Abstract
Background/Objectives: Rhamnolipids (RLs) are biosurfactants with significant industrial and environmental potential, which physicochemical properties depend greatly on their fatty acyl chain composition. This study investigated the impact of genetically modulating the fatty acid synthesis genes fabA and fabZ on RL composition and functionality in Pseudomonas aeruginosa PAO1. Methods and Results: Using temperature-sensitive mutants and suppressor strains for these essential genes, we successfully engineered RLs with altered fatty acyl chain lengths and saturation levels. LC–MS/MS analyses showed that deletion and overexpression of fabA and fabZ significantly shifted RL fatty acid profiles. Functional analyses indicated that these structural changes markedly influenced RL emulsification activity and critical micelle concentration (CMC). Conclusions: These findings demonstrate the feasibility of optimizing RL properties through targeted genetic manipulation, offering valuable insights for designing customized biosurfactants for diverse industrial and environmental applications.
1. Introduction
Rhamnolipids (RLs) are microbial biosurfactants with promising applications in bioremediation, pharmaceuticals, and industrial formulations due to their high surface activity, emulsification capacity, and biodegradability [1,2]. P. aeruginosa is one of the most efficient RL producers, synthesizing both mono-rhamnolipids (mRLs) and di-rhamnolipids (dRLs) [3,4]. The fatty acyl composition of RLs, determined by the bacterial fatty acid synthesis (FAS II) pathway, significantly influences their physicochemical properties, including emulsification potential and surface tension reduction [5,6,7,8]. While previous studies have focused on optimizing the RL yield through metabolic engineering and fermentation strategies [9,10,11], limited research has been conducted on tailoring RL functionality by modifying its fatty acyl composition through genetic manipulation.
The fatty acid synthesis pathway in P. aeruginosa is governed by the FAS II system, where fabA and fabZ encode β-hydroxyacyl-ACP dehydratases that regulate acyl chain elongation and desaturation [12,13]. FabA is a bifunctional enzyme catalyzing both the dehydration of β-hydroxyacyl-ACP and isomerization of trans-2-decenoyl-ACP, essential for unsaturated fatty acid biosynthesis [12]. FabZ, a functionally overlapping enzyme, primarily facilitates saturated fatty acid elongation [13,14]. Both fabA and fabZ genes are essential for bacterial viability [15,16]. Their inactivation leads to cell death, making direct deletion unfeasible under normal growth conditions.
To circumvent this challenge, we previously developed a three-step strategy to construct temperature-sensitive (ts) mutants of essential genes [17,18,19,20]. This method allowed us to generate fabA ts-mutants and subsequently isolate suppressor strains that rescued ΔfabA lethality, enabling the functional analysis of fabA deletion [18]. A similar approach was applied in this study to construct a fabZ ts-mutant and screen for suppressor strains that permitted fabZ deletion while maintaining bacterial viability. This suppressor-based strategy provides a unique opportunity to investigate how fabA and fabZ influence RL biosynthesis and its physicochemical properties.
While previous studies have primarily focused on improving the RL yield or general property characterization, relatively few have explored how a targeted modulation of fatty acid biosynthetic genes may influence RL composition and function. This study aims to elucidate the impact of modulating fabA and fabZ expression on RL acyl chain composition and its emulsification and surfactant properties. We selected P. aeruginosa PAO1 as the model organism based on its well-characterized RL biosynthesis pathway [21], which provides both a clearly defined regulatory network and robust genetic tools for precise metabolic engineering. Building on our laboratory’s prior development of a three-step conditional allele construction strategy specifically optimized for this strain, we generated five mutant variants with differential fabA/fabZ expression profiles while maintaining rhlC deletion to ensure exclusive mono-rhamnolipid (mRL) production [22]. Through LC–MS/MS analysis, emulsification assays, and critical micelle concentration (CMC) measurements, we characterized the structure–function relationship of these engineered RL variants. By integrating metabolic engineering with functional analysis, this research contributes to the advancement of customizable RLs biosurfactants, providing a foundation for optimizing RLs for industrial and environmental applications.
2. Materials and Methods
2.1. Oligonucleotides, Plasmids, and Bacterial Strains
The information on all oligonucleotides, plasmids, and bacterial strains used in this study is summarized in Table 1. The wild-type P. aeruginosa PAO1 strain (BioSciBio, Hangzhou, China) and its derivatives were cultured in LB medium (10 g/L tryptone, 10 g/L NaCl, and 5 g/L yeast extract, pH 7.0) or in minimum mineral (MS) medium containing 2% glycerol as the sole carbon source. The MS medium (per liter) consisted of 0.6 g Na2HPO4, 0.2 g KH2PO4, 4.0 g NaNO3, 0.3 g MgSO4, 0.01 g CaCl2, and 0.01 g FeSO4. The chemicals used in this study were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China).

Table 1.
Oligonucleotides, plasmids, and strains used in this study.
2.2. Plasmid Construction
The deletion and temperature-sensitive (ts) rescue plasmids used in this study were derived from our previously published work [17,18,19,20]. To generate deletion and rescue plasmids, corresponding gene cassettes were amplified by PCR and inserted into the corresponding deletion plasmids and rescue plasmid using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). For overexpression plasmids, the araC-PBAD promoter fragment [23] and the coding sequences of fabA or fabZ were cloned into the pBBR1MCS-5 vector [24] using the same cloning kit. All recombinant plasmids were confirmed by DNA sequencing prior to use.
