 5, e010. [Google Scholar] [CrossRef]
- Su, W.; Xu, M.; Radani, Y.; Yang, L. Technological Development and Application of Plant Genetic Transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Tang, Q.; Zhang, H.; Liu, T.; Wang, Y. Trends in the Global Commercialization of Genetically Modified Crops in 2023. J. Integr. Agric. 2024, 23, 3943–3952. [Google Scholar] [CrossRef]
- International Service for the Acquisition of Agri-biotech Applications. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA Briefs; ISAAA: Los Baños, Philippines, 2019. [Google Scholar]
- Agbio Investor. Agbio Investor Global GM Crop Area Review. 2024. Available online: https://gm.agbioinvestor.com (accessed on 18 February 2024).
- Kuiken, T.; Kuzma, J. Genome Editing in Latin America: Regional Regulatory Overview; IDB: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Biswas, S.; Srivastava, A.; Yadav, S.; Mishra, Y. Evolution of Genetically Modified (GM) Crops and The Scared World. In Policy Issues in Genetically Modified Crops; Elsevier: Singapore, 2020; pp. 317–334. [Google Scholar] [CrossRef]
- Karalis, D.T.; Karalis, T.; Karalis, S.; Kleisiari, A.S. Genetically Modified Products, Perspectives and Challenges. Cureus 2020, 12, e7306. [Google Scholar] [CrossRef]
- Lynch, M.; Conery, J.S. The Origins of Genome Complexity. Science 2003, 302, 1401–1404. [Google Scholar] [CrossRef]
- Lane, N.; Martin, W.F. Eukaryotes Really Are Special, and Mitochondria Are Why. Proc. Natl. Acad. Sci. USA 2015, 112, E4823. [Google Scholar] [CrossRef] [PubMed]
- Harkess, A. Smashing Barriers in Biolistic Plant Transformation. Plant Cell. Engl. Febr. 2019, 31, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Stapley, J.; Feulner, P.G.D.; Johnston, S.E.; Santure, A.W.; Smadja, C.M. Recombination: The Good, the Bad and the Variable. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2017, 372, 20170279. [Google Scholar] [CrossRef] [PubMed]
- Allshire, R.C.; Madhani, H.D. Ten Principles of Heterochromatin Formation and Function. Nat. Rev. Mol. Cell Biol. 2018, 19, 229–244. [Google Scholar] [CrossRef]
- Gu, P.; Yang, F.; Su, T.; Wang, Q.; Liang, Q.; Qi, Q. A Rapid and Reliable Strategy for Chromosomal Integration of Gene(s) with Multiple Copies. Sci. Rep. 2015, 5, 9684. [Google Scholar] [CrossRef]
- Schlötterer, C. The Evolution of Molecular Markers—Just a Matter of Fashion? Nat. Rev. Genet. 2004, 5, 63–69. [Google Scholar] [CrossRef]
- Tripodi, P. The Evolution of Molecular Genotyping in Plant Breeding. Agronomy 2023, 13, 2569. [Google Scholar] [CrossRef]
- Heather, J.M.; Chain, B. The Sequence of Sequencers: The History of Sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Chaney, L.; Sharp, A.R.; Evans, C.R.; Udall, J.A. Genome Mapping in Plant Comparative Genomics. Trends Plant Sci. 2016, 21, 770–780. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Deng, B.; Jiang, Y.; Zhu, J.; Chen, L.; Li, M.; Li, J. Applications and Prospects of CRISPR/Cas9-Mediated Base Editing in Plant Breeding. Curr. Issues Mol. Biol. 2023, 45, 918–935. [Google Scholar] [CrossRef]
