The microRNA Pathway of Macroalgae: Its Similarities and Differences to the Plant and Animal microRNA Pathways
Abstract
1. The microRNA Pathway and Its Evolution in Eukaryotes
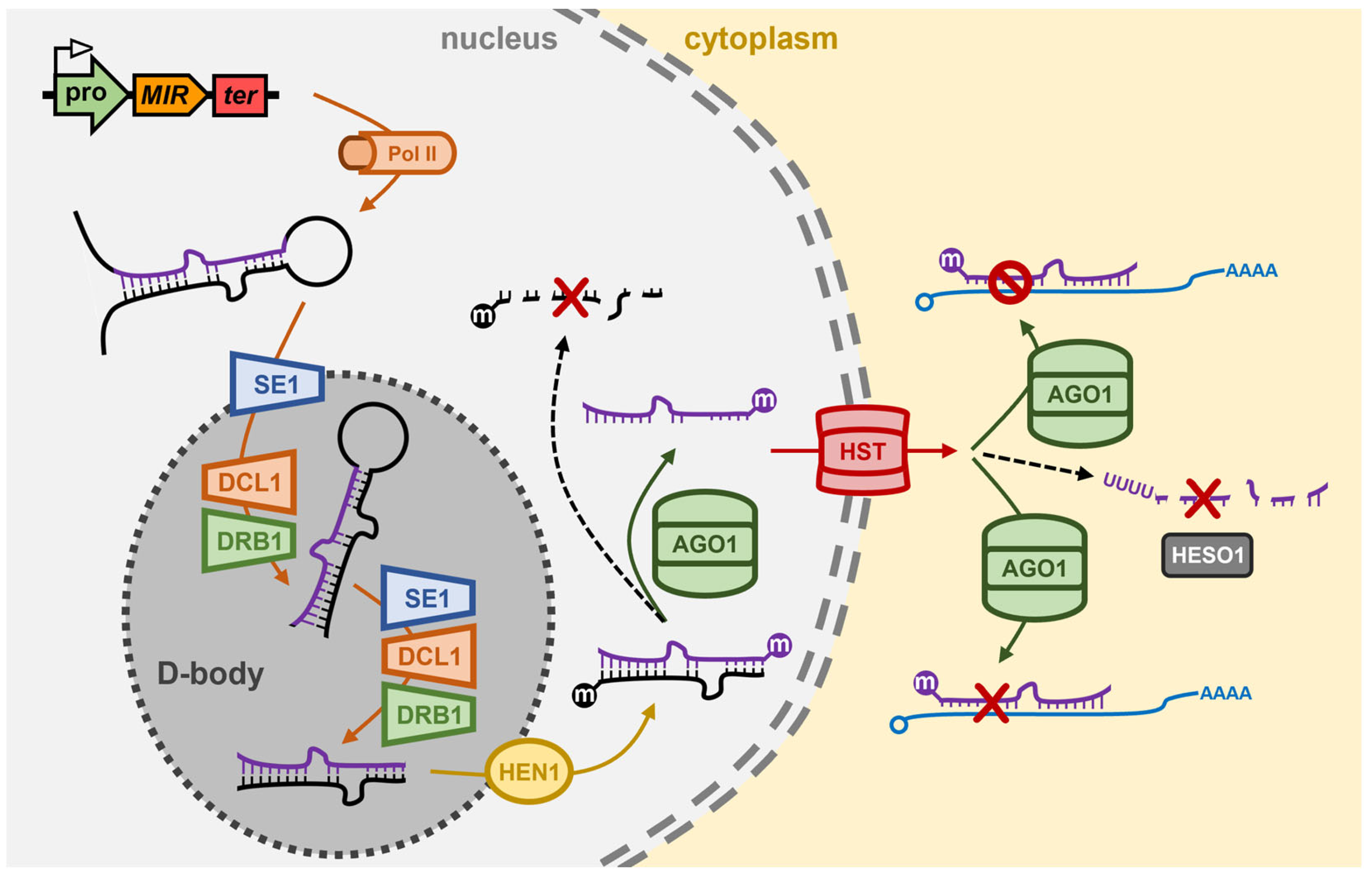
2. The miRNA Pathway of Arabidopsis thaliana
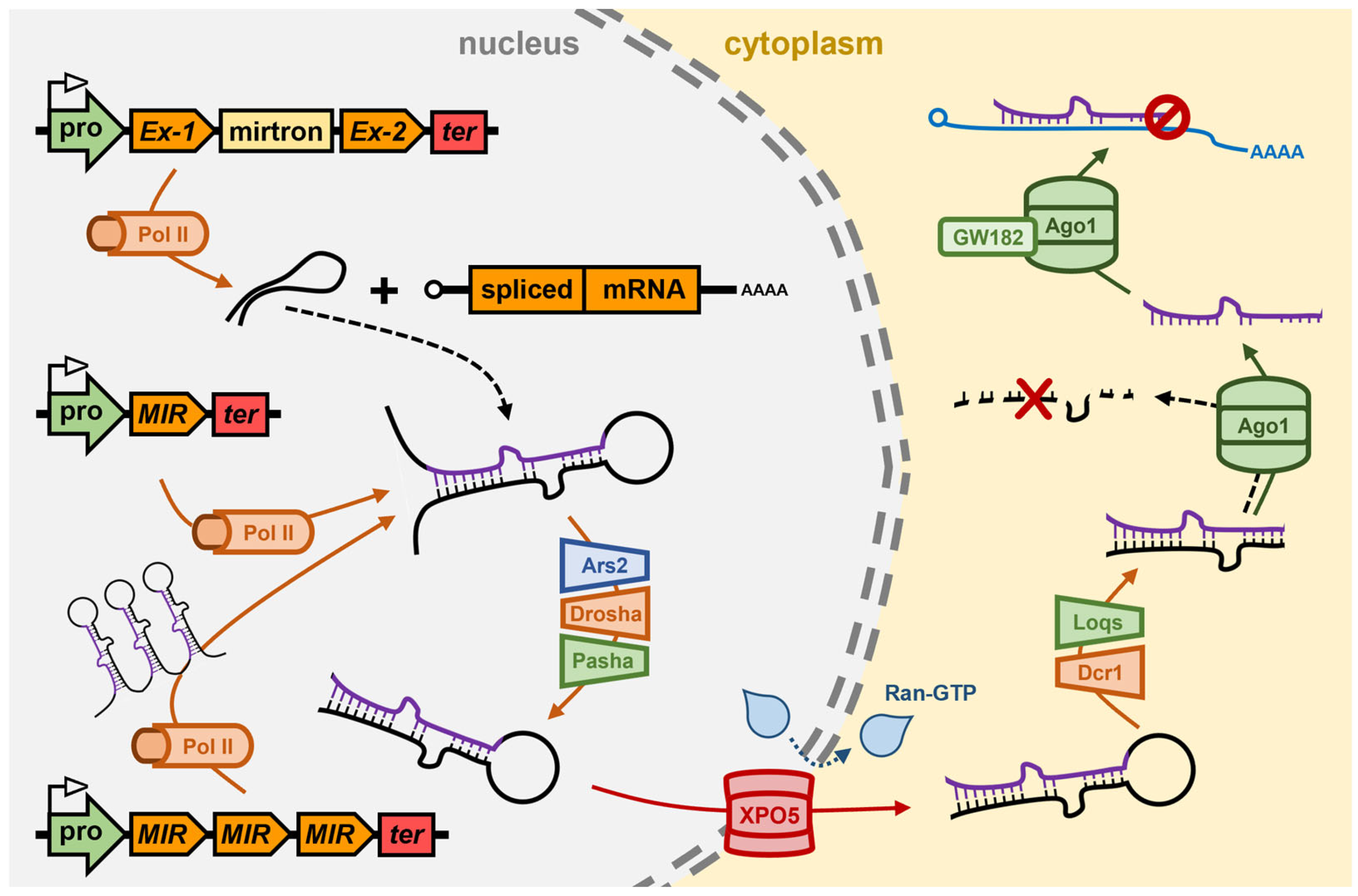
3. The miRNA Pathway of Drosophila melanogaster
4. The miRNA Pathway of Chlamydomonas reinhardtii
5. The microRNA Pathway of Green, Brown and Red Seaweed
5.1. microRNAs in the Chlorophyta
5.2. microRNAs in the Phaeophyceae
5.3. microRNAs in the Rhodophyta
5.3.1. microRNAs in Red Microalgae
5.3.2. microRNAs in Red Macroalgae
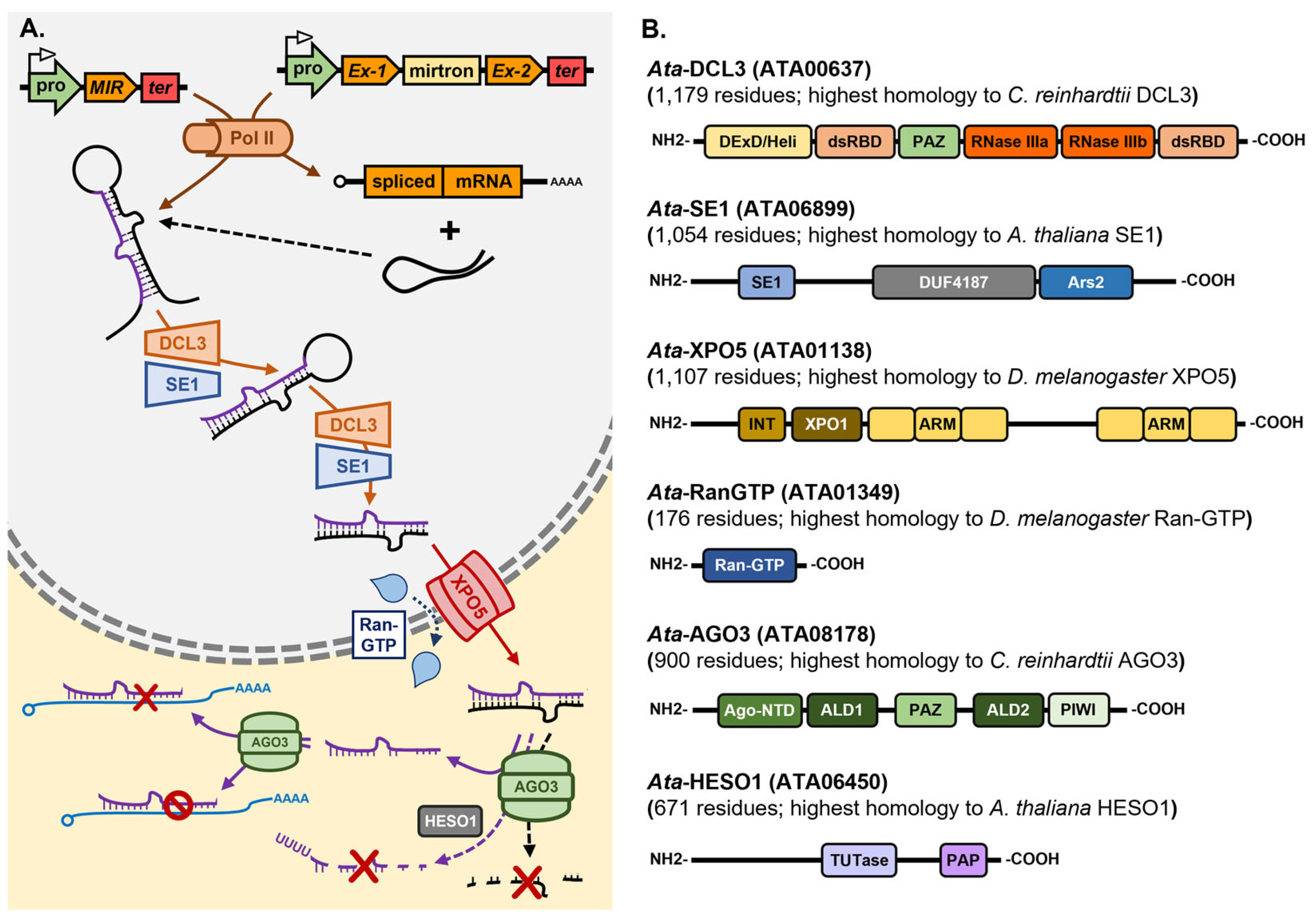
5.3.3. The miRNA Pathway of the Red Seaweed Asparagopsis taxiformis
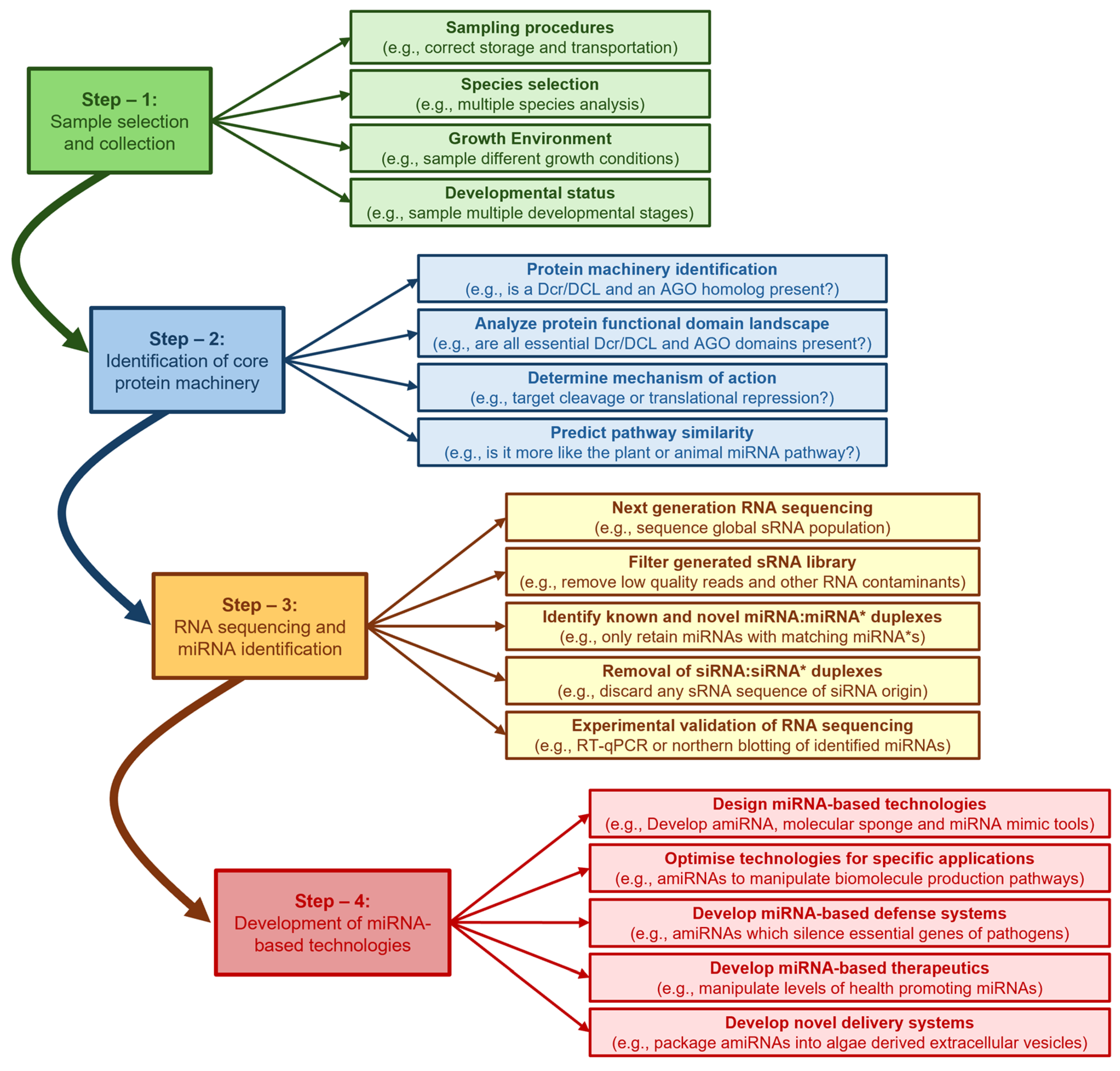
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Chiou, T.J.; Lin, S.I.; Aung, K.; Zhu, J.K. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 2005, 15, 2038–2043. [Google Scholar] [CrossRef]
- Boutet, S.; Vazquez, F.; Liu, J.; Béclin, C.; Fagard, M.; Gratias, A.; Morel, J.B.; Crété, P.; Chen, X.; Vaucheret, H. Arabidopsis HEN1: A genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. 2003, 13, 843–848. [Google Scholar] [CrossRef]
- Agorio, A.; Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 2007, 19, 3778–3790. [Google Scholar] [CrossRef]
- Eamens, A.; Vaistij, F.E.; Jones, L. NRPD1a and NRPD1b are required to maintain post-transcriptional RNA silencing and RNA-directed DNA methylation in Arabidopsis. Plant J. 2008, 55, 596–606. [Google Scholar] [CrossRef]
- Watahiki, A.; Wang, Y.; Morris, J.; Dennis, K.; O’Dwyer, H.M.; Gleave, M.; Gout, P.W.; Wang, Y. MicroRNAs associated with metastatic prostate cancer. PLoS ONE 2011, 6, e24950. [Google Scholar] [CrossRef]
- Pizzi, G.; Groppetti, D.; Brambilla, E.; Pecile, A.; Grieco, V.; Lecchi, C. MicroRNA as epigenetic regulators of canine cryptorchidism. Res. Vet. Sci. 2023, 162, 104961. [Google Scholar] [CrossRef]
- Clark, S.D.; Song, W.; Cianciolo, R.; Lees, G.; Nabity, M.; Liu, S. Abnormal Expression of miR-21 in Kidney Tissue of Dogs With X-Linked Hereditary Nephropathy: A Canine Model of Chronic Kidney Disease. Vet. Pathol. 2019, 56, 93–105. [Google Scholar] [CrossRef]
- Stefanon, B.; Cintio, M.; Sgorlon, S.; Scarsella, E.; Licastro, D.; Zecconi, A.; Colitti, M. Regulatory Role of microRNA of Milk Exosomes in Mastitis of Dairy Cows. Animals 2023, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Llave, C.; Kasschau, K.D.; Rector, M.A.; Carrington, J.C. Endogenous and silencing-associated small RNAs in plants. Plant Cell 2002, 14, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Krogh, A. Computational evidence for hundreds of non-conserved plant microRNAs. BMC Genom. 2005, 6, 119. [Google Scholar] [CrossRef]
- Talmor-Neiman, M.; Stav, R.; Klipcan, L.; Buxdorf, K.; Baulcombe, D.C.; Arazi, T. Identification of trans-acting siRNAs in moss and an RNA-dependent RNA polymerase required for their biogenesis. Plant J. 2006, 48, 511–521. [Google Scholar] [CrossRef]
- Jones, L. Revealing micro-RNAs in plants. Trends Plant Sci. 2002, 7, 473–475. [Google Scholar] [CrossRef]
