Identification of Differentially Expressed Genes in Spinal Cord Injury
Abstract
1. Introduction
1.1. The Burden of Spinal Cord Injury
1.2. Pathophysiology of SCI & Regeneration
1.2.1. Disease Progression
1.2.2. Central vs. Peripheral Nervous System
1.2.3. Regenerative Hypotheses
1.3. Genetic Analysis
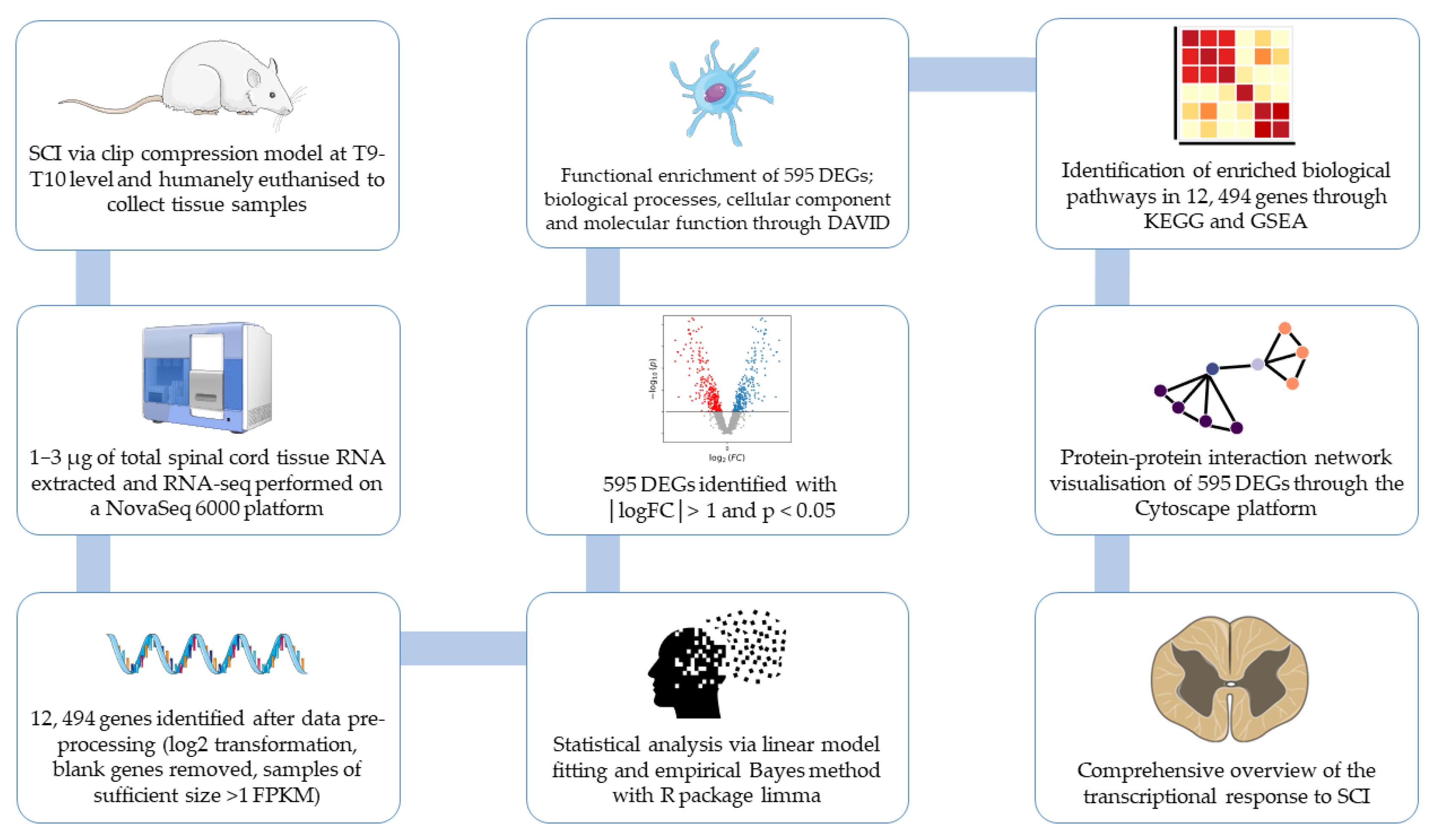
2. Method
2.1. Gene Collection
2.2. RNA-Sequencing
2.3. Data Pre-Processing
2.4. Statistical Analysis
2.5. Functional Enrichment Analysis of Downregulated DEGs
2.6. Protein–Protein Interaction (PPI) Network and Module Analysis
3. Results from Total Spinal Cord Tissue of Ratticus Norvegicus
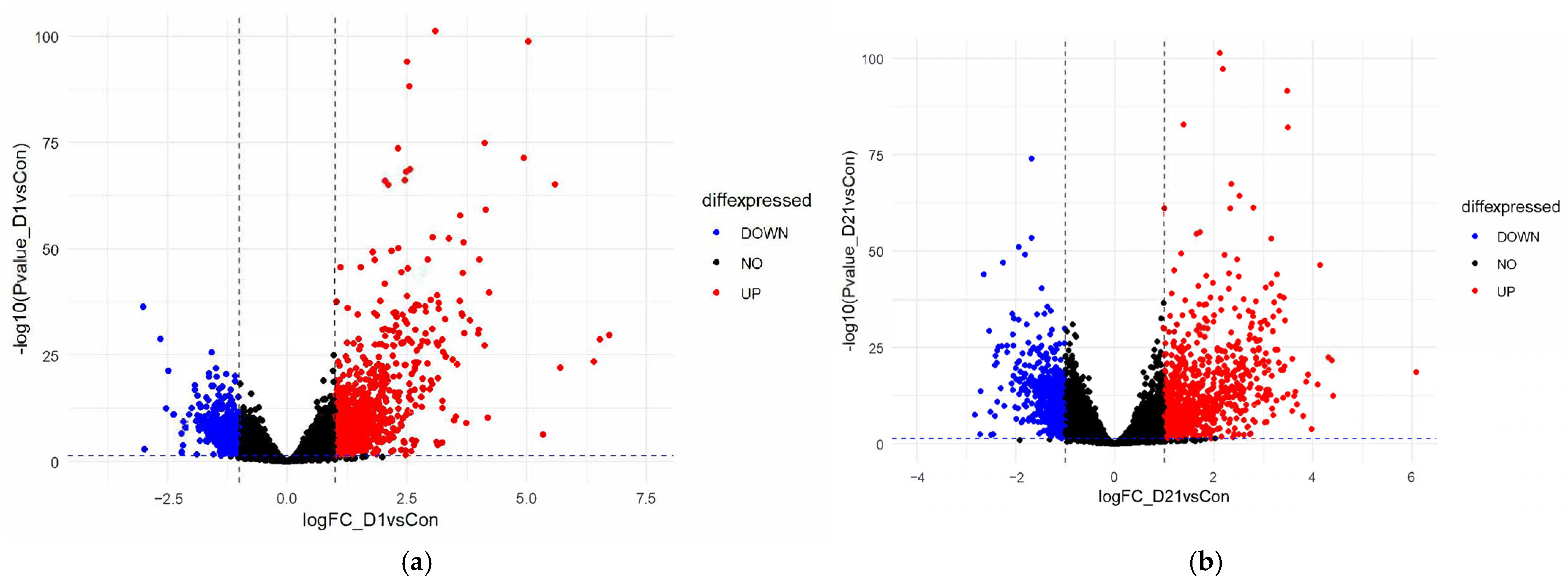
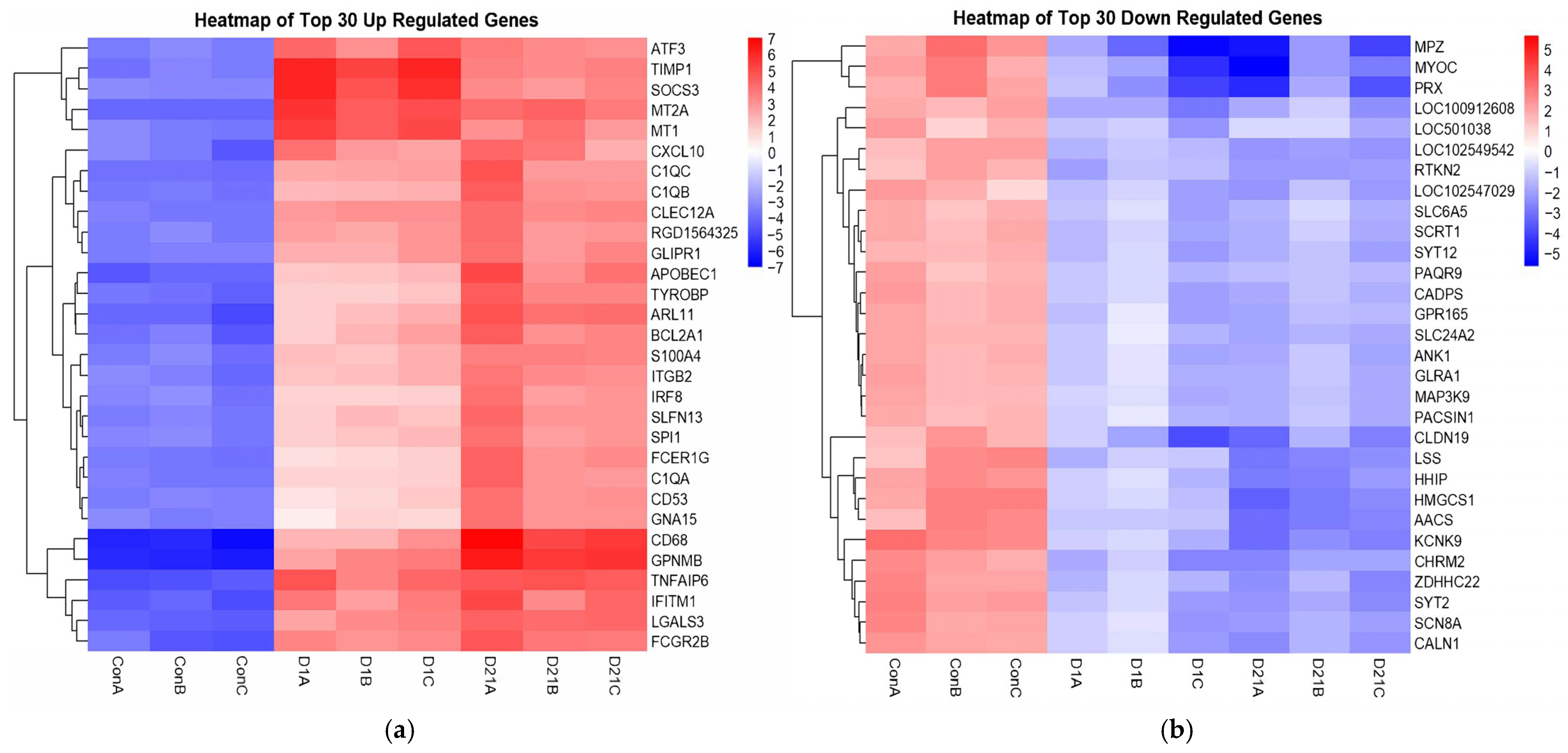
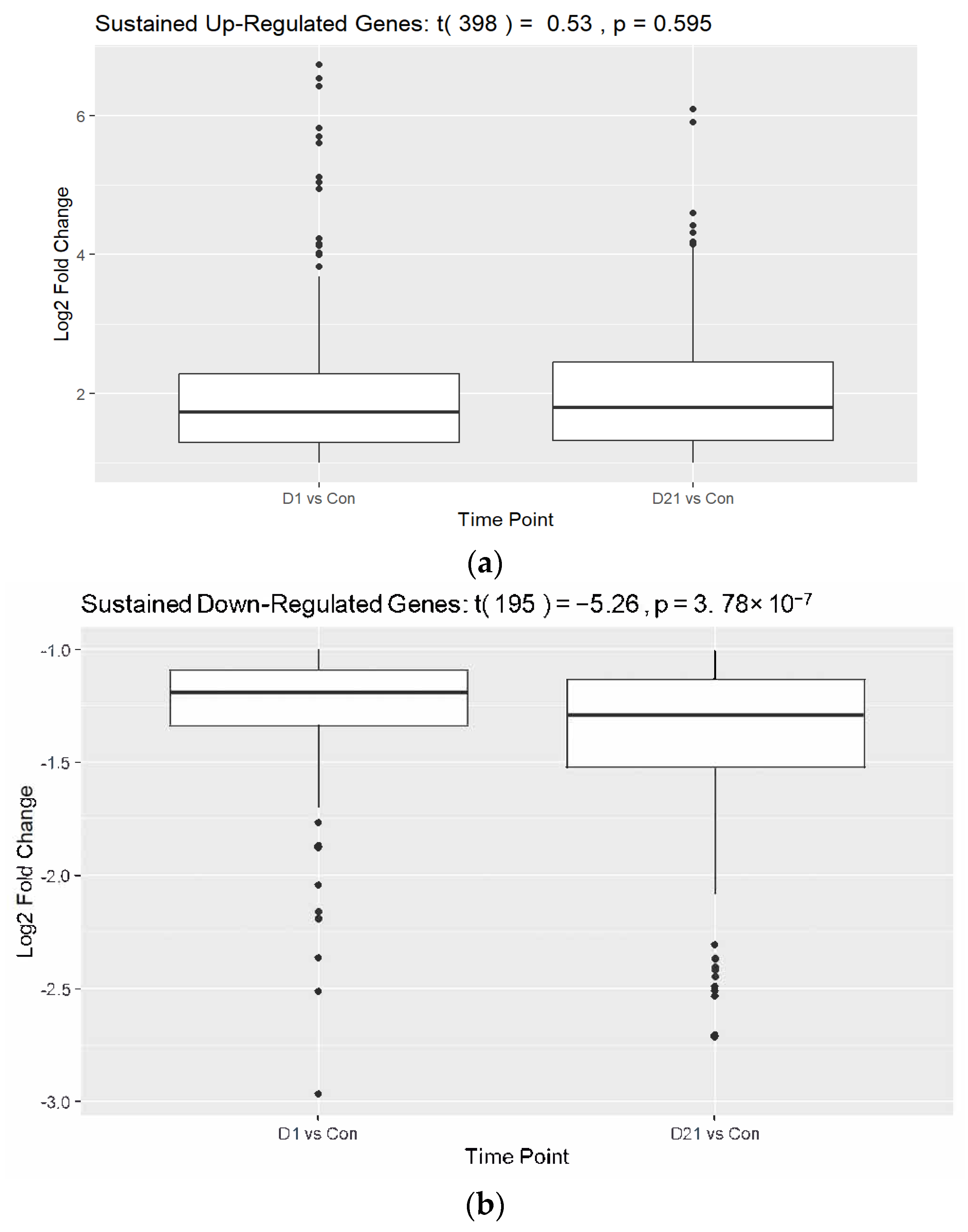
3.1. Differential Gene Expression Analysis Between SCI and Control Groups
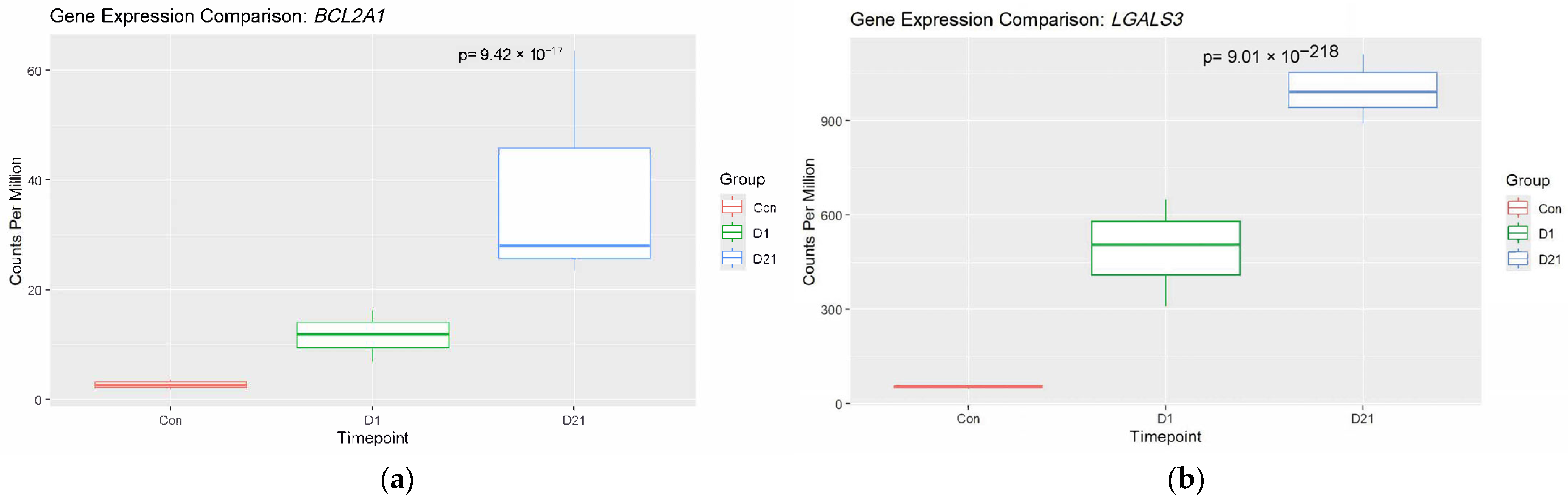
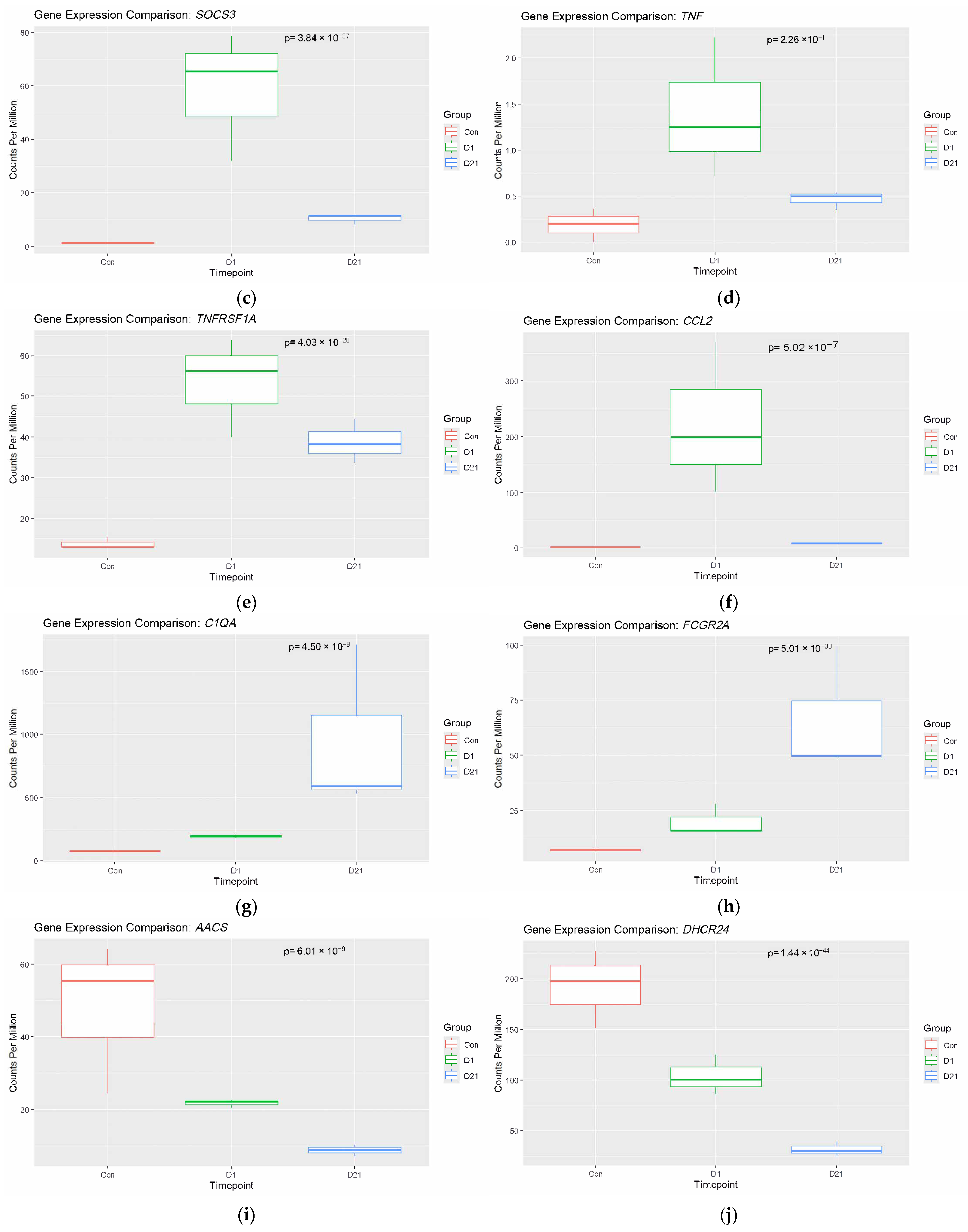
3.2. Analysis of Established Genes Involved in SCI
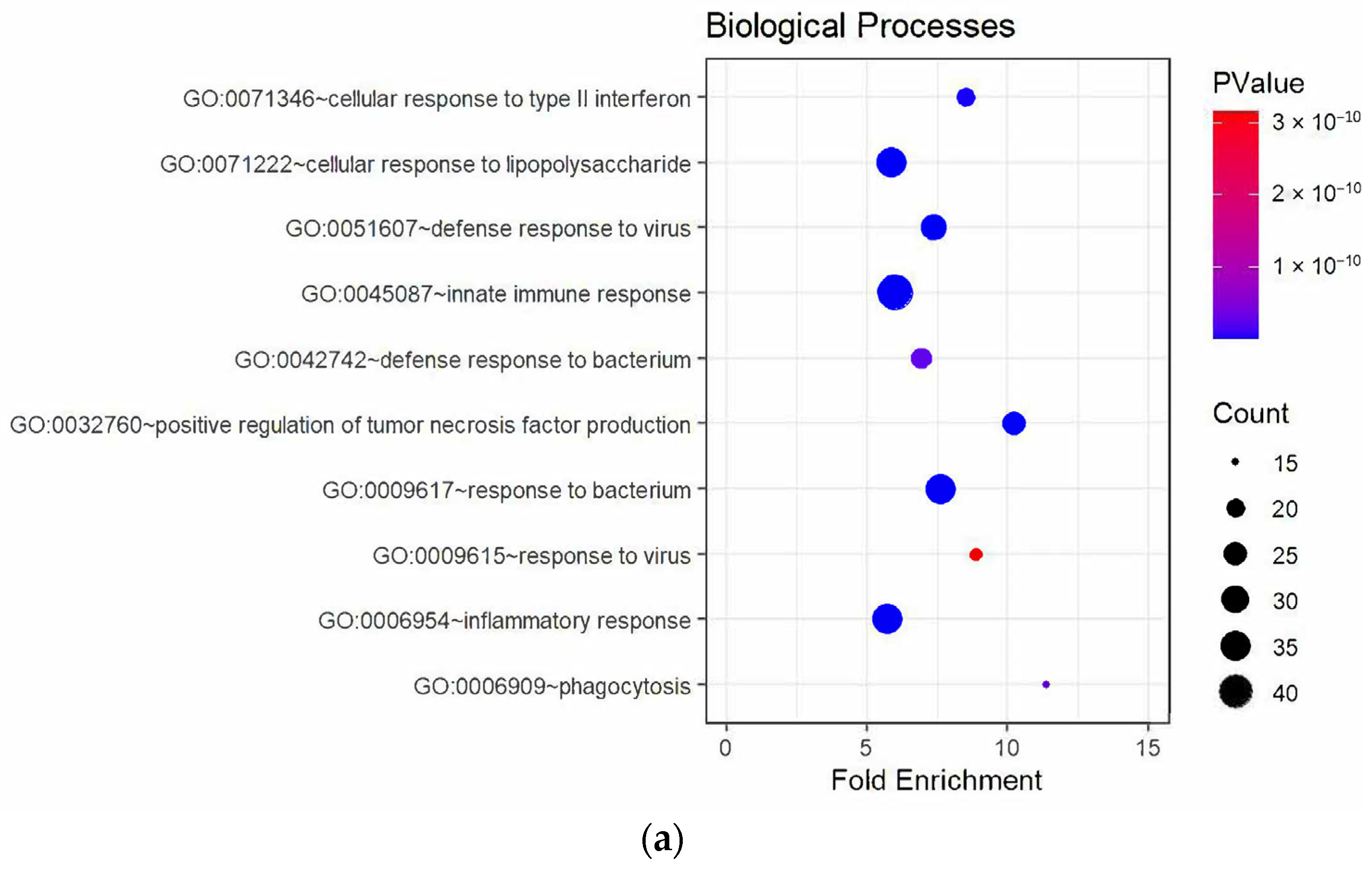
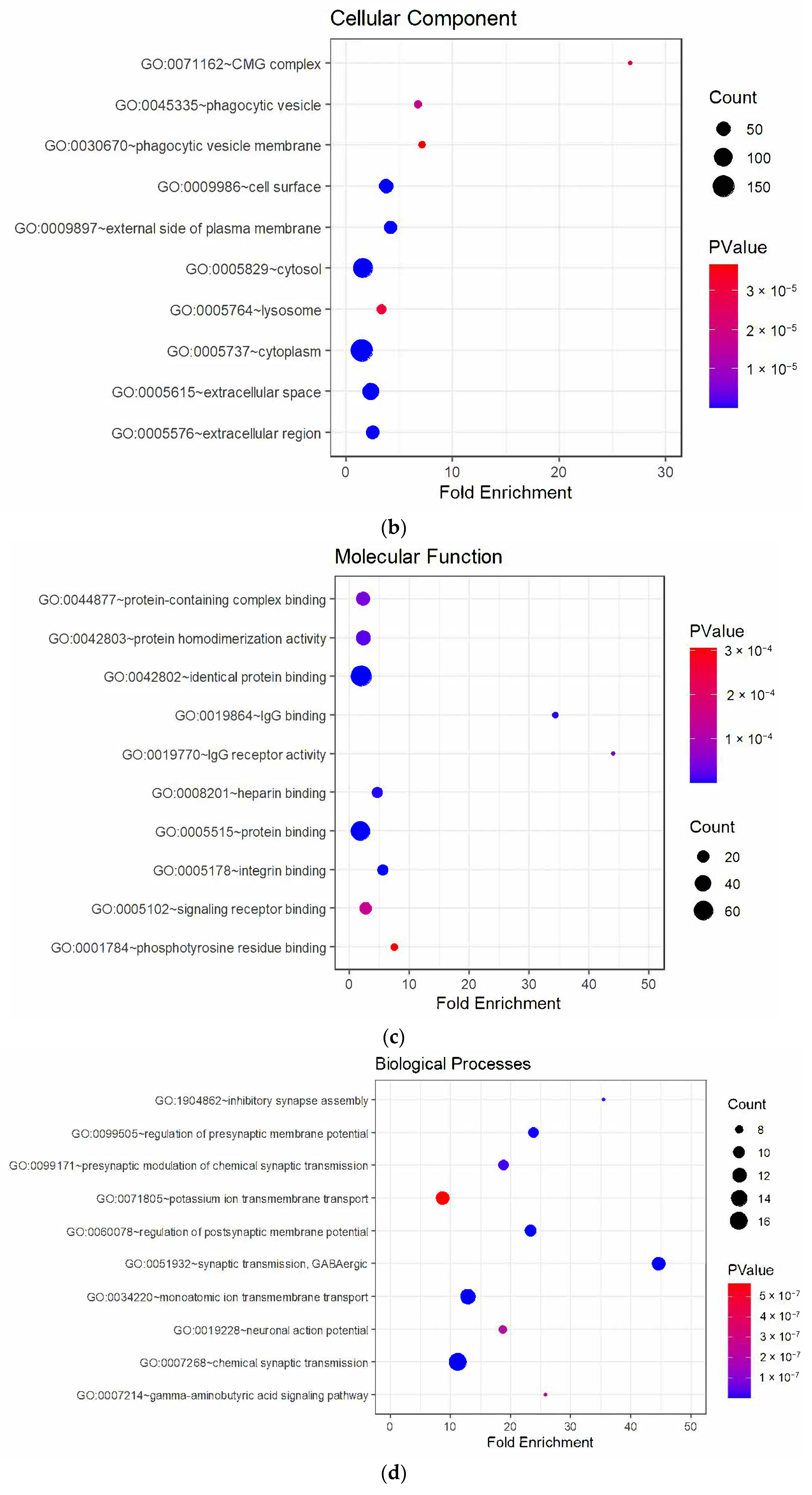
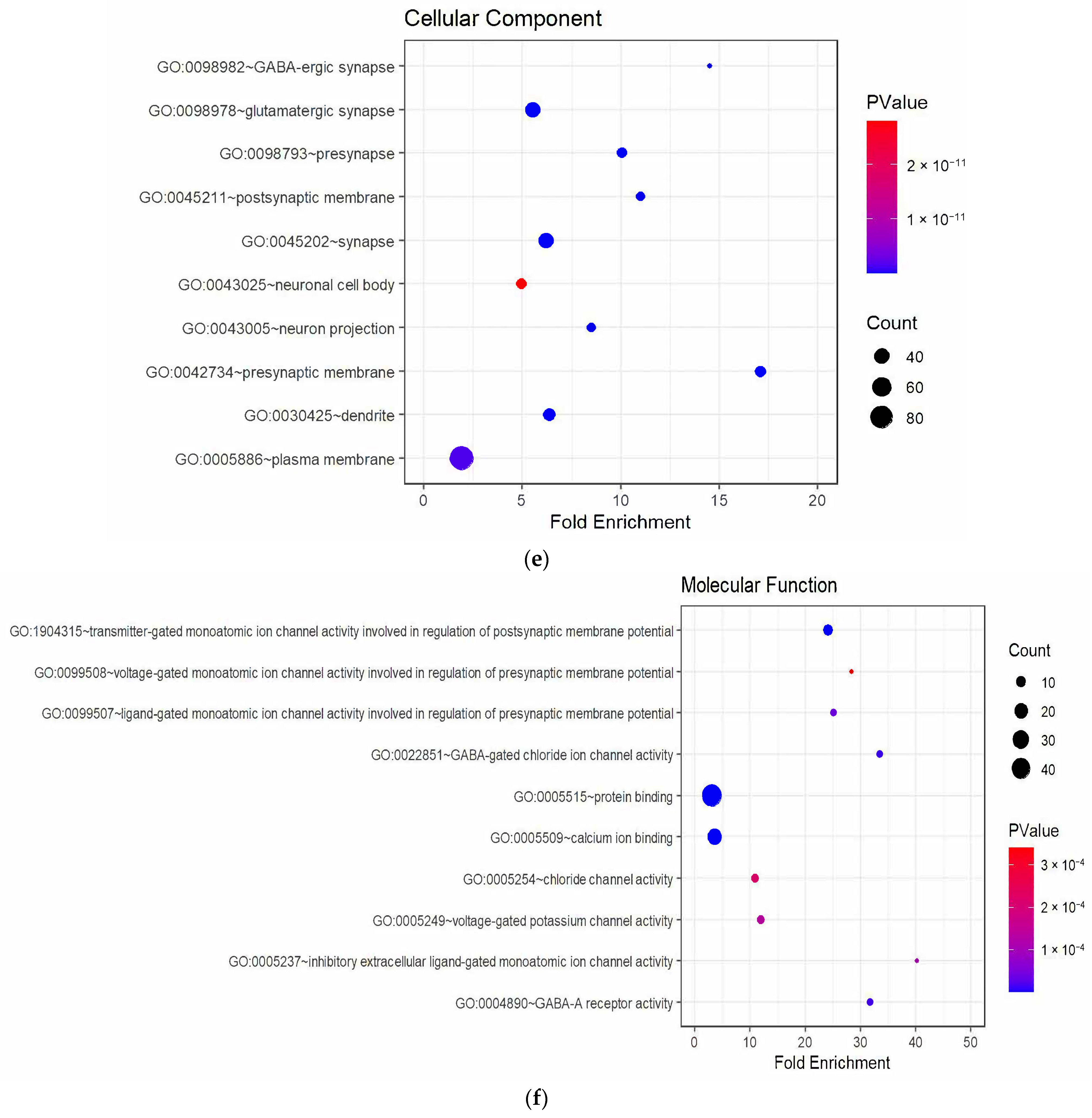
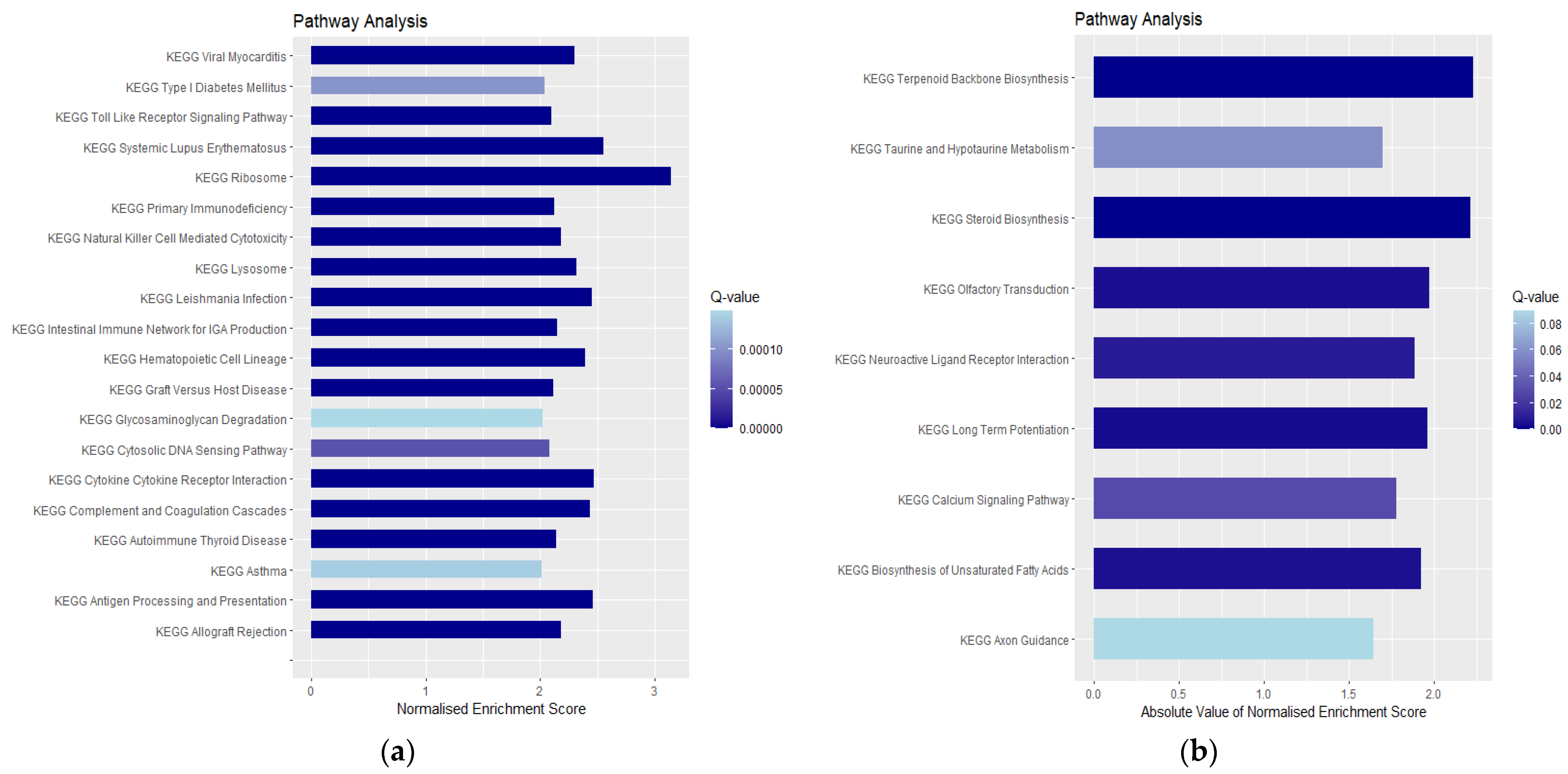
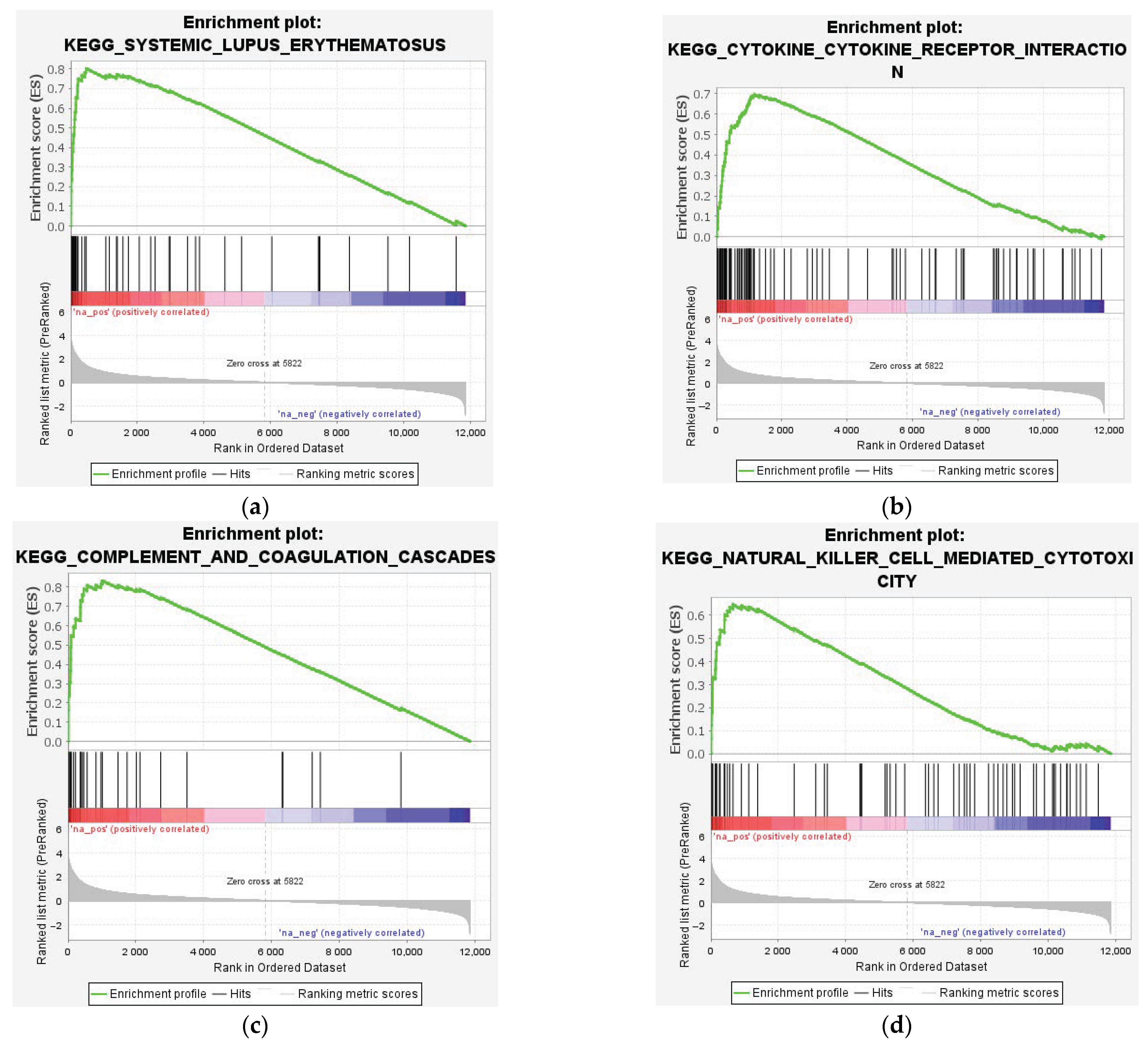
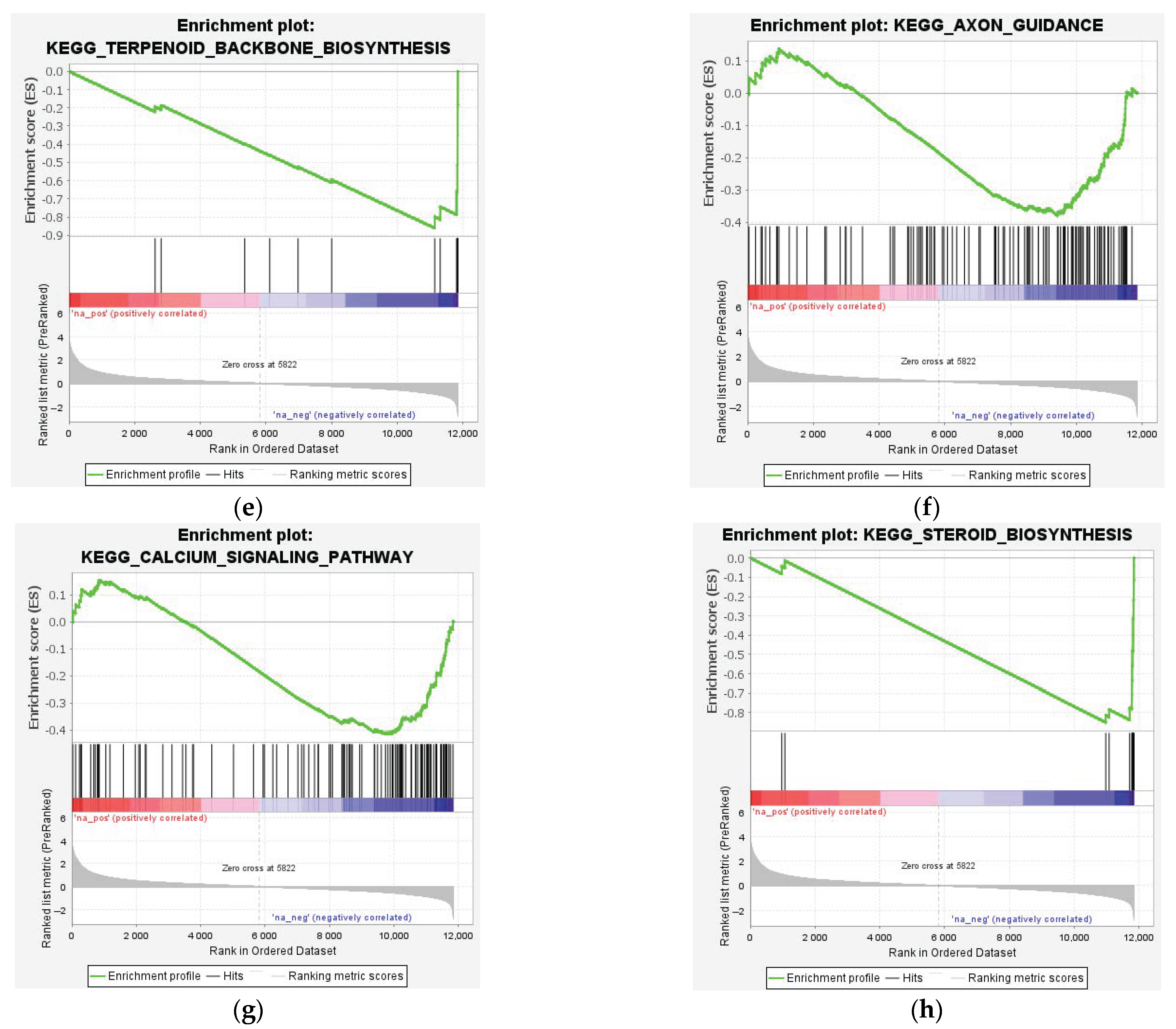
3.3. Enrichment Analysis of DEGs
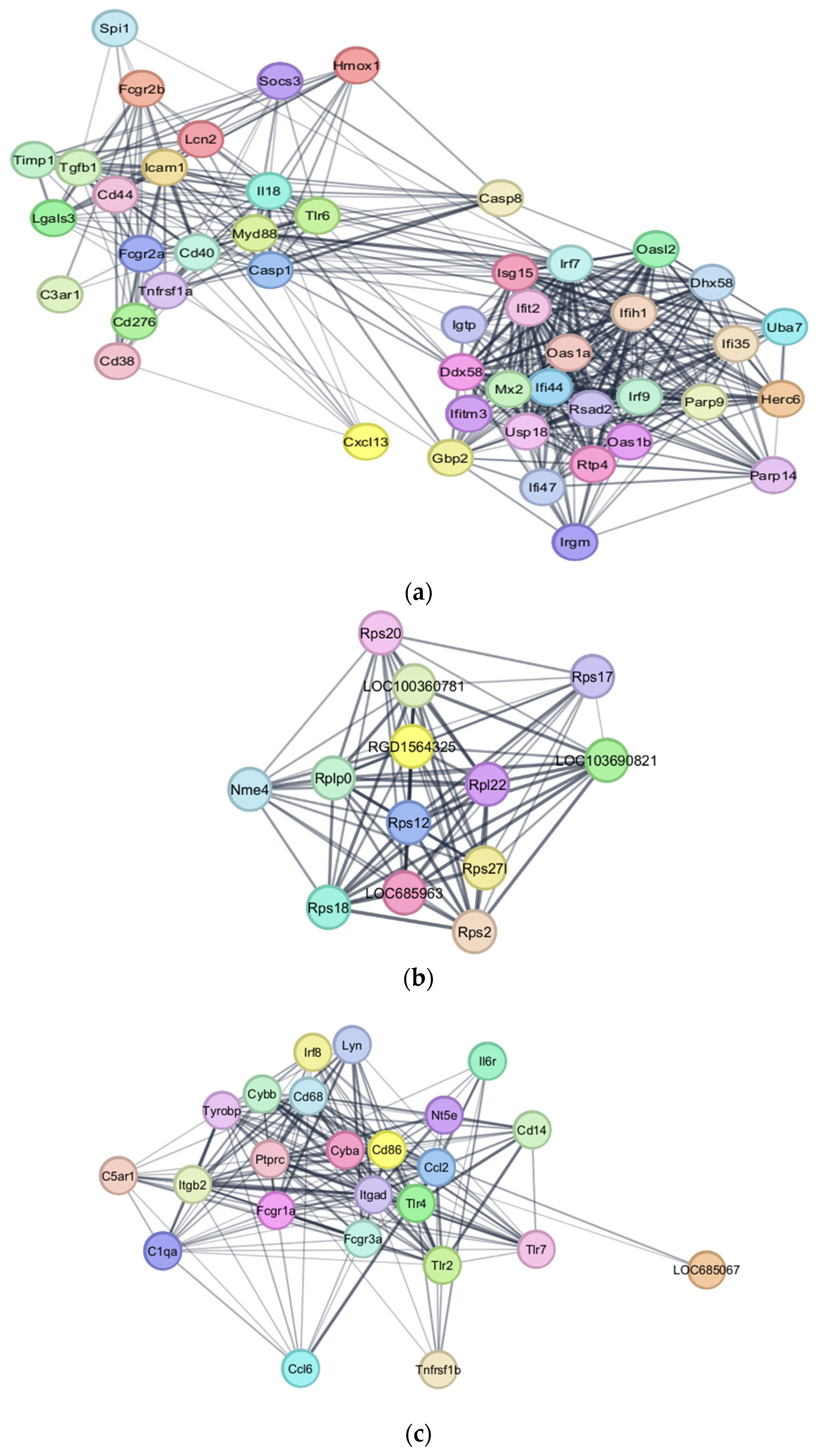
3.4. PPI Interaction Network Analysis
4. Discussion
4.1. Upregulated Genes and Neuroinflammation
4.1.1. Microglia Activity After SCI
4.1.2. Microglia and the Complement System
4.1.3. Tumor Necrosis Factor and Associated Pathways
4.2. Autoimmunity and Immune Dysregulation
Fc-γ Receptors and Autoantibody Production
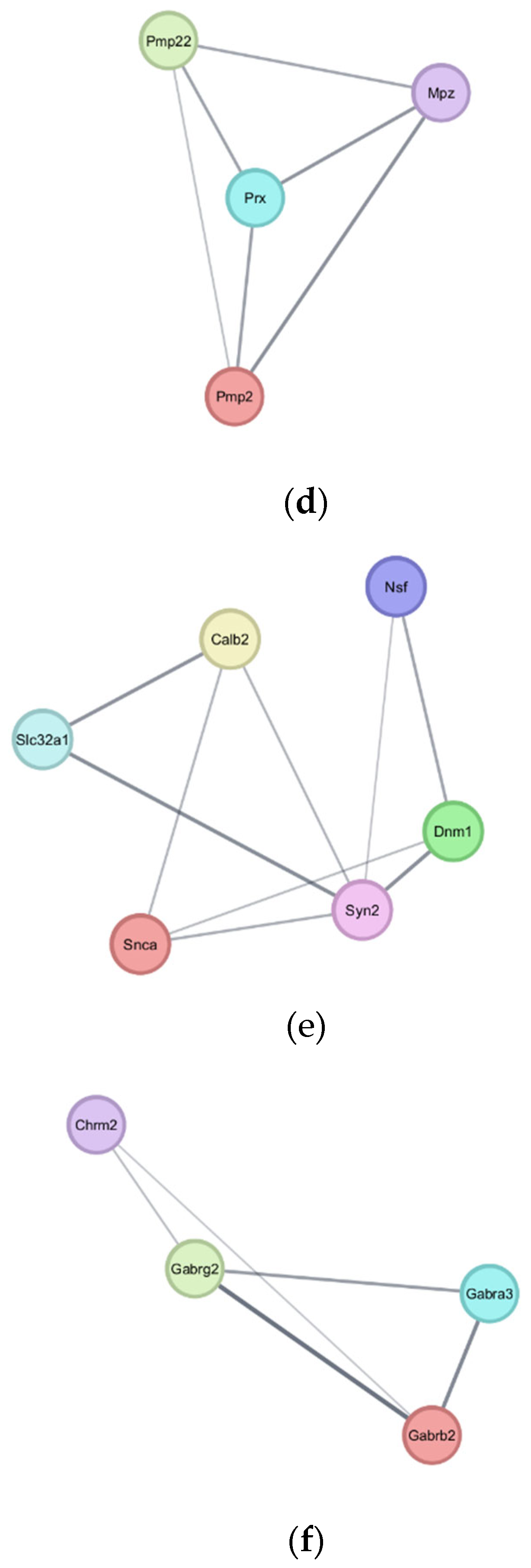
4.3. Downregulated Pathways and Regenerative Hypotheses
4.4. Key Downregulated Genes and Implications
4.4.1. Myelination and Lipid Metabolism
4.4.2. Synaptic Integrity and Neurotransmission
4.5. Treatment of Spinal Cord Injuries and Therapeutic Considerations
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hochman, S. Spinal cord. Curr. Biol. 2007, 17, R950–R955. [Google Scholar] [CrossRef] [PubMed]
- Nas, K.; Yazmalar, L.; Şah, V.; Aydın, A.; Öneş, K. Rehabilitation of spinal cord injuries. World J. Orthop. 2015, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M.; et al. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef]