- Ku, H.-K.; Ha, S.-H. Improving Nutritional and Functional Quality by Genome Editing of Crops: Status and Perspectives. Front. Plant Sci. 2020, 11, 577313. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.V.; Nguyen, N.T.; Kim, J.; Hong, J.C.; Kim, J.-Y. Prime Editing: Mechanism Insight and Recent Applications in Plants. Plant Biotechnol. J. 2024, 22, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.; Nain, V. TALENs-an Indispensable Tool in the Era of CRISPR: A Mini Review. J. Genet. Eng. Biotechnol. 2021, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed Breeding Is a Powerful Tool to Accelerate Crop Research and Breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Presidência da República Casa Civil. Lei Nº 11,105, de 24 de março de 2005. Regulamenta os Incisos II, IV e V do § 1º do art. 225 da Constituição Federal. Regulamenta os incisos II, IV e V do § 1º do art. 225 da Constituição Federal, Estabelece Normas de Segurança e Mecanismos de Fiscalização de Atividades que Envolvam Organismos Geneticamente Modificados–OGM e seus Derivados, cria o Conselho Nacional de Biossegurança–CNBS, Reestrutura a Comissão Técnica Nacional de Biossegurança–CTNBio, Dispõe Sobre a Política Nacional de Biossegurança–PNB, Revoga a Lei nº 8.974, de 5 de Janeiro de 1995, e a Medida Provisória nº 2.191-9, de 23 de Agosto de 2001, e os Arts. 5º, 6º, 7º, 8º, 9º, 10 e 16 da Lei nº 10.814, de 15 de Dezembro de 2003, e dá Outras Providências. Diário Oficial da União, 28 de Março, de 2005. p. 1. Available online: http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2005/lei/l11105.htm (accessed on 18 February 2024).
- Fontes, E.M.G. Legal and regulatory concerns about transgenic plants in Brazil. J. Invertebr Pathol. 2003, 83, 100–103. [Google Scholar] [CrossRef]
- Rios, L.V.; Natália, V.; Freitas, C.; Justen, F.; Fuganti-Pagliarini, R.; Soares, M.S.; Hugo, F.; Correa, B.A.; Domingues, M.E.; Romano, V.E.; et al. Regulatory Framework of Genome Editing in Brazil and Worldwide. In CRISPR Technology in Plant Genome Editing: Biotechnology Applied to Agriculture; Embrapa: Brasilia, Brazil, 2021. [Google Scholar]
- Alvarez, D.; Cerda-Bennasser, P.; Stowe, E.; Ramirez-Torres, F.; Capell, T.; Dhingra, A.; Christou, P. Fruit Crops in the Era of Genome Editing: Closing the Regulatory Gap. Plant Cell Rep. 2021, 40, 915–930. [Google Scholar] [CrossRef]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise Plant Genome Editing Using Base Editors and Prime Editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef]
- Velini, E.D.; Dagli, M.L.Z.; de Souza, G.D.; Nascimento, R.J.; Pinho, T.F.; de Andrade, P.P.; Carrer, H. The Brazilian GMO Regulatory System: A Historical View and Perspective. In Genetically Modified Organisms in Developing Countries: Risk Analysis and Governance; Adenle, A.A., Morris, E.J., Murphy, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 258–270. [Google Scholar] [CrossRef]
- Menz, J.; Modrzejewski, D.; Hartung, F.; Wilhelm, R.; Sprink, T. Genome Edited Crops Touch the Market: A View on the Global Development and Regulatory Environment. Front. Plant Sci. 2020, 11, 586027. [Google Scholar] [CrossRef]
- Comissão Técnica Nacional de Biossegurança–CTNBio. Resolução Normativa Nº 16, de 15 de janeiro de 2018. Estabelece os Requisitos Técnicos Para Apresentação de Consulta à CTNBio Sobre as Técnicas Inovadoras de Melhoramento de Precisão. Available online: http://ctnbio.mctic.gov.br/resolucoes-normativas/-/asset_publisher/OgW431Rs9dQ6/content/resolucao-normativa-n%C2%BA-16-de-15-de-janeiro-de-2018?redirect=http%3A%2F%2Fctnbio.mctic.gov.br%2Fresolucoes-normativas%3Fp_p_id%3D101_INSTANCE_OgW431Rs9dQ6%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn-2%26p_p_col_count%3D3 (accessed on 18 February 2024).
