Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Sample
2.2. Biological Sample Collection and Clinical Protocol
2.3. miRNA Analysis
2.4. Bioinformatic and Statistical Analysis
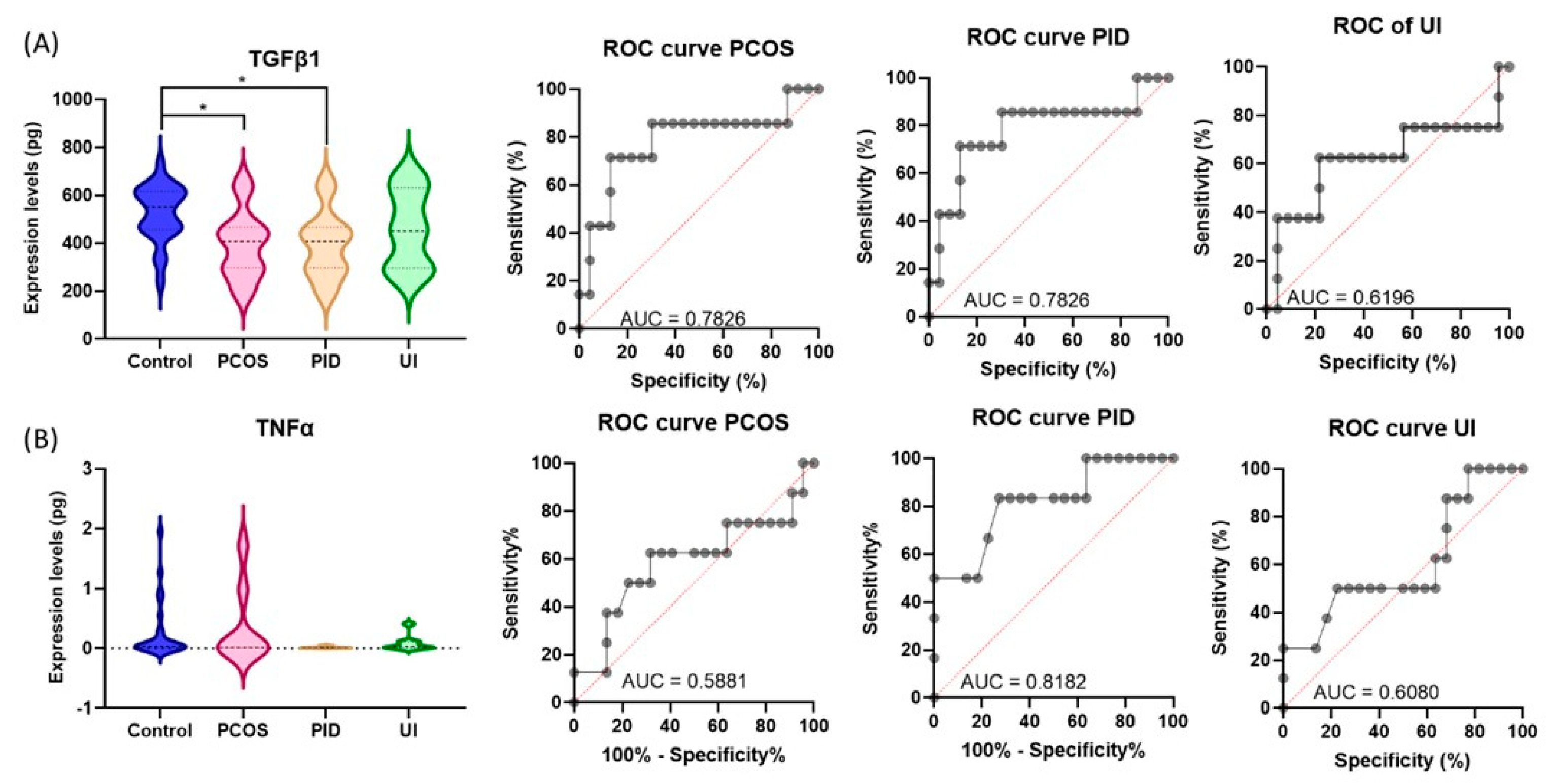
2.5. TGFβ1 and TNFα Protein Serum Quantification Using ELISA
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luke, B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: With an emphasis on US population-based studies. Am. J. Obstet. Gynecol. 2017, 217, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, R.; Mulligan, S.; Shah, J.; Korkidakis, A.; Penzias, A.; Vaughan, D.; Patrizio, P.; Sakkas, D. From oocytes to a live birth: Are we improving the biological efficiency? Fertil. Steril. 2023, 120, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Aghebati-Maleki, L.; Rashidiani, S.; Csabai, T.; Nnaemeka, O.B.; Szekeres-Bartho, J. Long-Term Effects of ART on the Health of the Offspring. Int. J. Mol. Sci. 2023, 24, 13564. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, W.; Liu, Y.; Zhang, W.; Ren, C.; Guan, Y. What Does Unexpected Suboptimal Response During Ovarian Stimulation Suggest, an Overlooked Group? Front. Endocrinol. 2021, 12, 795254. [Google Scholar] [CrossRef]
- Bosch, E.; Labarta, E.; Kolibianakis, E.; Rosen, M.; Meldrum, D. Regimen of ovarian stimulation affects oocyte and therefore embryo quality. Fertil. Steril. 2016, 105, 560–570. [Google Scholar] [CrossRef]
- Montano, L.; Raimondo, S.; Piscopo, M.; Ricciardi, M.; Guglielmino, A.; Chamayou, S.; Gentile, R.; Gentile, M.; Rapisarda, P.; Oliveri Conti, G.; et al. First evidence of microplastics in human ovarian follicular fluid: An emerging threat to female fertility. Ecotoxicol. Environ. Saf. 2025, 291, 117868. [Google Scholar] [CrossRef]
- Adamczak, R.; Ukleja-Sokołowska, N.; Lis, K.; Dubiel, M. Function of Follicular Cytokines: Roles Played during Maturation, Development and Implantation of Embryo. Medicina 2021, 57, 1251. [Google Scholar] [CrossRef]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein pathways working in human follicular fluid: The future for tailored IVF? Expert. Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Calin, G.A.; Berindan-Neagoe, I. MicroRNAs and cancer therapy—From bystanders to major players. Curr. Med. Chem. 2013, 20, 3561–3573. [Google Scholar] [CrossRef]