- Katoh, H.; Yokota, K.; Fehlings, M.G. Regeneration of Spinal Cord Connectivity Through Stem Cell Transplantation and Biomaterial Scaffolds. Front. Cell. Neurosci. 2019, 13, 248. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment Imbalance of Spinal Cord Injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Bieler, L.; Vogl, M. Pathophysiology of traumatic spinal cord injury. Neurol. Asp. Spinal Cord Inj. 2017, 503–528. [Google Scholar]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal cord injury: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Zhou, L.; Shao, J.; Hu, X.; Xu, W.; Ren, Y.; Zhu, X.; Ge, W.; Zhang, K.; et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct. Target. Ther. 2022, 7, 65. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Poplawski, G.H.D.; Kawaguchi, R.; Van Niekerk, E.; Lu, P.; Mehta, N.; Canete, P.; Lie, R.; Dragatsis, I.; Meves, J.M.; Zheng, B.; et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature 2020, 581, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lichtman, J.W. Motor axon regeneration and muscle reinnervation in young adult and aged animals. J. Neurosci. 2013, 33, 19480–19491. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.N. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 2010, 223, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Chandran, V.; Coppola, G.; Nawabi, H.; Omura, T.; Versano, R.; Huebner, E.A.; Zhang, A.; Costigan, M.; Yekkirala, A.; Barrett, L.; et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 2016, 89, 956–970. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef]
- GrandPré, T.; Li, S.; Strittmatter, S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 2002, 417, 547–551. [Google Scholar] [CrossRef]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Kanter, J.L.; He, Z. PTEN/mTOR and axon regeneration. Exp. Neurol. 2010, 223, 45–50. [Google Scholar] [CrossRef]
- Young, W. Spinal Cord Regeneration. Cell Transplant. 2014, 23, 573–611. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Zheng, B.; Ho, C.; Li, S.; Keirstead, H.; Steward, O.; Tessier-Lavigne, M. Lack of Enhanced Spinal Regeneration in Nogo-Deficient Mice. Neuron 2003, 38, 213–224. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Wu, K.L.; Hsu, C.; Chan, J.Y. Impairment of the mitochondrial respiratory enzyme activity triggers sequential activation of apoptosis-inducing factor-dependent and caspase-dependent signaling pathways to induce apoptosis after spinal cord injury. J. Neurochem. 2007, 101, 1552–1566. [Google Scholar] [CrossRef]