- Tachikawa, M.; Matsuo, M. Divergence and Convergence in International Regulatory Policies Regarding Genome-Edited Food: How to Find a Middle Ground. Front. Plant Sci. 2023, 14, 1105426. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- CTNBio. Tecnologias inovadoras de melhoramento genético (RN16). Available online: http://ctnbio.mctic.gov.br/tecnologias-inovadoras-de-melhoramento-genetico-rn16-/-/document_library_display/cuSvsid1CsUD/view/2304555?_110_INSTANCE_cuSvsid1CsUD_redirect=http%3A%2F%2Fctnbio.mctic.gov.br%2Ftecnologias-inovadoras-de-melhoramento-genetico-rn16-%3Fp_p_id%3D110_INSTANCE_cuSvsid1CsUD%26p_p_lifecycle%3D0%26p_p_state%3Dnormal%26p_p_mode%3Dview%26p_p_col_id%3Dcolumn-2%26p_p_col_count%3D1 (accessed on 27 February 2025).
- Li, H.; Zhu, Z.; Li, S.; Li, J.; Yan, L.; Zhang, C.; Ma, Y.; Xia, L. Multiplex Precision Gene Editing by a Surrogate Prime Editor in Rice. Mol. Plant. 2022, 15, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Z.; Mao, Y.; Lu, Y.; Yang, R.; Tao, X.; Zhu, J.-K. Optimizing Base Editors for Improved Efficiency and Expanded Editing Scope in Rice. Plant Biotechnology. J. 2019, 17, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L.; et al. Prime Genome Editing in Rice and Wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Hillary, V.E.; Ceasar, S.A. CRISPR/Cas System-Mediated Base Editing in Crops: Recent Developments and Future Prospects. Plant Cell Rep. 2024, 43, 271. [Google Scholar] [CrossRef]
- Molla, K.A.; Yang, Y. CRISPR/Cas-Mediated Base Editing: Technical Considerations and Practical Applications. Trends Biotechnol. 2019, 37, 1121–1142. [Google Scholar] [CrossRef]
- Shahwar, D.; Ahn, N.; Kim, D.; Ahn, W.; Park, Y. Mutagenesis-Based Plant Breeding Approaches and Genome Engineering: A Review Focused on Tomato. Mutat. Res.-Rev. Mutat. Res. 2023, 792, 108473. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, F.; Zhao, S.; Song, J.; Feng, F.; Zhao, J.; Yang, J. Expanding Base Editing Scope to Near-PAMless with Engineered CRISPR/Cas9 Variants in Plants. Mol. Plant 2021, 14, 191–194. [Google Scholar] [CrossRef]
- Hussain, B.; Raza, Q.; Atif, R.M.; Qadir Ahmad, M. New Breeding Techniques (NBTs) and Biotechnology for Boosting Rice Grain Yield to Feed 5 Billion in 2050. In Modern Techniques of Rice Crop Production; Springer: Singapore, 2022; pp. 681–700. [Google Scholar] [CrossRef]
- ISAAA GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase/advsearch/default.asp?CropID=Any&TraitTypeID=Any&DeveloperID=Any&CountryID=BR&ApprovalTypeID=Any (accessed on 15 February 2025).