2.3. Construction of Plasmid-Based Ts-Mutant fabZ(Ts) and ΔrhlC Strains
The fabZ temperature-sensitive (ts) mutant strain was constructed using our previously established three-step allelic exchange method [17,18,19,20]. First, a non-replicative fabZ deletion plasmid was introduced into P. aeruginosa PAO1 via electroporation, and single-crossover integrants were selected on LB plates containing gentamicin. The integrants were then transformed with a temperature-sensitive (ts) rescue plasmid carrying fabZ under its native promoter and selected on tetracycline-containing plates. Subsequently, counterselection on sucrose plates (via the sacB gene) facilitated excision of the integrated plasmid, generating the chromosomal deletion mutant (ΔfabZ). The resulting fabZ(Ts) strain was verified by PCR and assessed for temperature-sensitive growth. For rhlC gene deletion, a similar two-step allelic exchange approach was employed [25]. Since rhlC is a non-essential gene, transformation with a rescue plasmid was not required. The deletion plasmid was introduced into various P. aeruginosa strains (PAO1, fabA-OE, fabZ-OE, ΔfabA-sup, and ΔfabZ-sup), and single-crossover integrants were selected on tetracycline-containing plates. The final deletion mutants were obtained by sucrose counter-selection and verified by PCR analysis.
2.4. Isolation of Suppressors
To obtain spontaneous suppressor mutants, more than 1.0 × 10⁹ fabZ(Ts) cells were spread on LB plates and incubated at a semi-restrictive temperature of 40 °C for two weeks, as previously described [17,18]. The plates were kept in a humidified environment with filtered fresh air to support optimal growth. Emergent suppressor colonies were re-streaked on fresh LB plates and incubated at 42 °C to verify the temperature resistance. The presence of the fabZ deletion allele was confirmed by PCR using gene-specific primers.
2.5. Spot-Plating Assay
Growth of the fabZ(Ts) mutant was assessed using a spot-plating assay. Overnight cultures were adjusted to the same OD₆₀₀ and subjected to 10-fold serial dilutions. Diluted samples were spotted onto LB agar plates containing the indicated stress conditions using a 48-pin replicator (V&P Scientific, San Diego, CA, USA). The plates were incubated at 30 °C (permissive temperature) or 42 °C (restrictive temperature), and growth was recorded after appropriate incubation periods.
2.6. Rhamnolipid Extraction and Purification
Cell-free supernatants were acidified to pH 2 with HCl to precipitate rhamnolipids and incubated overnight at room temperature. The precipitate was harvested by centrifugation (5000× g, 20 min), followed by triple sequential extraction with 100 mL of a 2:1 (v/v) chloroform–methanol mixture (equal to the initial fermentation volume). After each extraction, the organic phase was collected and concentrated via rotary evaporation. The combined extracts were oven-dried at 70 °C for 12 h to obtain semisolid rhamnolipids. For functional characterization, purified rhamnolipids were dissolved in distilled water (for surface tension and emulsification assays) or chloroform (for TLC and LC–MS/MS analyses).
2.7. Quantification of Rhamnolipids via the Orcinol–Sulfuric Acid Method
The orcinol assay was performed using a previously described method [26]. Briefly, 300 μL of culture supernatant was subjected to diethyl ether extraction (1 mL each) after centrifugation. The combined organic phase was dried under vacuum centrifugation and redissolved in 0.5 mL sterile water. Subsequently, 100 μL aliquots were reacted with 900 μL freshly prepared 0.19% (w/v) orcinol reagent (dissolved in 53% sulfuric acid) for 30 min at 80 °C in a heating block, followed by equilibration to room temperature (15 min). Absorbance readings at 421 nm were acquired using a spectrophotometer. Parallel analysis of rhamnose standards (50–500 mg/L) enabled the construction of a calibration curve by correlating the OD421 values with known concentrations.
2.8. Thin-Layer Chromatography (TLC) Analysis
Purified rhamnolipids were first dissolved in methanol and then applied onto silica gel TLC plates. The plates were developed using chloroform/methanol/acetic acid (70:10:1.4, v/v/v). After development, the presence of rhamnolipids was detected through staining with anthrone–sulfuric acid and ninhydrin reagents.
2.9. Emulsification Capacity Assay
The emulsification index (E24%) was determined following a previously described method [27], with minor modifications. Briefly, equal volumes of two-fold diluted surfactant solution (adjusted to pH 7) and crude oil were mixed vigorously using a vortex mixer (IKA, Staufen, Germany) at maximum speed for 2 min. The mixture was then left undisturbed at 25 °C for 24 h. The E24% was calculated as the height of the emulsion layer divided by the total height of the liquid column, expressed as a percentage.
2.10. Determination of the Critical Micelle Concentration (CMC)
The critical micelle concentration (CMC) is defined as the solute concentration at which further increases no longer result in a significant decrease in surface tension. To determine the CMC, the extracted rhamnolipids were dissolved in distilled water to obtain a stock solution with an initial concentration of 96 mg/L. A series of dilutions was then prepared down to 3 mg/L. The surface tension of RL solutions at varying concentrations was measured using a BZY-102 tensiometer (Shanghai Fangrui Instrument Co., Shanghai, China) based on the du Noüy ring method to determine the critical micelle concentration (CMC), and the results were plotted to generate a curve of surface tension versus surfactant concentration. The CMC was identified as the point at which the curve plateaued.