- Kotipatruni, R.R.; Dasari, V.R.; Veeravalli, K.K.; Dinh, D.H.; Fassett, D.; Rao, J.S. p53- and Bax-Mediated Apoptosis in Injured Rat Spinal Cord. Neurochem. Res. 2011, 36, 2063–2074. [Google Scholar] [CrossRef]
- Park, K.W.; Lin, C.-Y.; Lee, Y.-S. Expression of Suppressor of Cytokine Signaling-3 (SOCS3) and its role in neuronal death after complete spinal cord injury. Exp. Neurol. 2014, 261, 65–75. [Google Scholar] [CrossRef]
- Schulze, A.; Downward, J. Navigating gene expression using microarrays—A technology review. Nat. Cell Biol. 2001, 3, E190–E195. [Google Scholar] [CrossRef]
- Singh, K.P.; Miaskowski, C.; Dhruva, A.A.; Flowers, E.; Kober, K.M. Mechanisms and Measurement of Changes in Gene Expression. Biol. Res. Nurs. 2018, 20, 369–382. [Google Scholar] [CrossRef]
- Rodriguez-Esteban, R.; Jiang, X. Differential gene expression in disease: A comparison between high-throughput studies and the literature. BMC Med. Genom. 2017, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.J.; Suter, R.K.; Ayad, N.G. An overview of human single-cell RNA sequencing studies in neurobiological disease. Neurobiol. Dis. 2023, 184, 106201. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. Immune response following traumatic spinal cord injury: Pathophysiology and therapies. Front. Immunol. 2022, 13, 1084101. [Google Scholar] [CrossRef]
- Holness, C.L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Liu, F.; Yang, K. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci. Rep. 2022, 12, 7844. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Feng, Z.-P. The good and bad of microglia/macrophages: New hope in stroke therapeutics. Acta Pharmacol. Sin. 2013, 34, 6–7. [Google Scholar] [CrossRef]
- Green, T.R.F.; Rowe, R.K. Quantifying microglial morphology: An insight into function. Clin. Exp. Immunol. 2024, 216, 221–229. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Ding, Y.; Wang, L.; Zhu, Y.J. Current Knowledge of Microglia in Traumatic Spinal Cord Injury. Front. Neurol. 2021, 12, 796704. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.M.; Wang, Y.J.; Zhou, Y.; Zhu, Z.J.; Chen, M.H.; Wang, Y.J.; Xu, H.; Wang, Y.H. Macrophage migration inhibitory factor facilitates astrocytic production of the CCL2 chemokine following spinal cord injury. Neural Regen. Res. 2023, 18, 1802–1808. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, M.; Bennett, S.; Wang, Z.; Pfleger, K.D.G.; Xu, J. The molecular structure and role of CCL2 (MCP-1) and C-C chemokine receptor CCR2 in skeletal biology and diseases. J. Cell. Physiol. 2021, 236, 7211–7222. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, S.; Park, J.-Y.; Lee, W.-H.; Mori, K.; Kim, S.-H.; Kim, I.K.; Suk, K. A Dual Role of Lipocalin 2 in the Apoptosis and Deramification of Activated Microglia1. J. Immunol. 2007, 179, 3231–3241. [Google Scholar] [CrossRef]
- Tong, Z.; Wu, X.; Ovcharenko, D.; Zhu, J.; Chen, C.S.; Kehrer, J.P. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem. J. 2005, 391 Pt 2, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zuo, X.; Liang, Z.; Ding, T.; Li, K.; Ma, Y.; Li, P.; Zhu, Z.; Ju, C.; et al. Photobiomodulation inhibits the activation of neurotoxic microglia and astrocytes by inhibiting Lcn2/JAK2-STAT3 crosstalk after spinal cord injury in male rats. J. Neuroinflamm. 2021, 18, 256. [Google Scholar] [CrossRef] [PubMed]
- Hopperton, K.E.; Mohammad, D.; Trépanier, M.O.; Giuliano, V.; Bazinet, R.P. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Mol. Psychiatry 2018, 23, 177–198. [Google Scholar] [CrossRef]
- Lynch, N.J.; Willis, C.L.; Nolan, C.C.; Roscher, S.; Fowler, M.J.; Weihe, E.; Ray, D.E.; Schwaeble, W.J. Microglial activation and increased synthesis of complement component C1q precedes blood–brain barrier dysfunction in rats. Mol. Immunol. 2004, 40, 709–716. [Google Scholar] [CrossRef]
- Hellenbrand, D.J.; Quinn, C.M.; Piper, Z.J.; Morehouse, C.N.; Fixel, J.A.; Hanna, A.S. Inflammation after spinal cord injury: A review of the critical timeline of signaling cues and cellular infiltration. J. Neuroinflamm. 2021, 18, 284. [Google Scholar] [CrossRef]
- Day, A.J.; Milner, C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019, 78–79, 60–83. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Lauer, M.E.; Soleman, S.; Zhao, C.; Hascall, V.C.; Day, A.J.; Fawcett, J.W. Tumor Necrosis Factor-stimulated Gene-6 (TSG-6) Is Constitutively Expressed in Adult Central Nervous System (CNS) and Associated with Astrocyte-mediated Glial Scar Formation following Spinal Cord Injury. J. Biol. Chem. 2016, 291, 19939–19952. [Google Scholar] [CrossRef]