- Andrade, P.; Ferreira, M.; Muniz, M.; Lira-Neto, A. GM Insect Pests under the Brazilian Regulatory Framework: Development and Perspectives. BMC Proc. 2018, 12, 16. [Google Scholar] [CrossRef]
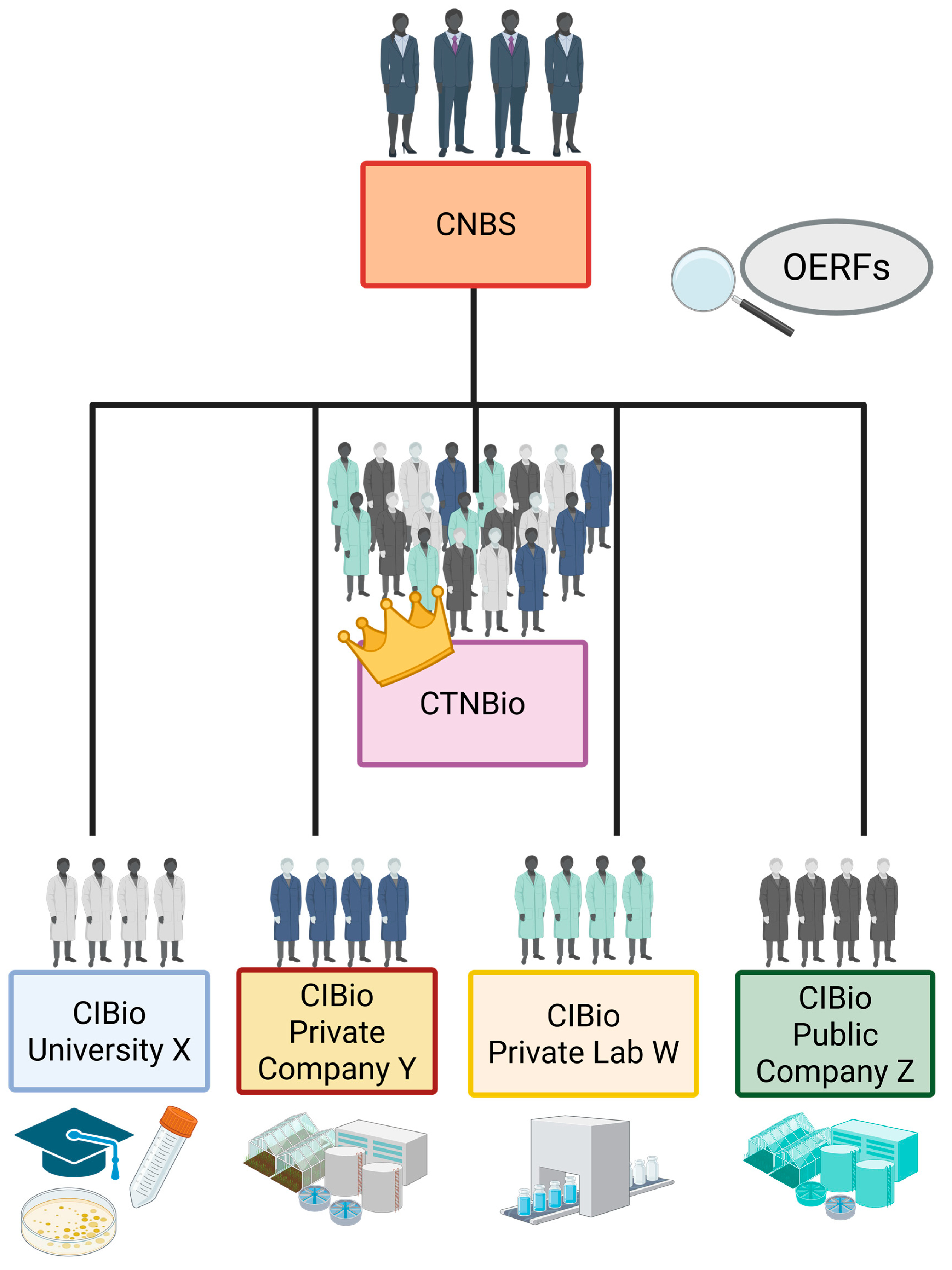



| Organ | Composition | Members | Characteristics and Attributions |
|---|---|---|---|
| CNBS | 11 high representatives of the Brazilian State | Chief of Staff of the Presidency (Council President) Minister of Justice Minister of Science, Technology and Innovations Minister of Agricultural Development Minister of Agriculture, Livestock and Supply Minister of Health Minister of the Environment Minister of Development, Industry and Foreign Trade Minister of Foreign Affairs Minister of Defense Secretary of Aquaculture and Fisheries | Highest authority in the Brazilian regulatory system Advises the President of the Republic on the formulation and implementation of NBP Establishes principles and guidelines for action on issues of commercial use of GMOs and their derivatives, based on issues of national, socioeconomic and political interest It speaks out whenever it wishes (quorum of 6 or more members), when requested by CTNBio or regulatory bodies If socio-economic and/or strategic policy decisions are required, the CNBS may issue a technical opinion (not technical judgment) in a final decision on the commercial release of GMOs Issues a final decision regarding the commercial release of GMOs and their derivatives. It has the power to refute, within 30 days, a technical decision recommending the commercial release of a GMO or derivative, issued by CTNBio. If it does not issue a refutation during the forementioned period, the GMO is automatically qualified for commercial registration |
| CTNBio | 27 full members and their alternates (all with doctorates), all Brazilian citizens The 12 scientific members are directly appointed by the MCTI minister The remaining members are recommended by the Ministries that form the CNB and appointed by the MCTI minister Members must present recognized technical competence and outstanding scientific backgrounds All members must be professionally active in the areas of Biology, Biotechnology, Biosafety, Microbiology, Health and the environment, Human/animal health, or related areas | 12 scientists: 3 experts in human health, 3 in zoo-health, 3 in plant health, 3 in the environment 9 representatives of ministries 1 expert in consumer protection 1 expert in health 1 expert in the environment 1 expert in biotechnology 1 expert in family farming 1 expert in worker health | Presents 4 permanent sectoral subcommittee: environment, plant health, animal health and human health Support the Federal Government in the establishment of the NBP Issue normative resolutions and biosafety guidelines for risk assessment of GMOs and their derivatives Establishes technical safety standards regarding the authorization of activities related to research, and the commercial release of GMOs Establishes standards for research with GMOs and derivatives Carry out, on a case-by-case basis, a risk assessment of activities