- Gasciolli, V.; Mallory, A.C.; Bartel, D.P.; Vaucheret, H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 2005, 15, 1494–1500. [Google Scholar] [CrossRef]
- Xie, Z.; Johansen, L.K.; Gustafson, A.M.; Kasschau, K.D.; Lellis, A.D.; Zilberman, D.; Jacobsen, S.E.; Carrington, J.C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004, 2, E104. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef]
- Baumberger, N.; Baulcombe, D.C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 11928–11933. [Google Scholar] [CrossRef]
- Ronemus, M.; Vaughn, M.W.; Martienssen, R.A. MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by argonaute, dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell 2006, 18, 1559–1574. [Google Scholar] [CrossRef]
- Banerjee, D.; Slack, F. Control of developmental timing by small temporal RNAs: A paradigm for RNA-mediated regulation of gene expression. Bioessays 2002, 24, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hutvágner, G.; Zamore, P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wang, L.J.; Mao, Y.B.; Cai, W.J.; Xue, H.W.; Chen, X.Y. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef]
- Nilsen, T.W. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007, 23, 243–249. [Google Scholar] [CrossRef]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Zhao, T.; Li, G.; Mi, S.; Li, S.; Hannon, G.J.; Wang, X.J.; Qi, Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007, 21, 1190–1203. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Chiang, H.R.; Schoenfeld, L.W.; Ruby, J.G.; Auyeung, V.C.; Spies, N.; Baek, D.; Johnston, W.K.; Russ, C.; Luo, S.; Babiarz, J.E.; et al. Mammalian microRNAs: Experimental evaluation of novel and previously annotated genes. Genes Dev. 2010, 24, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.K.; Griffiths-Jones, S.; Enright, A.J. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA 2007, 104, 17719–17724. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Schwach, F.; Studholme, D.J.; Thuenemann, E.C.; Baulcombe, D.C. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 2007, 447, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Alaba, S.; Piszczalka, P.; Pietrykowska, H.; Pacak, A.M.; Sierocka, I.; Nuc, P.W.; Singh, K.; Plewka, P.; Sulkowska, A.; Jarmolowski, A.; et al. The liverwort Pellia endiviifolia shares microtranscriptomic traits that are common to green algae and land plants. New Phytol. 2015, 206, 352–367. [Google Scholar] [CrossRef]
- Tarver, J.E.; Donoghue, P.C.; Peterson, K.J. Do miRNAs have a deep evolutionary history? Bioessays 2012, 34, 857–866. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef]
- Jiang, D.; Yin, C.; Yu, A.; Zhou, X.; Liang, W.; Yuan, Z.; Xu, Y.; Yu, Q.; Wen, T.; Zhang, D. Duplication and expression analysis of multicopy miRNA gene family members in Arabidopsis and rice. Cell Res. 2006, 16, 507–518. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Yu, D. Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 2012, 7, e48951. [Google Scholar] [CrossRef]
- Ben Amor, B.; Wirth, S.; Merchan, F.; Laporte, P.; d’Aubenton-Carafa, Y.; Hirsch, J.; Maizel, A.; Mallory, A.; Lucas, A.; Deragon, J.M.; et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009, 19, 57–69. [Google Scholar] [CrossRef]