- La Russa, D.; Di Santo, C.; Lizasoain, I.; Moraga, A.; Bagetta, G.; Amantea, D. Tumor Necrosis Factor (TNF)-α-Stimulated Gene 6 (TSG-6): A Promising Immunomodulatory Target in Acute Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1162. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Atretkhany, K.-S.N.; Gogoleva, V.S.; Drutskaya, M.S.; Nedospasov, S.A. Distinct modes of TNF signaling through its two receptors in health and disease. J. Leukoc. Biol. 2020, 107, 893–905. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O.; Siegemund, M.; Wajant, H.; Scheurich, P.; Pfizenmaier, K. A TNF receptor 2 selective agonist rescues human neurons from oxidative stress-induced cell death. PLoS ONE 2011, 6, e27621. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Martin, A.; Grassner, L.; Garcia-Ovejero, D.; Paniagua-Torija, B.; Barroso-Garcia, G.; Arandilla, A.G.; Mach, O.; Turrero, A.; Vargas, E.; Alcobendas, M.; et al. Elevated Autoantibodies in Subacute Human Spinal Cord Injury Are Naturally Occurring Antibodies. Front. Immunol. 2018, 9, 2365. [Google Scholar] [CrossRef] [PubMed]
- Allison, D.J.; Ditor, D.S. Immune dysfunction and chronic inflammation following spinal cord injury. Spinal Cord. 2015, 53, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Sanders, V.M.; Jones, T.B.; Malarkey, W.B.; Popovich, P.G. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 2007, 207, 75–84. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Popovich, P.G. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience 2009, 158, 1112–1121. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Lucin, K.M.; Sanders, V.M.; McGaughy, V.M.; Popovich, P.G. Spinal cord injury triggers systemic autoimmunity: Evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006, 99, 1073–1087. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.T.; Craft, J. The pathogenesis of systemic lupus erythematosus-an update. Curr. Opin. Immunol. 2012, 24, 651–657. [Google Scholar] [CrossRef]
- Li, X.; Ptacek, T.S.; Brown, E.E.; Edberg, J.C. Fcgamma receptors: Structure, function and role as genetic risk factors in SLE. Genes. Immun. 2009, 10, 380–389. [Google Scholar] [CrossRef]
- Smith, K.G.; Clatworthy, M.R. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef]
- Yan, L.; Fu, J.; Dong, X.; Chen, B.; Hong, H.; Cui, Z. Identification of hub genes in the subacute spinal cord injury in rats. BMC Neurosci. 2022, 23, 51. [Google Scholar] [CrossRef]