- Braicu, C.; Catana, C.; Calin, G.A.; Berindan-Neagoe, I. NCRNA combined therapy as future treatment option for cancer. Curr. Pharm. Des. 2014, 20, 6565–6574. [Google Scholar] [CrossRef]
- Shekibi, M.; Heng, S.; Nie, G. MicroRNAs in the Regulation of Endometrial Receptivity for Embryo Implantation. Int. J. Mol. Sci. 2022, 23, 6210. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wang, S.; Wang, Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Inoue, Y.; Hara, S.; Itou, J.; Shirasuna, K.; Iwata, H. microRNAs associated with the quality of follicular fluids affect oocyte and early embryonic development. Reprod. Med. Biol. 2024, 23, e12559. [Google Scholar] [CrossRef] [PubMed]
- Qasemi, M.; Amidi, F. Extracellular microRNA profiling in human follicular fluid: New biomarkers in female reproductive potential. J. Assist. Reprod. Genet. 2020, 37, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Redis, R.S.; Berindan-Neagoe, I.; Pop, V.I.; Calin, G.A. Non-coding RNAs as theranostics in human cancers. J. Cell. Biochem. 2012, 113, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Cojocneanu, R.; Braicu, C.; Raduly, L.; Jurj, A.; Zanoaga, O.; Magdo, L.; Irimie, A.; Muresan, M.S.; Ionescu, C.; Grigorescu, M.; et al. Plasma and Tissue Specific miRNA Expression Pattern and Functional Analysis Associated to Colorectal Cancer Patients. Cancers 2020, 12, 843. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Chen, B.; Yu, X.; Zhou, Y.; Ni, D.; Zhang, X.; Zhang, J.; Ling, X.; Zhang, Z.; et al. Follicular fluid C3a-peptide promotes oocyte maturation through F-actin aggregation. BMC Biol. 2023, 21, 285. [Google Scholar] [CrossRef]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef]
- Butler, A.E.; Ramachandran, V.; Sathyapalan, T.; David, R.; Gooderham, N.J.; Benurwar, M.; Dargham, S.R.; Hayat, S.; Hani Najafi-Shoushtari, S.; Atkin, S.L. microRNA Expression in Women with and Without Polycystic Ovarian Syndrome Matched for Body Mass Index. Front. Endocrinol. 2020, 11, 206. [Google Scholar]
- Hon, J.X.; Wahab, N.A.; Karim, A.K.A.; Mokhtar, N.M.; Mokhtar, M.H. MicroRNAs in Endometriosis: Insights into Inflammation and Progesterone Resistance. Int. J. Mol. Sci. 2023, 24, 15001. [Google Scholar] [CrossRef]