- Jian, X.; Zhang, L.; Li, G.; Zhang, L.; Wang, X.; Cao, X.; Fang, X.; Chen, F. Identification of novel stress-regulated microRNAs from Oryza sativa L. Genomics 2010, 95, 47–55. [Google Scholar] [CrossRef]
- Fahlgren, N.; Jogdeo, S.; Kasschau, K.D.; Sullivan, C.M.; Chapman, E.J.; Laubinger, S.; Smith, L.M.; Dasenko, M.; Givan, S.A.; Weigel, D.; et al. MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana. Plant Cell 2010, 22, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Fromm, B.; Billipp, T.; Peck, L.E.; Johansen, M.; Tarver, J.E.; King, B.L.; Newcomb, J.M.; Sempere, L.F.; Flatmark, K.; Hovig, E.; et al. A Uniform System for the Annotation of Vertebrate microRNA Genes and the Evolution of the Human microRNAome. Annu. Rev. Genet. 2015, 49, 213–242. [Google Scholar] [CrossRef] [PubMed]
- Tarver, J.E.; Cormier, A.; Pinzón, N.; Taylor, R.S.; Carré, W.; Strittmatter, M.; Seitz, H.; Coelho, S.M.; Cock, J.M. microRNAs and the evolution of complex multicellularity: Identification of a large, diverse complement of microRNAs in the brown alga Ectocarpus. Nucleic Acids Res. 2015, 43, 6384–6398. [Google Scholar] [CrossRef]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Casas-Mollano, J.A. On the origin and functions of RNA-mediated silencing: From protists to man. Curr. Genet. 2006, 50, 81–99. [Google Scholar] [CrossRef]
- Millar, A.A.; Waterhouse, P.M. Plant and animal microRNAs: Similarities and differences. Funct. Integr. Genomics 2005, 5, 129–135. [Google Scholar] [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Qi, Y. MicroRNAs in a multicellular green alga Volvox carteri. Sci. China Life Sci. 2014, 57, 36–45. [Google Scholar] [CrossRef]
- Valli, A.A.; Santos, B.A.; Hnatova, S.; Bassett, A.R.; Molnar, A.; Chung, B.Y.; Baulcombe, D.C. Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs. Genome Res. 2016, 26, 519–529. [Google Scholar] [CrossRef]
- Voshall, A.; Kim, E.J.; Ma, X.; Moriyama, E.N.; Cerutti, H. Identification of AGO3-associated miRNAs and computational prediction of their targets in the green alga Chlamydomonas reinhardtii. Genetics 2015, 200, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef]
- Meyers, B.C.; Green, P.J.; Lu, C. miRNAs in the plant genome: All things great and small. Genome Dyn. 2008, 4, 108–118. [Google Scholar]
- Xie, Z.; Khanna, K.; Ruan, S. Expression of microRNAs and its regulation in plants. Semin. Cell Dev. Biol. 2010, 21, 790–797. [Google Scholar] [CrossRef]
- Kim, S.; Yang, J.Y.; Xu, J.; Jang, I.C.; Prigge, M.J.; Chua, N.H. Two cap-binding proteins CBP20 and CBP80 are involved in processing primary MicroRNAs. Plant Cell Physiol. 2008, 49, 1634–1644. [Google Scholar] [CrossRef]
- Fang, X.; Cui, Y.; Li, Y.; Qi, Y. Transcription and processing of primary microRNAs are coupled by Elongator complex in Arabidopsis. Nat Plants. 2015, 1, 15075. [Google Scholar] [CrossRef]
- Bielewicz, D.; Kalak, M.; Kalyna, M.; Windels, D.; Barta, A.; Vazquez, F.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Introns of plant pri-miRNAs enhance miRNA biogenesis. EMBO Rep. 2013, 14, 622–628. [Google Scholar] [CrossRef]