2.11. LC–MS/MS Analysis
Purified rhamnolipids were dissolved in chloroform (0.1 g/mL). Analysis was performed using a Waters UPLC system (Waters Corp., Milford, MA, USA) with an Acquity UPLC BEH C18 column (1.7 μm, 2.1 × 50 mm), coupled to an AB Sciex TripleTOF 5600+ mass spectrometer (Sciex, Framingham, MA, USA). The mobile phases were 0.1% formic acid in water (A) and in acetonitrile (B), with a gradient of 20% to 95% B from 0 to 20 min, held until 35 min, and returned to 20% at 36 min. The injection volume was 2 μL, the flow rate 0.4 mL/min, the column temperature 35 °C, and UV detection at 220 nm.
Mass spectrometry was conducted in the negative ion mode for rhamnolipids (scan range m/z 100–2000), with a source voltage of −4.5 kV and source temperature of 550 °C. Gas pressures were set to 50 psi for Gas 1 and 2 (Air) and 35 psi for Curtain Gas (N2). Mass tolerance was ±5 ppm, with a declustering potential of 100 V. Collision energy (CE) was 10 V for MS and 50 ± 20 V for MS/MS, with IRD and IRW set at 67 and 25, respectively. Data acquisition and processing were performed using Analyst TF 1.6 and PeakView v1.2 (AB Sciex, Framingham, MA, USA).
2.12. Statistical Analysis
Data are presented as the mean ± standard error. The statistical significance of differences was assessed using an unpaired, two-tailed Student’s t-test. A p-value < 0.05 was deemed statistically significant.
2.13. Data Availability
All the data presented in this document can be found within the manuscript and accompanying Supplementary Files.
3. Results
3.1. Construction of rhlC-Deleted Strains to Investigate fabA and fabZ Effects on Mono-Rhamnolipid Fatty Acyl Composition
The fabA and fabZ genes encode β-hydroxyacyl-ACP dehydratases, which are essential for fatty acid biosynthesis and membrane lipid homeostasis in P. aeruginosa [12]. Specifically, β-hydroxyacyl-ACP, a precursor for rhamnolipid fatty acyl chains [7], is generated during the fatty acid elongation cycle, thereby influencing the structure of rhamnolipids. Due to their essential roles, the deletion of fabA or fabZ is lethal. In our previous study, we identified a suppressor strain that rescues the lethal phenotype of the ΔfabA mutant (ΔfabA-sup), enabling genetic analysis of fabA-deficient strains [18]. Similarly, using the same plasmid-based temperature-sensitive (ts) approach, we constructed a fabZ ts-mutant fabZ(Ts) and subsequently isolated a ΔfabZ suppressor mutant, ΔfabZ-sup (Supplementary Figure S1), analogous to ΔfabA-sup. Additionally, we generated fabA-overexpressing (fabA-OE) and fabZ-overexpressing (fabZ-OE) strains [18] using an arabinose-inducible araC-PBAD promoter system [23].
The rhlC gene encodes rhamnosyltransferase 2, which converts mono-rhamnolipids (mRLs) into di-rhamnolipids (dRLs) [22]. Therefore, rhlC deletion forces the strain to produce only mRLs, providing a suitable model to examine how fabA and fabZ perturbations affect their fatty acyl chain composition. Using a two-step rapid method for the knockout of dispensable genes in the P. aeruginosa method [25] (see Section 2), we deleted rhlC in P. aeruginosa PAO1 wild-type, as well as in the fabA-OE, fabZ-OE, ΔfabA-sup, and ΔfabZ-sup backgrounds, generating five mutant strains: ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE. PCR verification confirmed successful rhlC deletion in all strains (Figure 1A), and TLC (thin-layer chromatography) analysis demonstrated that, while wild-type P. aeruginosa PAO1 produced both mRLs and dRLs, all mutant strains exclusively synthesized mRLs (Figure 1B).
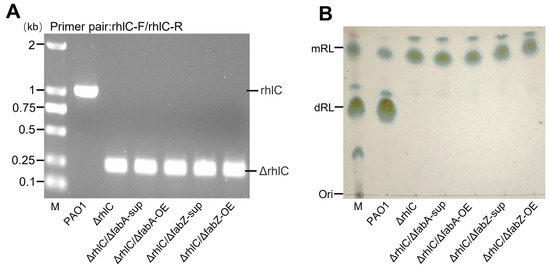
Figure 1.
Confirmation of rhlC deletion and rhamnolipid composition analysis in mutant strains. (A) PCR verification of rhlC deletion using the primer pair rhlC-F/rhlC-R. The wild-type P. aeruginosa PAO1 strain shows an intact rhlC band (~1 kb), while the ΔrhlC-derived mutant strains exhibit a smaller ΔrhlC amplicon (~0.2 kb), confirming successful gene deletion. M: DNA marker. (B) TLC analysis of rhamnolipid production. The PAO1 produces both mono-rhamnolipids (mRLs) and di-rhamnolipids (dRLs), while ΔrhlC-derived mutant strains exclusively produce mRL, confirming the functional loss of rhlC. M: rhamnolipid standard marker.
3.2. Effects of fabA and fabZ Deletion and Overexpression on Bacterial Growth and RL Production
To evaluate the impact of fabA and fabZ deletion and overexpression on bacterial growth and RLs production, we compared the growth curves and rhamnolipid yields of the ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains. All strains were cultured in MS medium supplemented with 2% glycerol, and overexpression of fabA and fabZ was induced with 0.02% arabinose. The OD600 was measured every 12 h, and mRL production was quantified daily.