- Hoeffel, G.; Debroas, G.; Roger, A.; Rossignol, R.; Gouilly, J.; Laprie, C.; Chasson, L.; Barbon, P.V.; Balsamo, A.; Reynders, A.; et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 2021, 594, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hu, X.; Bennett, S.; Mai, Y.; Xu, J. Molecular Structure, Expression and Role of TAFA4 and its Receptor FPR1 in the Spinal Cord. Front. Cell Dev. Biol. 2022, 10, 911414. [Google Scholar] [CrossRef] [PubMed]
- Ziff, O.J.; Clarke, B.E.; Taha, D.M.; Crerar, H.; Luscombe, N.M.; Patani, R. Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res. 2022, 32, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Schwab, J.M. Determinants of Axon Growth, Plasticity, and Regeneration in the Context of Spinal Cord Injury. Am. J. Pathol. 2018, 188, 53–62. [Google Scholar] [CrossRef]
- Martin, J.H. Neuroplasticity of spinal cord injury and repair. Handb. Clin. Neurol. 2022, 184, 317–330. [Google Scholar] [CrossRef]
- Auld, D.S.; Robitaille, R. Perisynaptic Schwann cells at the neuromuscular junction: Nerve- and activity-dependent contributions to synaptic efficacy, plasticity, and reinnervation. Neuroscientist 2003, 9, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Kalafatakis, I.; Karagogeos, D. Oligodendrocytes and Microglia: Key Players in Myelin Development, Damage and Repair. Biomolecules 2021, 11, 1058. [Google Scholar] [CrossRef]
- Guest, J.D.; Rao, A.; Olson, L.; Bunge, M.B.; Bunge, R.P. The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp. Neurol. 1997, 148, 502–522. [Google Scholar] [CrossRef]
- Jensen, S.K.; Michaels, N.J.; Ilyntskyy, S.; Keough, M.B.; Kovalchuk, O.; Yong, V.W. Multimodal Enhancement of Remyelination by Exercise with a Pivotal Role for Oligodendroglial PGC1α. Cell Rep. 2018, 24, 3167–3179. [Google Scholar] [CrossRef]
- Badner, A.; Siddiqui, A.M.; Fehlings, M.G. Spinal cord injuries: How could cell therapy help? Expert. Opin. Biol. Ther. 2017, 17, 529–541. [Google Scholar] [CrossRef]
- Sharma, R.; Grover, A. Myocilin-associated Glaucoma: A Historical Perspective and Recent Research Progress. Mol. Vis. 2021, 27, 480–493. [Google Scholar] [PubMed]
- Hasel, P.; Cooper, M.L.; Marchildon, A.E.; Rufen-Blanchette, U.A.; Kim, R.D.; Ma, T.C.; Kang, U.J.; Chao, M.V.; Liddelow, S.A. Defining the molecular identity and morphology of glia limitans superficialis astrocytes in mouse and human. bioRxiv 2023. [Google Scholar] [CrossRef]