- Knop, K.; Stepien, A.; Barciszewska-Pacak, M.; Taube, M.; Bielewicz, D.; Michalak, M.; Borst, J.W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Active 5′ splice sites regulate the biogenesis efficiency of Arabidopsis microRNAs derived from intron-containing genes. Nucleic Acids Res. 2016, 45, 2757–2775. [Google Scholar] [CrossRef]
- Lobbes, D.; Rallapalli, G.; Schmidt, D.D.; Martin, C.; Clarke, J. SERRATE: A new player on the plant microRNA scene. EMBO Rep. 2006, 7, 1052–1058. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Lu, F.; Dong, A.; Huang, H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2006, 47, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Spector, D.L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 2007, 17, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Utsumi, M.; Ohba, Y.; Watanabe, Y. Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis. Plant Cell Physiol. 2007, 48, 1243–1453. [Google Scholar] [CrossRef]
- Vazquez, F.; Gasciolli, V.; Crété, P.; Vaucheret, H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 2004, 14, 346–351. [Google Scholar] [CrossRef]
- Kurihara, Y.; Takashi, Y.; Watanabe, Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA 2006, 12, 206–212. [Google Scholar] [CrossRef]
- Song, L.; Axtell, M.J.; Fedoroff, N.V. RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr. Biol. 2010, 20, 37–41. [Google Scholar] [CrossRef]
- Werner, S.; Wollmann, H.; Schneeberger, K.; Weigel, D. Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr. Biol. 2010, 20, 42–48. [Google Scholar] [CrossRef]
- Dong, Z.; Han, M.H.; Fedoroff, N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl. Acad. Sci. USA 2008, 105, 9970–9975. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Cheng, Y.; Jia, D. HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 2012, 129, 1085–1094. [Google Scholar] [CrossRef]
- Baranauskė, S.; Mickutė, M.; Plotnikova, A.; Finke, A.; Venclovas, Č.; Klimašauskas, S.; Vilkaitis, G. Functional mapping of the plant small RNA methyltransferase: HEN1 physically interacts with HYL1 and DICER-LIKE1 proteins. Nucleic Acids Res. 2015, 43, 2802–2812. [Google Scholar] [CrossRef]
- Eamens, A.L.; Smith, N.A.; Curtin, S.J.; Wang, M.B.; Waterhouse, P.M. The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA 2009, 15, 2219–2235. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Brioudes, F.; Jay, F.; Sarazin, A.; Grentzinger, T.; Devers, E.A.; Voinnet, O. HASTY, the Arabidopsis EXPORTIN-5 ortholog, regulates cell-to-cell and vascular microRNA movement. EMBO J. 2021, 40, e107455. [Google Scholar] [CrossRef] [PubMed]
- Lanet, E.; Delannoy, E.; Sormani, R.; Floris, M.; Brodersen, P.; Crété, P.; Voinnet, O.; Robaglia, C. Biochemical evidence for translational repression by Arabidopsis microRNAs. Plant Cell 2009, 21, 1762–1768. [Google Scholar] [CrossRef]
- Reis, R.S.; Hart-Smith, G.; Eamens, A.L.; Wilkins, M.R.; Waterhouse, P.M. Gene regulation by translational inhibition is determined by Dicer partnering proteins. Nat. Plants. 2015, 1, 1402. [Google Scholar] [CrossRef]