Growth curve analysis showed that both fabA and fabZ deletion, as well as their overexpression, resulted in a slight reduction in growth compared to the control strain ΔrhlC (Figure 2A,B and Supplementary Figure S2). Despite these mild differences, all strains reached a stationary phase at approximately 60 h, indicating that fabA and fabZ mutations do not drastically impair bacterial growth. Similarly, mRL production analysis using an orcinol method [26,28] revealed that fabA and fabZ suppression or overexpression caused a slight reduction in rhamnolipid yield (Figure 2C,D). However, the differences were relatively minor, suggesting that fabA and fabZ perturbations do not substantially disrupt rhamnolipid biosynthesis.
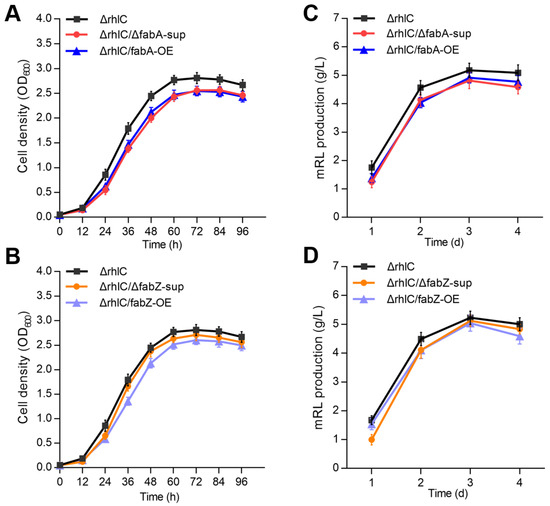
Figure 2.
Growth curve analysis and rhamnolipids production of ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains. (A,B) Growth curves of the ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains, with time (h) on the x-axis and cell density (OD600) on the y-axis. (C,D) Quantification of rhamnolipids by the orcinol method produced by various mutant stains.
3.3. Effects of fabA and fabZ Deletion and Overexpression on the Fatty Acyl Composition of mRLs
To investigate how fabA and fabZ deletion or overexpression affects the fatty acyl composition of mRLs, we extracted mRLs from the five strains (ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE) and analyzed their fatty acyl chain structures using LC–MS/MS. Seven major mRL species were identified based on their negative ion mass spectra (Supplementary Figure S3), and their relative abundances in each strain are summarized in Table 2 and Supplementary Table S1. The results showed that the ΔrhlC strain exhibited a balanced distribution of saturated and unsaturated acyl chains, with the most abundant structures being Rha-C10-C10 (27.61%) and Rha-C10-C12:1/Rha-C12:1-C10 (30.31%), followed by Rha-C10-C12/Rha-C12-C10 (17.35%) (Table 2 and Figure 3). However, in ΔrhlC/ΔfabA-sup, there was a pronounced shift toward longer unsaturated acyl chains, with Rha-C10-C12:1/Rha-C12:1-C10 increasing to 59.30%, while Rha-C10-C10 decreased to 12.94% and shorter acyl chain species (Rha-C8-C8/Rha-C10-C6) were nearly depleted (0.48%) (Table 2 and Figure 3). Conversely, overexpression of fabA (ΔrhlC/fabA-OE) resulted in an increase in Rha-C10-C14:1/Rha-C14:1-C10 (23.40%) and Rha-C8-C8/Rha-C10-C6 (27.54%) compared to their proportions in ΔrhlC, while medium-chain fatty acids mRLs such as Rha-C10-C10, Rha-C10-C12:1, and Rha-C12-C10 showed a concurrent decrease (Table 2 and Figure 3).

Table 2.
Relative abundance of seven mono-rhamnolipid structures in different strains.
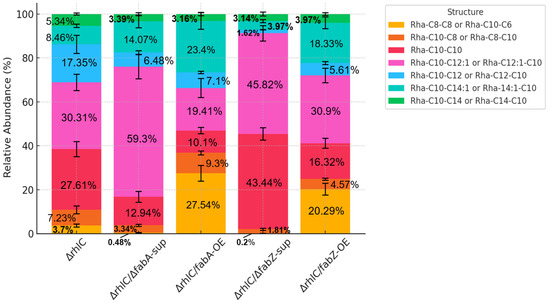
Figure 3.
Relative abundance of seven mRL structures in different strains. Stacked bar chart showing the relative abundance of seven major mRL species identified in five P. aeruginosa strains: ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE. The composition of mRL fatty acyl chains was analyzed using LC–MS/MS.
Similarly, the deletion of fabZ also exhibited distinct acyl chain profiles. In ΔrhlC/ΔfabZ-sup, Rha-C10-C10 increased significantly to 43.44%, accompanied by a notable accumulation of Rha-C10-C12 or Rha-C12-C10 (45.82%) and a sharp decline in longer-chain species such as Rha-C10-C14:1/Rha-14:1-C10 (3.97%) and Rha-C10-C12:1/Rha-12:1-C10 (1.62%) compared to ΔrhlC (Table 2 and Figure 3). Overexpression of fabZ (ΔrhlC/fabZ-OE) resulted in a noticeable increase in shorter-chain species of Rha-C8-C8 or Rha-C10-C6 (20.29%) and longer-chain unsaturated species of Rha-C10-C14:1 or Rha-C14:1-C10 (18.33%) compared to ΔrhlC (Table 2 and Figure 3). These findings suggest that fabA and fabZ play regulatory roles in determining the rhamnolipid fatty acid chain length and saturation, likely by modulating the availability of precursor β-hydroxyacyl-ACPs in the fatty acid synthesis pathway.