- Torii, T.; Miyamoto, Y.; Yamauchi, J. Cellular Signal-Regulated Schwann Cell Myelination and Remyelination. Adv. Exp. Med. Biol. 2019, 1190, 3–22. [Google Scholar] [CrossRef]
- Louit, A.; Beaudet, M.J.; Pépin, R.; Berthod, F. Differentiation of Human Induced Pluripotent Stem Cells into Mature and Myelinating Schwann Cells. Tissue Eng. Part. C Methods 2023, 29, 134–143. [Google Scholar] [CrossRef]
- Chelyshev, Y.A.; Muhamedshina, Y.O.; Povysheva, T.V.; Shaymardanova, G.F.; Rizvanov, A.A.; Nigmetzyanova, M.V.; Tiapkina, O.V.; Bondarenko, N.I.; Nikolskiy, E.E.; Islamov, R.R. Characterization of spinal cord glial cells in a model of hindlimb unloading in mice. Neuroscience 2014, 280, 328–339. [Google Scholar] [CrossRef]
- Wang, I.H.; Huang, T.T.; Chen, J.L.; Chu, L.W.; Ping, Y.H.; Hsu, K.W.; Huang, K.H.; Fang, W.L.; Lee, H.C.; Chen, C.F.; et al. Mevalonate Pathway Enzyme HMGCS1 Contributes to Gastric Cancer Progression. Cancers 2020, 12, 1088. [Google Scholar] [CrossRef]
- Saher, G.; Brügger, B.; Lappe-Siefke, C.; Möbius, W.; Tozawa, R.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef]
- Chen, G.; Fang, X.; Yu, M. Regulation of gene expression in rats with spinal cord injury based on microarray data. Mol. Med. Rep. 2015, 12, 2465–2472. [Google Scholar] [CrossRef]
- Breuer, S.; Pech, K.; Buss, A.; Spitzer, C.; Ozols, J.; Hol, E.M.; Heussen, N.; Noth, J.; Schwaiger, F.W.; Schmitt, A.B. Regulation of stearoyl-CoA desaturase-1 after central and peripheral nerve lesions. BMC Neurosci. 2004, 5, 15. [Google Scholar] [CrossRef][Green Version]
- Lu, X.; Jia, D.; Zhao, C.; Wang, W.; Liu, T.; Chen, S.; Quan, X.; Sun, D.; Gao, B. Recombinant adenovirus-mediated overexpression of 3β-hydroxysteroid-Δ24 reductase. Neural Regen. Res. 2014, 9, 504–512. [Google Scholar] [CrossRef]
- Hasegawa, S.; Kume, H.; Iinuma, S.; Yamasaki, M.; Takahashi, N.; Fukui, T. Acetoacetyl-CoA synthetase is essential for normal neuronal development. Biochem. Biophys. Res. Commun. 2012, 427, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Cooksey, E.; Duncan, I.D. Myelin Loss Does Not Lead to Axonal Degeneration in a Long-Lived Model of Chronic Demyelination. J. Neurosci. 