- Biemar, F.; Zinzen, R.; Ronshaugen, M.; Sementchenko, V.; Manak, J.R.; Levine, M.S. Spatial regulation of microRNA gene expression in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 2005, 102, 15907–15911. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, Z.; Liang, J.; Ge, Q.; Duan, X.; Ma, F.; Li, F. The full-length transcripts and promoter analysis of intergenic microRNAs in Drosophila melanogaster. Genomics 2011, 97, 294–303. [Google Scholar] [CrossRef][Green Version]
- Church, V.A.; Pressman, S.; Isaji, M.; Truscott, M.; Cizmecioglu, N.T.; Buratowski, S.; Frolov, M.V.; Carthew, R.W. Microprocessor Recruitment to Elongating RNA Polymerase II Is Required for Differential Expression of MicroRNAs. Cell Rep. 2017, 20, 3123–3134. [Google Scholar] [CrossRef][Green Version]
- Ryazansky, S.S.; Gvozdev, V.A.; Berezikov, E. Evidence for post-transcriptional regulation of clustered microRNAs in Drosophila. BMC Genomics 2011, 12, 371. [Google Scholar] [CrossRef]
- Vodala, S.; Pescatore, S.; Rodriguez, J.; Buescher, M.; Chen, Y.W.; Weng, R.; Cohen, S.M.; Rosbash, M. The oscillating miRNA 959-964 cluster impacts Drosophila feeding time and other circadian outputs. Cell Metab. 2012, 16, 601–612. [Google Scholar] [CrossRef]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Agius, P.; Westholm, J.O.; Chen, M.; Okamura, K.; Robine, N.; Leslie, C.S.; Lai, E.C. Computational and experimental identification of mirtrons in Drosophila melanogaster and Caenorhabditis elegans. Genome Res. 2011, 21, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Natalin, P.; Stark, A.; Brennecke, J.; Cohen, S.M.; Izaurralde, E. Genome-wide analysis of mRNAs regulated by Drosha and Argonaute proteins in Drosophila melanogaster. Mol. Cell Biol. 2006, 26, 2965–2975. [Google Scholar] [CrossRef]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef]
- Martin, R.; Smibert, P.; Yalcin, A.; Tyler, D.M.; Schäfer, U.; Tuschl, T.; Lai, E.C. A Drosophila pasha mutant distinguishes the canonical microRNA and mirtron pathways. Mol. Cell Biol. 2009, 29, 861–870. [Google Scholar] [CrossRef]
- Sabin, L.R.; Zhou, R.; Gruber, J.J.; Lukinova, N.; Bambina, S.; Berman, A.; Lau, C.K.; Thompson, C.B.; Cherry, S. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 2009, 138, 340–351. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Shibata, S.; Sasaki, M.; Miki, T.; Shimamoto, A.; Furuichi, Y.; Katahira, J.; Yoneda, Y. Exportin-5 orthologues are functionally divergent among species. Nucleic Acids Res. 2006, 34, 4711–4721. [Google Scholar] [CrossRef]
- Park, J.K.; Liu, X.; Strauss, T.J.; McKearin, D.M.; Liu, Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr. Biol. 2007, 17, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Processing of pre-microRNAs by the Dicer-1-Loquacious complex in Drosophila cells. PLoS Biol. 2005, 3, e235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ye, X.; Liu, X.; Fincher, L.; McKearin, D.; Liu, Q. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 2005, 19, 1674–1679. [Google Scholar] [CrossRef]
- Förstemann, K.; Tomari, Y.; Du, T.; Vagin, V.V.; Denli, A.M.; Bratu, D.P.; Klattenhoff, C.; Theurkauf, W.E.; Zamore, P.D. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 2005, 3, e236. [Google Scholar] [CrossRef]