- Begum, M.I.A.; Chuan, L.; Hong, S.T.; Chae, H.S. The Pathological Role of miRNAs in Endometriosis. Biomedicines 2023, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Neamtiu, I.A.; Surcel, M.; Begum, T.F.; Gurzau, E.S.; Berindan-Neagoe, I.; Braicu, C.; Rotar, I.; Muresan, D.; Bloom, M.S. Specific lifestyle factors and in vitro fertilization outcomes in Romanian women: A pilot study. PeerJ 2022, 10, e14189. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Ciocan-Cartita, C.A.; Jurj, A.; Zanoaga, O.; Cojocneanu, R.; Pop, L.A.; Moldovan, A.; Moldovan, C.; Zimta, A.A.; Raduly, L.; Pop-Bica, C.; et al. New insights in gene expression alteration as effect of doxorubicin drug resistance in triple negative breast cancer cells. J. Exp. Clin. Cancer Res. 2020, 39, 241. [Google Scholar] [CrossRef]
- Roth, L.W.; McCallie, B.; Alvero, R.; Schoolcraft, W.B.; Minjarez, D.; Katz-Jaffe, M.G. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2014, 31, 355–362. [Google Scholar] [CrossRef]
- Sang, Q.; Yao, Z.; Wang, H.; Feng, R.; Wang, H.; Zhao, X.; Xing, Q.; Jin, L.; He, L.; Wu, L.; et al. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013, 98, 3068–3079. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Greco, C.; Lazzaretti, C.; Paradiso, E.; Casarini, L.; Potì, F.; Brigante, G.; Simoni, M. The “Hitchhiker’s Guide to the Galaxy” of Endothelial Dysfunction Markers in Human Fertility. Int. J. Mol. Sci. 2021, 22, 2584. [Google Scholar] [CrossRef]
- Singh, S.; Pal, N.; Shubham, S.; Sarma, D.K.; Verma, V.; Marotta, F.; Kumar, M. Polycystic Ovary Syndrome: Etiology, Current Management, and Future Therapeutics. J. Clin. Med. 2023, 12, 1454. [Google Scholar] [CrossRef]
- Fahs, D.; Salloum, D.; Nasrallah, M.; Ghazeeri, G. Polycystic Ovary Syndrome: Pathophysiology and Controversies in Diagnosis. Diagnostics 2023, 13, 1559. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Zhao, H.; Yang, Z.; Kang, Y. Aberrant miRNA-mRNA regulatory network in polycystic ovary syndrome is associated with markers of insulin sensitivity and inflammation. Ann. Transl. Med. 2021, 9, 1405. [Google Scholar] [CrossRef]