and projects with GMOs and derivatives Authorize, register and monitor research activities with GMOs or GMO derivatives Provide technical assistance to research organizations Inspect research organizations Authorize the import of GMOs and their derivatives for research activities Advise the CNBS in formulating the NBP Issue Biosafety Quality Certificates (in Portuguese CQBs – Quality Certificates in Biosafety), an official authorization for the research institution to operate with Recombinant DNA Technology methods and be under government control Assessment of new technologies and their possible impacts on human and animal health and the environment Propose new regulations for emerging technologies Evaluate risks associated with activities with GMOs and prepare a technical report on commercial release Issue a technical decision/judgment on the biosafety and commercial release of GMOs and their derivatives, which is binding on other bodies and entities of the Public Administration Minimum quorum of 14 members at meetings, including at least one representative from each of the 4 subcommittees The committee may invite members of the scientific community, representatives of civil entities and the public sector to participate in the meetings, but without voting power All meetings are open to citizens The committee presents a public agenda and all bulletins and opinions produced are published on the official CTNBio website Decisions by absolute majority of votes (14 votes) For the commercial release of GMOs and derivatives, the required majority is 18 votes The president of CTNBio is appointed by the MCTI Minister for a 2-year term, renewable for another 2 years All members have a two-year term, renewable for two consecutive terms The commission has a permanent executive secretariat capable of technically and administratively advising members and organizes monthly meetings (except in January and July) All decisions are published in the official journal (Diário Oficial da União) and open for public comment within 30 days Decides whether there is a need for environmental licensing for research if the GMO presents a risk to the environment or human health |
| CIBio | Composed of 4 members, one of whom is the chairman of the committee All members are scientists from a research institution | Members with adequate training and knowledge in the areas of Biotechnology, Recombinant DNA Technology, Biosafety and other related areas | Required for all public or private research institutions that deal with Recombinant DNA Technology and GMOs and their derivatives To operate, the commission must receive the CQB from CTNBio Registers all projects and project leaders that involve research activities with GMOs at the institution Keeps a record of monitoring all activities involving GMOs and their derivatives at the institution Responsible for ensuring the biosafety conditions of the entity’s facilities Responsible for conducting regular inspections (at least 1 inspection per year) and inspections at the research institution to attest compliance with Biosafety rules