- Förstemann, K.; Horwich, M.D.; Wee, L.; Tomari, Y.; Zamore, P.D. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell 2007, 130, 287–297. [Google Scholar] [CrossRef]
- Kawamata, T.; Seitz, H.; Tomari, Y. Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nat. Struct. Mol. Biol. 2009, 16, 953–960. [Google Scholar] [CrossRef]
- Okamura, K.; Liu, N.; Lai, E.C. Distinct mechanisms for microRNA strand selection by Drosophila Argonautes. Mol. Cell 2009, 36, 431–444. [Google Scholar] [CrossRef]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kawamata, T.; Tomari, Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol. Cell 2009, 34, 58–67. [Google Scholar] [CrossRef]
- Iwasaki, S.; Tomari, Y. Argonaute-mediated translational repression (and activation). Fly 2009, 3, 204–206. [Google Scholar] [CrossRef]
- Parker, M.S.; Park, E.A.; Sallee, F.R.; Parker, S.L. Canonical Matches of Human MicroRNAs with mRNAs: A Broad Matrix of Position and Size. Microrna 2016, 5, 211–221. [Google Scholar] [CrossRef]
- Wee, L.M.; Flores-Jasso, C.F.; Salomon, W.E.; Zamore, P.D. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 2012, 151, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Nahvi, A.; Shoemaker, C.J.; Green, R. An expanded seed sequence definition accounts for full regulation of the hid 3′ UTR by bantam miRNA. RNA 2009, 15, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fu, H.; Zhang, X.; Li, Y. Identifications of conserved 7-mers in 3′-UTRs and microRNAs in Drosophila. BMC Bioinform. 2007, 8, 432. [Google Scholar] [CrossRef]
- Matsuura-Suzuki, E.; Kiyokawa, K.; Iwasaki, S.; Tomari, Y. miRNA-mediated gene silencing in Drosophila larval development involves GW182-dependent and independent mechanisms. EMBO J. 2024, 43, 6161–6179. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Huntzinger, E.; Izaurralde, E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 2008, 15, 346–353. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Ciniglia, C.; Pinto, G.; Bhattacharya, D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 2004, 21, 809–818. [Google Scholar] [CrossRef]
- Casas-Mollano, J.A.; Rohr, J.; Kim, E.J.; Balassa, E.; Van Dijk, K.; Cerutti, H. Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing. Genetics 2008, 179, 69–81. [Google Scholar] [CrossRef]
- Sun, T.; Tao, M.; Di, Q.; Hu, Z.; Li, H.; Lou, S. Identification of CrDCL1-mediated microRNA biogenesis in green alga Chlamydomonas reinhardtii. Front Microbiol. 2025, 16, 1487584. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Onishi, M.; Kim, E.J.; Cerutti, H.; Ohama, T. RNA-binding protein DUS16 plays an essential role in primary miRNA processing in the unicellular alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 10720–10725. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Cerutti, H. Cooperative processing of primary miRNAs by DUS16 and DCL3 in the unicellular green alga Chlamydomonas reinhardtii. Commun. Integr. Biol. 2017, 10, e1280208. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kim, E.J.; Cerutti, H.; Ohama, T. Argonaute3 is a key player in miRNA-mediated target cleavage and translational repression in Chlamydomonas. Plant J. 2016, 85, 258–268. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, W.; Bai, X.; Qi, Y. Gene silencing by artificial microRNAs in Chlamydomonas. Plant J. 2009, 58, 157–164. [Google Scholar] [CrossRef]