2013, 33, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- McPhail, L.T.; Stirling, D.P.; Tetzlaff, W.; Kwiecien, J.M.; Ramer, M.S. The contribution of activated phagocytes and myelin degeneration to axonal retraction/dieback following spinal cord injury. Eur. J. Neurosci. 2004, 20, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Berton, F.; Iborra, C.; Boudier, J.A.; Seagar, M.J.; Marquèze, B. Developmental regulation of synaptotagmin I, II, III, and IV mRNAs in the rat CNS. J. Neurosci. 1997, 17, 1206–1216. [Google Scholar] [CrossRef]
- Williams, A.C.; Brophy, P.J. The function of the Periaxin gene during nerve repair in a model of CMT4F. J. Anat. 2002, 200, 323–330. [Google Scholar] [CrossRef]
- Jeong, J.; McMahon, A.P. Growth and pattern of the mammalian neural tube are governed by partially overlapping feedback activities of the hedgehog antagonists patched 1 and Hhip1. Development 2005, 132, 143–154. [Google Scholar] [CrossRef]
- Tang, F.; Yang, C.; Li, F.P.; Yu, D.H.; Pan, Z.Y.; Wang, Z.F.; Li, Z.Q. Palmitoyl transferases act as potential regulators of tumor-infiltrating immune cells and glioma progression. Mol. Ther. Nucleic Acids 2022, 28, 716–731. [Google Scholar] [CrossRef]
- Tian, L.; McClafferty, H.; Knaus, H.G.; Ruth, P.; Shipston, M.J. Distinct acyl protein transferases and thioesterases control surface expression of calcium-activated potassium channels. J. Biol. Chem. 2012, 287, 14718–14725. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84s–94s. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, A.; Dias Abeyagunawardene, S.; Zheng, X.; Jin, H.; Wang, Q.; Xu, J. Identification of Differentially Expressed Genes in Spinal Cord Injury. Genes 2025, 16, 514. https://doi.org/10.3390/genes16050514
Chang A, Dias Abeyagunawardene S, Zheng X, Jin H, Wang Q, Xu J. Identification of Differentially Expressed Genes in Spinal Cord Injury. Genes. 2025; 16(5):514. https://doi.org/10.3390/genes16050514
Chicago/Turabian StyleChang, Andrew, Shevanka Dias Abeyagunawardene, Xiaohang Zheng, Haiming Jin, Qingqing Wang, and Jiake Xu. 2025. "Identification of Differentially Expressed Genes in Spinal Cord Injury" Genes 16, no. 5: 514. https://doi.org/10.3390/genes16050514
APA StyleChang, A., Dias Abeyagunawardene, S., Zheng, X., Jin, H., Wang, Q., & Xu, J. (2025). Identification of Differentially Expressed Genes in Spinal Cord Injury. Genes, 16(5), 514. https://doi.org/10.3390/genes16050514