and standards Holds at least 2 annual meetings Writes and issues the annual report of its activities and projects to CTNBio Trains human resources in biosafety, conducting periodic training Takes the first appropriate measures to avoid/contain adverse effects in the event of an accident Notifies the responsible agencies in the event of accidents CIBios have the autonomy to authorize projects with GMOs and derivatives of risk class 1 Edits the institution’s Biosafety Manual |
| OERFs | There are 4 ministries that make up the CNBS | Ministry of Agriculture, Livestock, and Supply Ministry of Health Ministry of Environment Secretariat of Aquaculture and Fisheries | Responsible for monitoring GMOs and their by-products Inspect research activities Register and inspect the commercial use of GMOs Authorize the import of products for research and commercial use Update researchers and research institutions on Biosafety information Advise CTNBio in defining parameters for assessing biosafety Grant registrations and authorizations for commercial use of GMOs Enforce the law and apply penalties in cases of non-compliance with biosafety parameters |
| Criterion | Classification |
|---|---|
| (I) Absence of recombinant DNA/RNA | non-GMO |
| (II) Presence of genetic elements that could be obtained by crossing | non-GMO |
| (III) Presence of induced mutations that could also be obtained by standard techniques | non-GMO |
| (IV) Presence of mutations that could occur naturally | non-GMO |
| (V) Presence of mutations by SDN-1 * | non-GMO |
| (VI) Presence of mutations by SDN-2 * | non-GMO |
| (VII) Presence of mutations by SDN-3 * | GMO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Cunha, N.B.; Silva Junior, J.J.d.; Araújo, A.M.M.; de Souza, L.R.; Leite, M.L.; Medina, G.d.S.; Rodriguez, G.R.; dos Anjos, R.M.; Rodrigues, J.C.M.; Costa, F.F.; et al. Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Revolution in Brazilian Agribusiness. Genes 2025, 16, 553. https://doi.org/10.3390/genes16050553
da Cunha NB, Silva Junior JJd, Araújo AMM, de Souza LR, Leite ML, Medina GdS, Rodriguez GR, dos Anjos RM, Rodrigues JCM, Costa FF, et al. Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Revolution in Brazilian Agribusiness. Genes. 2025; 16(5):553. https://doi.org/10.3390/genes16050553
Chicago/Turabian Styleda Cunha, Nicolau B., Jaim J. da Silva Junior, Amanda M. M. Araújo, Ludmila R. de Souza, Michel L. Leite, Gabriel da S. Medina, Gustavo R. Rodriguez, Renan M. dos Anjos, Júlio C. M. Rodrigues, Fabrício F. Costa, and et al. 2025. "Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Revolution in Brazilian Agribusiness" Genes 16, no. 5: 553. https://doi.org/10.3390/genes16050553
APA Styleda Cunha, N. B., Silva Junior, J. J. d., Araújo, A. M. M., de Souza, L. R., Leite, M. L., Medina, G. d. S., Rodriguez, G. R., dos Anjos, R. M., Rodrigues, J. C. M., Costa, F. F., Dias, S. C., Rech, E. L., & Vianna, G. R. (2025). Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Revolution in Brazilian Agribusiness. Genes, 16(5), 553. https://doi.org/10.3390/genes16050553