- Molnar, A.; Bassett, A.; Thuenemann, E.; Schwach, F.; Karkare, S.; Ossowski, S.; Weigel, D.; Baulcombe, D. Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J. 2009, 58, 165–174. [Google Scholar] [CrossRef]
- Schmollinger, S.; Strenkert, D.; Schroda, M. An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Curr. Genet. 2010, 56, 383–389. [Google Scholar] [CrossRef]
- Yamasaki, T.; Voshall, A.; Kim, E.J.; Moriyama, E.; Cerutti, H.; Ohama, T. Complementarity to a miRNA seed region is sufficient to induce moderate repression of a target transcript in the unicellular green alga Chlamydomonas reinhardtii. Plant J. 2013, 76, 1045–1056. [Google Scholar] [CrossRef]
- Lenz, D.; May, P.; Walther, D. Comparative analysis of miRNAs and their targets across four plant species. BMC Res. Notes. 2011, 4, 483. [Google Scholar]
- Hu, J.; Deng, X.; Shao, N.; Wang, G.; Huang, K. Rapid construction and screening of artificial microRNA systems in Chlamydomonas reinhardtii. Plant J. 2014, 79, 1052–1064. [Google Scholar]
- Chung, B.Y.; Valli, A.; Deery, M.J.; Navarro, F.J.; Brown, K.; Hnatova, S.; Howard, J.; Molnar, A.; Baulcombe, D.C. Distinct roles of Argonaute in the green alga Chlamydomonas reveal evolutionary conserved mode of miRNA-mediated gene expression. Sci. Rep. 2019, 9, 11091. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Rymarquis, L.A.; Kim, E.J.; Becker, J.; Balassa, E.; Green, P.J.; Cerutti, H. Uridylation of mature miRNAs and siRNAs by the MUT68 nucleotidyltransferase promotes their degradation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2010, 107, 3906–3911. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Rohr, J.; Jeong, W.J.; Hesson, J.; Cerutti, H. Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science 2006, 314, 1893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Zhai, J.; Ramachandran, V.; Dinh, T.T.; Meyers, B.C.; Mo, B.; Chen, X. The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr. Biol. 2012, 22, 689–694. [Google Scholar] [CrossRef]
- Bortolamiol-Becet, D.; Hu, F.; Jee, D.; Wen, J.; Okamura, K.; Lin, C.J.; Ameres, S.L.; Lai, E.C. Selective Suppression of the Splicing-Mediated MicroRNA Pathway by the Terminal Uridyltransferase Tailor. Mol. Cell. 2015, 59, 217–228. [Google Scholar] [CrossRef]
- Shu, L.; Hu, Z. Characterization and differential expression of microRNAs elicited by sulfur deprivation in Chlamydomonas reinhardtii. BMC Genom. 2012, 13, 108. [Google Scholar] [CrossRef]
- Matt, G.; Umen, J. Volvox: A simple algal model for embryogenesis, morphogenesis and cellular differentiation. Dev. Biol. 2016, 419, 99–113. [Google Scholar] [CrossRef]
- Biswal, D.P.; Panigrahi, K.C.S. Light- and hormone-mediated development in non-flowering plants: An overview. Planta 2020, 253, 1. [Google Scholar] [CrossRef]