- Yang, Y.; Lang, P.; Zhang, X.; Wu, X.; Cao, S.; Zhao, C.; Shen, R.; Ling, X.; Yang, Y.; Zhang, J. Molecular characterization of extracellular vesicles derived from follicular fluid of women with and without PCOS: Integrating analysis of differential miRNAs and proteins reveals vital molecules involving in PCOS. J. Assist. Reprod. Genet. 2023, 40, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, B.; Kong, Y.; Yang, Y.; Tian, C.; Chen, L.; Liao, Y.; Ma, L. Polycystic ovary syndrome: Identification of novel and hub biomarkers in the autophagy-associated mRNA-miRNA-lncRNA network. Front. Endocrinol. 2022, 13, 1032064. [Google Scholar] [CrossRef] [PubMed]
- Palagiano, A.; Cozzolino, M.; Ubaldi, F.M.; Palagiano, C.; Coccia, M.E. Effects of Hydrosalpinx on Endometrial Implantation Failures: Evaluating Salpingectomy in Women Undergoing in vitro fertilization. Rev. Bras. Ginecol. Obstet. 2021, 43, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Cheong, Y. Hydrosalpinx—Salpingostomy, salpingectomy or tubal occlusion. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 59, 41–47. [Google Scholar] [CrossRef]
- Batushansky, A.; Zacharia, A.; Shehadeh, A.; Bruck-Haimson, R.; Saidemberg, D.; Kogan, N.M.; Thomas Mannully, C.; Herzberg, S.; Ben-Meir, A.; Moussaieff, A. A Shift in Glycerolipid Metabolism Defines the Follicular Fluid of IVF Patients with Unexplained Infertility. Biomolecules 2020, 10, 1135. [Google Scholar] [CrossRef]
- Vaigauskaitė-Mažeikienė, B.; Baušytė, R.; Valatkaitė, E.; Maželytė, R.; Kazėnaitė, E.; Ramašauskaitė, D.; Navakauskienė, R. Assisted reproductive technology outcomes and gene expression in unexplained infertility patients. Front. Cell Dev. Biol. 2023, 11, 1217808. [Google Scholar] [CrossRef]
- Müller, M.; Fazi, F.; Ciaudo, C. Argonaute Proteins: From Structure to Function in Development and Pathological Cell Fate Determination. Front. Cell Dev. Biol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Kusumaningtyas, I.; Dasuki, D.; Harjana, S.M.; Sadewa, A.H.; Sweety, M.C.; Septiani, L. Unraveling the microRNAs, key players in folliculogenesis and ovarian diseases. Middle East Fertil. Soc. J. 2024, 29, 13. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Ku, B.J.; Kim, T.H.; Il Ahn, J.; Ahn, J.Y.; Yang, W.S.; Lim, J.M.; Taketo, M.M.; Shin, J.H.; Jeong, J.W. β-catenin activates TGF-β-induced epithelial-mesenchymal transition in adenomyosis. Exp. Mol. Med. 2020, 52, 1754–1765. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kishi, Y.; Matsubara, S. Mechanisms Underlying Adenomyosis-Related Fibrogenesis. Gynecol. Obstet. Investig. 2019, 85, 1–12. [Google Scholar] [CrossRef]
- Walters, K.A.; Rodriguez Paris, V.; Aflatounian, A.; Handelsman, D.J. Androgens and ovarian function: Translation from basic discovery research to clinical impact. J. Endocrinol. 2019, 242, R23–R50. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Chen, Y. Androgen excess: A hallmark of polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1273542. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Norouzi, M.; McCulloch, C.A. Impact of Vimentin on Regulation of Cell Signaling and Matrix Remodeling. Front. Cell Dev. Biol. 2022, 10, 869069. [Google Scholar] [CrossRef] [PubMed]

| Variable | N = Number of Subjects (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 35.2 ± 4.8 |
| Range | 26–44 |
| BMI (kg/m2) | |
| <18.5 | 2 (4.3) |
| 18.5–25 | 28 (59.6) |
| 25–30 | 13 (27.6) |
| ≥30 | 4 (8.5) |
| Education | |
| College | 1 (2.1) |
| Faculty | 36 (76.6) |
| High school | 4 (8.5) |
| Post high school | 4 (8.5) |
| Secondary school | 1 (2.1) |
| Vocational school | 1 (2.1) |
| Infertility diagnosis | |
| PCOS | 5 (10.6) |
| PID | 7 (14.9) |
| Endometriosis | 3 (6.4) |
| UI | 21 (44.7) |
| Male factor | 9 (19.1) |
| Mixed (female and male factor) | 2 (4.3) |
| ICSI | |
| YES | 27 (57.4) |
| NO | 20 (42.6) |
| No. of fertilized oocytes | |
| Mean ± SD | 4.8 ± 2.8 |
| Range | 1–12 |
| No. of blastocytes | |
| Mean ± SD | 2.2 ± 1.4 |
| Range | 0–6 |
| Pregnancy | |
| YES | 20 (42.6) |
| NO | 26 (55.3) |
| Abortion | 1 (2.1) |
| Smoking | |
| YES | 9 (19.1) |
| NO | 38 (80.9) |
| Male Factor Infertility Diagnosis | PCOS Diagnosis | PID Diagnosis | UI Diagnosis | |
|---|---|---|---|---|
| Sample ID | C015 | ID003 | ID005 | ID001 |
| C031 | ID010 | ID008 | ID006 | |
| C046 | ID013 | ID023 | ID009 | |
| ID016 | ID026 | ID012 | ||
| ID025 | ID033 | ID017 | ||
| ID028 | ID036 | ID018 | ||
| ID044 | ID039 | ID019 | ||