3.4. Emulsification Activity of mRLs with Modified Acyl Chain Composition Due to fabA and fabZ Perturbations
To assess whether the changes in rhamnolipid acyl chain composition induced by fabA and fabZ perturbations influence their physicochemical properties, we evaluated the emulsification activity of mRLs produced by the ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains. The emulsification index (E24%) was determined by mixing purified rhamnolipids with crude oil, with SDS (sodium dodecyl sulfate) serving as a reference surfactant (Figure 4A,B).
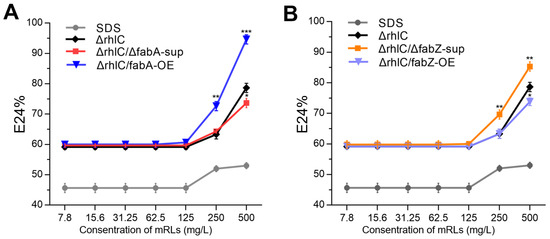
Figure 4.
Emulsification activity (E24%) of mRLs produced by different stains. (A) Emulsification efficiency of mRLs from the ΔrhlC, ΔrhlC/ΔfabA-sup, and ΔrhlC/fabA-OE strains. SDS was used as a reference surfactant. Data are the mean ± SD (n = 3), Asterisks indicate significant differences (* p < 0.05, ** p < 0.01, and *** p < 0.001; Student’s t-test). (B) Emulsification efficiency of mRLs from the ΔrhlC, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains.
The results showed that emulsification activity varied significantly among the strains, correlating with their respective acyl chain compositions. At lower concentrations (7.8–125 mg/L), all rhamnolipids exhibited minimal emulsification (E24% ≈ 60%), indicating that higher surfactant concentrations are required for efficient emulsification (Figure 4A,B). However, at concentrations of 250 mg/L and above, clear differences emerged. Among all the strains, ΔrhlC/fabA-OE (fabA overexpression) exhibited the highest emulsification activity, reaching an E24% of nearly 100% at 500 mg/L, significantly higher than the control strain (ΔrhlC) (Figure 4A). Conversely, ΔrhlC/ΔfabA-sup (fabA deletion) led to reduced emulsification efficiency. For fabZ mutants, ΔrhlC/ΔfabZ-sup also showed higher emulsification activity than the control (Figure 4B). In contrast, ΔrhlC/fabZ-OE displayed an emulsification pattern similar to the control ΔrhlC strain, with a slight decrease observed at the 500 mg/L RL concentration (Figure 4B). These findings confirm that rhamnolipid emulsification properties can be modulated by altering fabA and fabZ expressions.
3.5. Critical Micelle Concentration (CMC) Analysis of mRLs
Surfactants function by reducing surface tension, and their efficiency is often characterized by the critical micelle concentration (CMC), which represents the concentration at which micelles begin to form. A lower CMC value indicates a more efficient surfactant, as micelles are formed at lower concentrations, allowing for more effective surface tension reduction. To evaluate the impact of fabA and fabZ modifications on rhamnolipid surface activity, we determined the CMC of mRLs produced by the ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains. The results showed that, before reaching the CMC, the surface tension of the rhamnolipid solutions decreased sharply with the increasing concentration. Once a threshold concentration was reached, the reduction in surface tension slowed, eventually stabilizing at a minimum value, beyond which further increases in rhamnolipid concentration had little effect on the surface tension (Figure 5). The intersection of these two trends was used to determine the CMC values of each strain’s rhamnolipids.
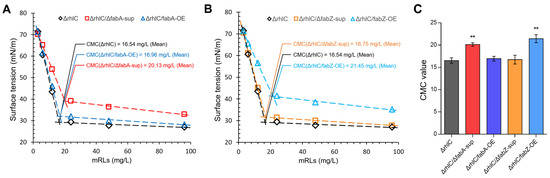
Figure 5.
Critical micelle concentration (CMC) of mRLs produced by different stains. (A,B) The CMC was determined for rhamnolipids extracted from the ΔrhlC, ΔrhlC/ΔfabA-sup, ΔrhlC/fabA-OE, ΔrhlC/ΔfabZ-sup, and ΔrhlC/fabZ-OE strains. CMC values were calculated based on the intersection of two trend lines: the rapid decline in surface tension before micelle formation and the plateau phase after micelle formation. (C) Comparison of the CMC values among the five tested strains. Data are the mean ± SD (n = 3). ** Indicates a significant difference (p < 0.01, Student’s t-test).
The results showed that the CMC values varied considerably among the tested strains, which may correlate with differences in their respective fatty acyl compositions. The control strain (ΔrhlC) exhibited a CMC of 16.54 mg/L, reflecting typical rhamnolipid surface activity. fabA deletion (ΔrhlC/ΔfabA-sup) resulted in an elevated CMC of 20.13 mg/L (Figure 5A,C). Conversely, fabA overexpression (ΔrhlC/fabA-OE) reduced the CMC to 16.96 mg/L (Figure 5A,C). Similarly, fabZ deletion (ΔrhlC/ΔfabZ-sup) yielded a resemble CMC (16.75 mg/L) with the ΔrhlC strain (Figure 5B,C). In contrast, fabZ overexpression (ΔrhlC/fabZ-OE) resulted in the highest CMC (21.45 mg/L). These results confirm that rhamnolipid surface activity can be affected by altering fabA and fabZ expression.