| ID045 | ID024 |
| PCOS | PID | UI | ||||||
|---|---|---|---|---|---|---|---|---|
| miRNA | p-Value | FC | miRNA | p-Value | FC | miRNA | p-Value | FC |
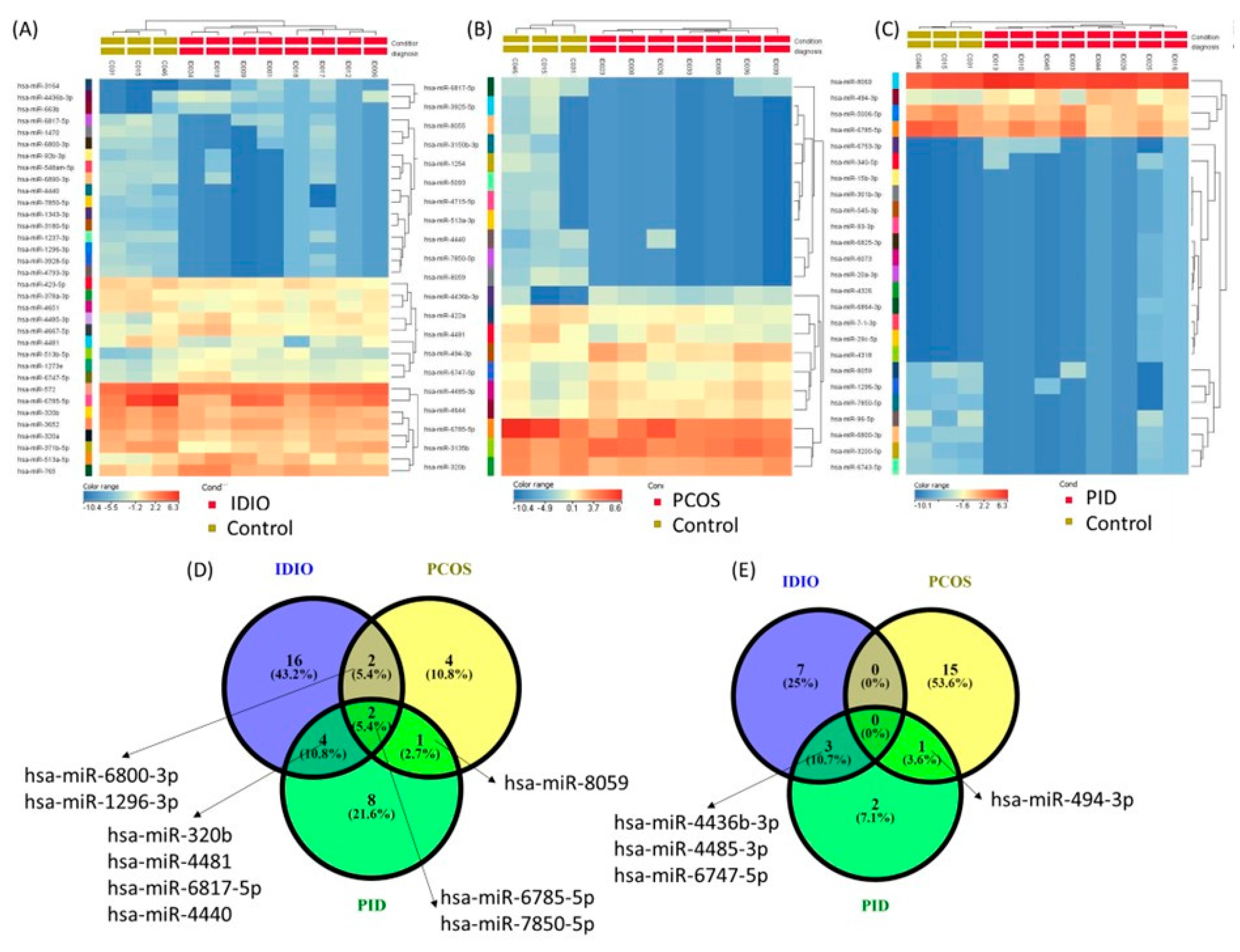
| hsa-miR-5006-5p | 0.048818 | −2.26296 | hsa-miR-320b | 0.039884 | −1.66046 | hsa-miR-3652 | 0.032759 | −1.5348632 |
| hsa-miR-6785-5p | 0.048797 | −4.35155 | hsa-miR-422a | 0.030223 | −2.08079 | hsa-miR-423-5p | 0.021881 | −1.6792842 |
| hsa-miR-3200-5p | 0.041602 | −6.29675 | hsa-miR-4481 | 0.035729 | −3.10321 | hsa-miR-320a | 0.010301 | −1.6942542 |
| hsa-miR-6800-3p | 0.020221 | −8.68356 | hsa-miR-6785-5p | 0.045551 | −4.25992 | hsa-miR-378a-3p | 0.035554 | −1.7705184 |
| hsa-miR-1296-3p | 0.021707 | −8.71487 | hsa-miR-3150b-3p | 0.030758 | −6.51924 | hsa-miR-4651 | 0.032146 | −1.9902356 |
| hsa-miR-6743-5p | 0.013994 | −8.78707 | hsa-miR-513a-3p | 0.026792 | −7.21145 | hsa-miR-320b | 0.003563 | −2.05183 |
| hsa-miR-7850-5p | 0.006439 | −9.30464 | hsa-miR-5093 | 0.025999 | −7.30568 | hsa-miR-572 | 0.037317 | −2.1064086 |
| hsa-miR-8059 | 0.035159 | −11.789 | hsa-miR-8055 | 0.027552 | −8.00561 | hsa-miR-371b-5p | 0.042022 | −2.7818508 |
| hsa-miR-96-5p | 0.032253 | −13.3004 | hsa-miR-4715-5p | 0.025002 | −8.11684 | hsa-miR-4481 | 0.037089 | −4.7878485 |
| hsa-miR-6753-3p | 0.042607 | 12.67912 | hsa-miR-3925-5p | 0.027265 | −8.56468 | hsa-miR-6785-5p | 0.02951 | −5.4503827 |
| hsa-miR-340-5p | 0.046759 | 9.009871 | hsa-miR-4440 | 0.035014 | −9.58227 | hsa-miR-1343-3p | 0.02673 | −5.9446187 |
| hsa-miR-6864-3p | 0.043599 | 5.416498 | hsa-miR-1254 | 0.023006 | −10.3288 | hsa-miR-92b-3p | 0.04412 | −6.4570365 |
| hsa-miR-29c-5p | 0.037662 | 5.181044 | hsa-miR-6817-5p | 0.015223 | −13.2246 | hsa-miR-6817-5p | 0.039526 | −6.7536287 |
| hsa-miR-4318 | 0.037197 | 5.16057 | hsa-miR-7850-5p | 1.91 × 10−5 | −15.3778 | hsa-miR-3180-5p | 0.018344 | −7.153791 |
| hsa-miR-7-1-3p | 0.036099 | 5.110514 | hsa-miR-8059 | 1.37 × 10−5 | −32.1081 | hsa-miR-6800-3p | 0.028772 | −7.775571 |
| hsa-miR-6825-3p | 0.033382 | 4.972778 | hsa-miR-4436b-3p | 0.012431 | 15.05348 | hsa-miR-6890-3p | 0.038843 | −9.462893 |
| hsa-miR-6073 | 0.032759 | 4.937158 | hsa-miR-4485-3p | 0.020422 | 3.270453 | hsa-miR-548am-5p | 0.019529 | −10.179389 |