4. Discussion
With advances in analytical techniques, more than 60 rhamnolipid (RL) congeners have been identified [7], each differing in their glycosyl groups and hydrophobic fatty acyl chains. Variations in the fatty acyl chain length and saturation among these congeners result in distinct physicochemical properties [29,30,31], such as solubility, surface activity, and emulsification efficiency, thus influencing their industrial and environmental applications [8]. Although previous metabolic engineering efforts in RL-producing strains were primarily aimed at increasing RL yields [32,33,34,35], recent findings highlight that precise modulation of the RL fatty acyl composition is equally essential for optimizing their functionality [36,37,38]. In this study, targeted modulation of the fatty acid synthesis genes fabA and fabZ within the FAS II pathway was shown to influence the fatty acyl chain length and saturation of RLs, offering a novel strategy for customizing RL properties to meet specific application requirements. Critically, this genetic reprogramming strategy maintained host viability, as the modulation of fabA and fabZ expression exerted only modest effects on bacterial growth and RL output (Figure 2). Both deletion (with suppressors) and mild overexpression caused a slight reduction in the specific growth rate and final RL titer compared to the ΔrhlC controls (Figure 2), demonstrating the feasibility of essential gene tuning without compromising RL biosynthesis.
Our results showed that changes in fabA and fabZ expression do not simply shift RL fatty acyl chains uniformly toward shorter or longer species. Instead, they selectively alter the abundance of specific RL variants. These structural shifts may be mechanistically explained by the altered availability of β-hydroxyacyl-ACP intermediates for RhlA, driven by FabA- and FabZ-mediated fatty acid flux redistribution in the FAS II cycle [5,6,7,8]. For instance, the deletion of fabA significantly increased the proportion of medium-chain unsaturated RLs (particularly C10–C12:1) while reducing shorter-chain (C6–C8) and saturated RL species (C10–C10) (Figure 3). Conversely, overexpression of fabA increased the proportions of both shorter-chain unsaturated (C8–C10) and longer-chain unsaturated (C14:1) RLs (Figure 3). This likely results from an increased flux through the FabA-mediated dehydration/isomerization step, generating diverse unsaturated intermediates [12,39].
The regulatory effect of fabZ exhibited a different pattern. Deletion of fabZ enriched the RL populations with medium-length saturated and partially unsaturated fatty acids (notably Rha-C10-C10 and Rha-C10-C12:1/Rha-C12:1-C10), accompanied by reduced longer-chain RLs (Figure 3). Overexpression of fabZ, however, produced a bimodal distribution of RL fatty acid lengths (Figure 3), suggesting that excessive FabZ activity disrupts the balance of fatty acid elongation intermediates [13,40], resulting in diverse RL species.
Alterations in the RL structure clearly impacted the emulsification performance. RLs from strains enriched in medium-chain fatty acids (C10–C12), such as ΔrhlC/ΔfabZ-sup, displayed enhanced emulsification ability (Figure 4B), which may be attributed to the favorable balance between hydrophilic and hydrophobic interactions provided by intermediate-length, partially unsaturated fatty acid chains at the oil–water interface. Conversely, RL mixtures with broader acyl-chain distributions, as in ΔrhlC/fabA-OE, showed superior emulsification only at higher concentrations, while RLs from ΔrhlC/fabZ-OE exhibited limited improvements. Critically, while the overall emulsification activity differed among strains, the structural complexity of rhamnolipid mixtures limits the attribution of activity changes to individual congeners alone. Such variability may arise from suboptimal molecular packing at interfaces due to heterogeneity in RL acyl chains [41,42]. However, although both the ΔrhlC/ΔfabA-sup and ΔrhlC/ΔfabZ-sup strains produced mRLs enriched in medium-chain fatty acids, only the latter exhibited improved emulsification, suggesting that additional factors such as congener saturation or acyl chain positioning may also contribute to functional differences. Critical micelle concentration (CMC) analysis further supported these observations (Figure 5). RLs from the ΔrhlC/ΔfabZ-sup and ΔrhlC/fabA-OE strains maintained relatively low CMC values similar to the control RLs, indicating high surface activity. In contrast, RLs from ΔrhlC/fabZ-OE and ΔrhlC/fabA-sup showed a notably higher CMC, reflecting less efficient micelle formation due to diverse fatty acid chain lengths [43,44].
Furthermore, this study highlights the utility of temperature-sensitive alleles and suppressor mutations in functional analyses of essential genes. As the direct deletion of fabA or fabZ is lethal in P. aeruginosa [15], employing suppressor screening methods [17,18] facilitated clear elucidation of their roles in RLs synthesis. Notably, the ΔrhlC/ΔfabZ-sup and ΔrhlC/fabA-OE strains exhibited enhanced emulsification activity while maintaining CMC values comparable to the control, highlighting their potential as promising candidates for high-activity RL production. Future studies could extend this approach to other essential fatty acid biosynthesis genes (e.g., fabB, fabG, or fabF) to further diversify RL structures and optimize their properties. In summary, our study demonstrates that the modulation of fabA and fabZ significantly influences RL fatty acid composition and functionality, providing both theoretical insights and practical guidelines for engineering RL biosurfactants tailored for specific industrial purposes.
5. Conclusions
In this study, targeted genetic modulation of the fatty acid synthesis pathway genes fabA and fabZ effectively altered the fatty acyl composition of rhamnolipids (RLs) produced by P. aeruginosa PAO1. Deletion or overexpression of fabA and fabZ resulted in distinct and controllable shifts in the RL acyl-chain length and saturation, leading to significant changes in their emulsification performance and surface activity. Additionally, the suppressor-based approach used for analyzing these essential genes provided an effective strategy to dissect their roles in RL biosynthesis. This research provides useful insights into how the genetic modulation of fatty acid synthesis pathways can influence RL structures and function, offering a potential strategy for the development of biosurfactants with improved or application-specific properties.