| hsa-miR-20a-3p | 0.032314 | 4.91043 | hsa-miR-494-3p | 0.033067 | 2.803433 | hsa-miR-3928-5p | 0.013132 | −10.692548 |
| hsa-miR-4326 | 0.032242 | 4.906011 | hsa-miR-6747-5p | 0.044561 | 2.320432 | hsa-miR-1470 | 0.02218 | −11.310488 |
| hsa-miR-15b-3p | 0.028673 | 4.571666 | hsa-miR-4644 | 0.008974 | 2.22851 | hsa-miR-4440 | 0.006532 | −11.646117 |
| hsa-miR-301b-3p | 0.028673 | 4.571666 | hsa-miR-3135b | 0.049657 | 1.9634 | hsa-miR-1296-3p | 0.004099 | −11.660315 |
| hsa-miR-545-3p | 0.028673 | 4.571666 | hsa-miR-7850-5p | 0.004139 | −12.055969 | |||
| hsa-miR-93-3p | 0.028673 | 4.571666 | hsa-miR-1237-3p | 0.013679 | −12.200047 | |||
| hsa-miR-494-3p | 0.034822 | 4.073813 | hsa-miR-4793-3p | 0.00522 | −13.793538 | |||
| hsa-miR-8069 | 0.020086 | 2.061847 | hsa-miR-4436b-3p | 0.040373 | 10.764503 | |||
| hsa-miR-3164 | 0.014509 | 10.348244 | ||||||
| hsa-miR-663b | 0.043826 | 5.7668953 | ||||||
| hsa-miR-513a-5p | 0.014961 | 3.638524 | ||||||
| hsa-miR-513b-5p | 0.036334 | 3.6029956 | ||||||
| hsa-miR-4485-3p | 0.031918 | 2.792712 | ||||||
| hsa-miR-6747-5p | 0.047025 | 2.4140615 | ||||||
| hsa-miR-4667-5p | 0.048273 | 2.1719646 | ||||||
| hsa-miR-765 | 0.0452 | 2.1134443 | ||||||
| hsa-miR-1273e | 0.048152 | 2.0018563 | ||||||
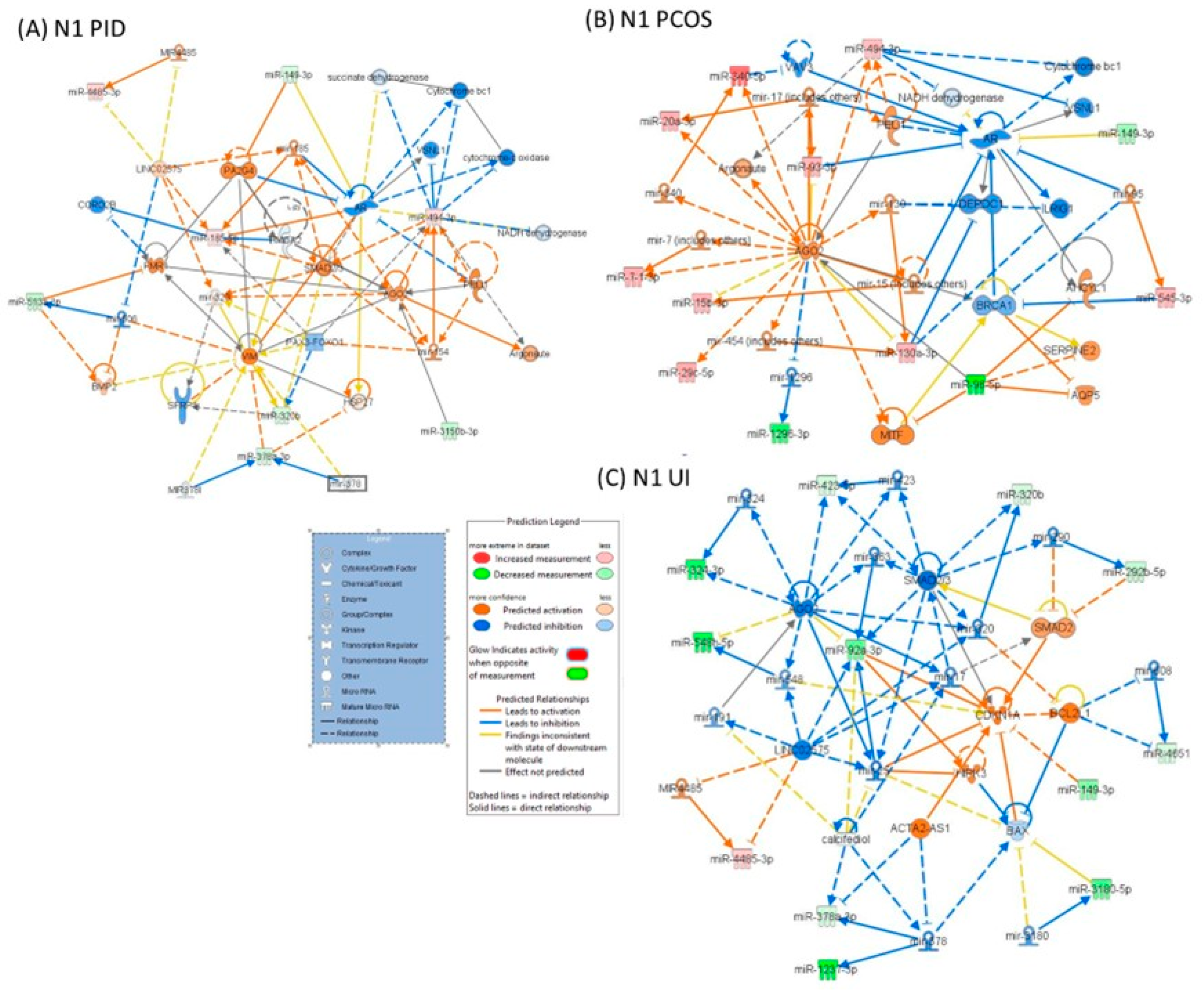
| Biological Process | Group | Name | p-Value Range | # Molecules |
|---|---|---|---|---|
| diseases and disorders | PCOS | Organismal Injury and Abnormalities | 4.61 × 10−2–4.06 × 10−6 | 10 |
| Reproductive System Disease | 3.65 × 10−2–4.06 × 10−6 | 7 | ||
| Cancer | 4.61 × 10−2–6.38 × 10−4 | 8 | ||
| Auditory Disease | 9.06 × 10−4–9.06 × 10−4 | 1 | ||
| Hereditary Disorder | 1.75 × 10−2–9.06 × 10−4 | 2 | ||
| PID | Inflammatory Disease | 1.99 × 10−2–6.16 × 10−6 | 4 | |
| Inflammatory Response | 1.99 × 10−2–6.16 × 10−6 | 3 | ||
| Organismal Injury and Abnormalities | 4.97 × 10−2–6.16 × 10−6 | 8 | ||
| Renal and Urological Disease | 6.16 × 10−6–6.16 × 10−6 | 3 | ||
| Cancer | 4.97 × 10−2–3.92 × 10−5 | 5 | ||
| UI | Organismal Injury and Abnormalities | 4.57 × 10−2–2.14 × 10−6 | 15 | |
| Psychological Disorders | 3.32 × 10−2–2.14 × 10−6 | 8 | ||
| Inflammatory Disease | 3.41 × 10−2–2.98 × 10−6 | 7 | ||
| Inflammatory Response | 1.27 × 10−3–2.98 × 10−6 | 5 | ||