Nevertheless, this study has certain limitations. First, since P. aeruginosa is a human pathogen, large-scale applications necessitate strict containment; future studies should explore transferring these genetic modifications into non-pathogenic hosts. Second, the fatty acid synthesis pathway is regulated by a network of enzymes and factors, and focusing exclusively on fabA and fabZ may not capture the full spectrum of metabolic control; genes such as fabB, fabG, or fabF may also play significant roles. Addressing these aspects will be essential to further refine and generalize this engineering strategy for broader application.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes16050515/s1: Figure S1: Construction and characterization of the fabZ temperature-sensitive (ts) mutant fabZ(Ts) and suppressor mutant (ΔfabZ-sup) in P. aeruginosa; Figure S2: Maximum specific growth rates (μmax) of ΔrhlC-derived strains; Figure S3: MS2 spectra of various mRL congeners detected; Table S1: Replicate abundance data for mRLs Composition in different strains.
Author Contributions
J.L., Z.C., H.Z., and Q.T. conducted biological and biochemical experiments. Z.Y. conceptualized the study, contributed to its design and coordination, and drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China under Grant No. LQ24C010003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Irfan-Maqsood, M.; Seddiq-Shams, M. Rhamnolipids: Well-characterized glycolipids with potential broad applicability as biosurfactants. Ind. Biotechnol. 2014, 10, 285–291. [Google Scholar] [CrossRef]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Factories 2021, 20, 1. [Google Scholar] [CrossRef]
- Soberón-Chávez, G.; Lépine, F.; Déziel, E. Production of rhamnolipids by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2005, 68, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.; Rahman, T.J.; McClean, S.; Marchant, R.; Banat, I.M. Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol. Prog. 2002, 18, 1277–1281. [Google Scholar] [CrossRef]
- White, S.W.; Zheng, J.; Zhang, Y.-M.; Rock, C.O. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 2005, 74, 791–831. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Rock, C.O. RhlA converts β-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the β-hydroxydecanoyl-β-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J. Bacteriol. 2008, 190, 3147–3154. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Costa, S.G.; Nitschke, M.; Lépine, F.; Déziel, E.; Contiero, J. Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem. 2010, 45, 1511–1516. [Google Scholar] [CrossRef]
- Gong, Z.; He, Q.; Che, C.; Liu, J.; Yang, G. Optimization and scale-up of the production of rhamnolipid by Pseudomonas aeruginosa in solid-state fermentation using high-density polyurethane foam as an inert support. Bioprocess Biosyst. Eng. 2020, 43, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Blunt, W.; Blanchard, C.; Morley, K. Effects of environmental parameters on microbial rhamnolipid biosynthesis and bioreactor strategies for enhanced productivity. Biochem. Eng. J. 2022, 182, 108436. [Google Scholar] [CrossRef]
- Zhao, F.; Cui, Q.; Han, S.; Dong, H.; Zhang, J.; Ma, F.; Zhang, Y. Enhanced rhamnolipid production of Pseudomonas aeruginosa SG by increasing copy number of rhlAB genes with modified promoter. RSC Adv. 2015, 5, 70546–70552. [Google Scholar] [CrossRef]
- Heath, R.J.; Rock, C.O. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 1996, 271, 27795–27801. [Google Scholar] [CrossRef]
- Kimber, M.S.; Martin, F.; Lu, Y.; Houston, S.; Vedadi, M.; Dharamsi, A.; Fiebig, K.M.; Schmid, M.; Rock, C.O. The structure of (3R)-hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 52593–52602. [Google Scholar] [CrossRef]
- Ruppe, S.; Mains, K.; Fox, J.M. A kinetic rationale for functional redundancy in fatty acid biosynthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 23557–23564. [Google Scholar] [CrossRef]
- Lee, S.A.; Gallagher, L.A.; Thongdee, M.; Staudinger, B.J.; Lippman, S.; Singh, P.K.; Manoil, C. General and condition-specific essential functions of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2015, 112, 5189–5194. [Google Scholar] [CrossRef]
- Goodall, E.C.; Robinson, A.; Johnston, I.G.; Jabbari, S.; Turner, K.A.; Cunningham, A.F.; Lund, P.A.; Cole, J.A.; Henderson, I.R. The essential genome of Escherichia coli K-12. mBio 2018, 9, e02096-17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Zhu, J.; Ma, Y.; Wang, J.; Liu, J. Analysis of the plasmid-based ts allele of PA0006 reveals its function in regulation of cell morphology and biosynthesis of core lipopolysaccharide in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2022, 88, e00480-22. [Google Scholar] [CrossRef]
- Tian, L.; Yang, Z.; Wang, J.; Liu, J. Analysis of the Plasmid-Based ts-Mutant Δ fabA/pTS-fabA Reveals Its Lethality under Aerobic Growth Conditions That Is Suppressed by Mild Overexpression of desA at a Restrictive Temperature in Pseudomonas aeruginosa. Microbiol. Spectr. 2023, 11, e01338-23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Z.; Liu, J. Genetic Analysis of the Plasmid-Based Temperature-Lethal Mutant pa1792| lpxH (Ts) in Pseudomonas aeruginosa. Genes 2024, 15, 784. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, H.; Yang, Z. Genetic Analysis of the ts-Lethal Mutant Δpa0665/pTS-pa0665 Reveals Its Role in Cell Morphology and Oxidative Phosphorylation in Pseudomonas aeruginosa. Genes 2024, 15, 590. [Google Scholar] [CrossRef]
- Müller, M.M.; Hörmann, B.; Syldatk, C.; Hausmann, R. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl. Microbiol. Biotechnol. 2010, 87, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rahim, R.; Ochsner, U.A.; Olvera, C.; Graninger, M.; Messner, P.; Lam, J.S.; Soberón-Chávez, G. Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyltransferase 2, an enzyme responsible for di-rhamnolipid biosynthesis. Mol. Microbiol. 2001, 40, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.-M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop II, R.M.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Huang, W.; Wilks, A. A rapid seamless method for gene knockout in Pseudomonas aeruginosa. BMC Microbiol. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laabei, M.; Jamieson, W.D.; Lewis, S.E.; Diggle, S.P.; Jenkins, A.T.A. A new assay for rhamnolipid detection—Important virulence factors of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2014, 98, 7199–7209. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Brückner, J. Estimation of monosaccharides by the orcinol–sulphuric acid reaction. Biochem. J. 1955, 60, 200. [Google Scholar] [CrossRef]
- Abalos, A.; Pinazo, A.; Infante, M.R.; Casals, M.; Garcia, F.; Manresa, A. Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas a eruginosa AT10 from soybean oil refinery wastes. Langmuir 2001, 17, 1367–1371. [Google Scholar] [CrossRef]
- Déziel, É.; Lépine, F.; Dennie, D.; Boismenu, D.; Mamer, O.A.; Villemur, R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 1999, 1440, 244–252. [Google Scholar] [CrossRef]
- Monteiro, S.A.; Sassaki, G.L.; de Souza, L.M.; Meira, J.A.; de Araújo, J.M.; Mitchell, D.A.; Ramos, L.P.; Krieger, N. Molecular and structural characterization of the biosurfactant produced by Pseudomonas aeruginosa DAUPE 614. Chem. Phys. Lipids 2007, 147, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, Y.; Tian, Y.; Hao, Z.; Chen, F.; Ma, Y. Enhanced rhamnolipid production of Pseudomonas aeruginosa DN1 by metabolic engineering under diverse nutritional factors. J. Pet. Environ. Biotechnol. 2018, 9, 384. [Google Scholar]
- Sinumvayo, J.P.; Ishimwe, N. Agriculture and food applications of rhamnolipids and its production by Pseudomonas aeruginosa. J. Chem. Eng. Process Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Chong, H.; Li, Q. Microbial production of rhamnolipids: Opportunities, challenges and strategies. Microb. Cell Factories 2017, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pang, A.-P.; Wang, Y.; Zhang, T.; Gao, F.; Shen, J.-D.; Huang, L.; Zhou, J.; Zhang, B.; Liu, Z.-Q.; Zheng, Y.-G. Highly efficient production of rhamnolipid in P. putida using a novel sacB-based system and mixed carbon source. Bioresour. Technol. 2024, 394, 130220. [Google Scholar] [CrossRef] [PubMed]
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Biosurfactant from Pseudomonas species with waxes as carbon source–Their production, modeling and properties. J. Ind. Eng. Chem. 2015, 31, 100–111. [Google Scholar] [CrossRef]
- Wittgens, A.; Santiago-Schuebel, B.; Henkel, M.; Tiso, T.; Blank, L.M.; Hausmann, R.; Hofmann, D.; Wilhelm, S.; Jaeger, K.-E.; Rosenau, F. Heterologous production of long-chain rhamnolipids from Burkholderia glumae in Pseudomonas putida—A step forward to tailor-made rhamnolipids. Appl. Microbiol. Biotechnol. 2018, 102, 1229–1239. [Google Scholar] [CrossRef]
- Tiso, T.; Zauter, R.; Tulke, H.; Leuchtle, B.; Li, W.-J.; Behrens, B.; Wittgens, A.; Rosenau, F.; Hayen, H.; Blank, L.M. Designer rhamnolipids by reduction of congener diversity: Production and characterization. Microb. Cell Factories 2017, 16, 1–14. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, X.; Khosla, C. Metabolic flux between unsaturated and saturated fatty acids is controlled by the FabA: FabB ratio in the fully reconstituted fatty acid biosynthetic pathway of Escherichia coli. Biochemistry 2013, 52, 8304–8312. [Google Scholar] [CrossRef][Green Version]
- Dodge, G.J.; Patel, A.; Jaremko, K.L.; McCammon, J.A.; Smith, J.L.; Burkart, M.D. Structural and dynamical rationale for fatty acid unsaturation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2019, 116, 6775–6783. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the world of rhamnolipids: A critical review of their production, interfacial properties, and potential application. Curr. Opin. Colloid Interface Sci. 2024, 69, 101780. [Google Scholar] [CrossRef]
- Palos Pacheco, R.; Kegel, L.L.; Pemberton, J.E. Interfacial and solution aggregation behavior of a series of bioinspired rhamnolipid congeners rha-C14-c x (x= 6, 8, 10, 12, 14). J. Phys. Chem. B 2021, 125, 13585–13596. [Google Scholar] [CrossRef] [PubMed]
- Mitrinova, Z.; Tcholakova, S.; Popova, Z.; Denkov, N.; Dasgupta, B.R.; Ananthapadmanabhan, K. Efficient control of the rheological and surface properties of surfactant solutions containing C8–C18 fatty acids as cosurfactants. Langmuir 2013, 29, 8255–8265. [Google Scholar] [CrossRef]
- Youssef, N.H.; Nguyen, T.; Sabatini, D.A.; McInerney, M.J. Basis for formulating biosurfactant mixtures to achieve ultra low interfacial tension values against hydrocarbons. J. Ind. Microbiol. Biotechnol. 2007, 34, 497–507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).