| Renal and Urological Disease | 2.98 × 10−6–2.98 × 10−6 | 4 | ||
| molecular and cellular function alteration | PCOS | Cellular Growth and Proliferation | 3.13 × 10−2–1.61 × 10−4 | 8 |
| Cellular Movement | 1.34 × 10−2–4.92 × 10−4 | 8 | ||
| Cell Death and Survival | 2.38 × 10−2–9.57 × 10−4 | 3 | ||
| Cellular Development | 3.13 × 10−2–1.36 × 10−3 | 6 | ||
| Cell Morphology | 2.38 × 10−2–4.52 × 10−3 | 1 | ||
| UI | Cellular Movement | 3.82 × 10−2–6.68 × 10−4 | 6 | |
| Cell Death and Survival | 4.17 × 10−2–3.33 × 10−3 | 3 | ||
| Cellular Development | 3.76 × 10−2–3.57 × 10−3 | 5 | ||
| Cellular Function and Maintenance | 9.85 × 10−3–3.57 × 10−3 | 3 | ||
| Cellular Growth and Proliferation | 3.76 × 10−2–3.57 × 10−3 | 36 | ||
| UI | Cellular Movement | 4.07 × 10−2–4.91 × 10−4 | 9 | |
| Cellular Development | 4.57 × 10−2–1.53 × 10−3 | 9 | ||
| Cellular Growth and Proliferation | 4.57 × 10−2–1.53 × 10−3 | 9 | ||
| Cell-To-Cell Signaling and Interaction | 1.73 × 10−3–1.73 × 10−3 | 1 | ||
| Cell Death and Survival | 3.74 × 10−2–2.59 × 10−3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braicu, C.; Ciocan, C.; Bica, C.; Zanoaga, O.; Pop, L.A.; Strilciuc, S.; Staicu, A.; Goidescu, I.; Muresan, D.; Surcel, M.; et al. Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies. Genes 2025, 16, 537. https://doi.org/10.3390/genes16050537
Braicu C, Ciocan C, Bica C, Zanoaga O, Pop LA, Strilciuc S, Staicu A, Goidescu I, Muresan D, Surcel M, et al. Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies. Genes. 2025; 16(5):537. https://doi.org/10.3390/genes16050537
Chicago/Turabian StyleBraicu, Cornelia, Cristina Ciocan, Cecilia Bica, Oana Zanoaga, Laura Ancuta Pop, Stefan Strilciuc, Adelina Staicu, Iulian Goidescu, Daniel Muresan, Mihai Surcel, and et al. 2025. "Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies" Genes 16, no. 5: 537. https://doi.org/10.3390/genes16050537
APA StyleBraicu, C., Ciocan, C., Bica, C., Zanoaga, O., Pop, L. A., Strilciuc, S., Staicu, A., Goidescu, I., Muresan, D., Surcel, M., & Berindan-Neagoe, I. (2025). Altered miRNA Signatures in Follicular Fluid: Insights into Infertility Etiologies. Genes, 16(5), 537. https://doi.org/10.3390/genes16050537