- Dueck, A.; Evers, M.; Henz, S.R.; Unger, K.; Eichner, N.; Merkl, R.; Berezikov, E.; Engelmann, J.C.; Weigel, D.; Wenzl, S.; et al. Gene silencing pathways found in the green alga Volvox carteri reveal insights into evolution and origins of small RNA systems in plants. BMC Genom. 2016, 17, 853. [Google Scholar] [CrossRef]
- Azaman, S.N.A.; Satharasinghe, D.A.; Tan, S.W.; Nagao, N.; Yusoff, F.M.; Yeap, S.K. Identification and Analysis of microRNAs in Chlorella sorokiniana Using High-Throughput Sequencing. Genes 2020, 11, 1131. [Google Scholar] [CrossRef]
- Yang, R.; Chen, G.; Peng, H.; Wei, D. Identification and Characterization of MiRNAs in Coccomyxa subellipsoidea C-169. Int. J. Mol. Sci. 2019, 20, 3448. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Hu, X.L.; Li, D.; Wang, L.; Cheng, J.; Gao, K. Identification and analysis of microRNAs in Botryococcus braunii using high-throughput sequencing. Aquat. Biol. 2017, 26, 41–48. [Google Scholar] [CrossRef]
- Gao, X.; Cong, Y.; Yue, J.; Xing, Z.; Wang, Y.; Chai, X. Small RNA, transcriptome, and degradome sequencing to identify salinity stress responsive miRNAs and target genes in Dunaliella salina. J. App. Phycol. 2019, 31, 1175–1183. [Google Scholar] [CrossRef]
- Lou, S.; Zhu, X.; Zeng, Z.; Wang, H.; Jia, B.; Li, H.; Hu, Z. Identification of microRNAs response to high light and salinity that involved in beta-carotene accumulation in microalga Dunaliella salina. Algal Res. 2020, 48, 101925. [Google Scholar] [CrossRef]
- Wang, X.; Miao, X.; Chen, G.; Cui, Y.; Sun, F.; Fan, J.; Gao, Z.; Meng, C. Identification of microRNAs involved in astaxanthin accumulation responding to high light and high sodium acetate (NaAC) stresses in Haematococcus pluvialis. Algal Res. 2021, 54, 102179. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Z.; Yan, X.; Lu, J.; Wang, C. Extracellular vesicles involved in growth regulation and metabolic modulation in Haematococcus pluvialis. Biotechnol. Biofuels Bioprod. 2024, 17, 15. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Arun, A.; Coelho, S.M.; De Clerck, O. Brown algae as a model for plant organogenesis. Methods Mol Biol. 2013, 959, 97–125. [Google Scholar]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Billoud, B.; Nehr, Z.; Le Bail, A.; Charrier, B. Computational prediction and experimental validation of microRNAs in the brown alga Ectocarpus siliculosus. Nucleic Acids Res. 2014, 42, 417–429. [Google Scholar] [CrossRef]
- Tseng, C.K. Algal biotechnology industries and research activities in China. J. Appl. Phycol. 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Liu, F.; Wang, W.; Sun, X.; Liang, Z.; Wang, F. Conserved and novel heat stress-responsive microRNAs were identified by deep sequencing in Saccharina japonica (Laminariales, Phaeophyta). Plant Cell Environ. 2015, 38, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.M.; Liu, F.; Duan, D.; Bourdareau, S.; Lipinska, A.P.; Coelho, S.M.; Tarver, J.E. Rapid Evolution of microRNA Loci in the Brown Algae. Genome Biol. Evol. 2017, 9, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, M.; Miura, S.; Nei, M. Origins and evolution of microRNA genes in plant species. Genome Biol Evol. 2012, 4, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Fredericq, S.; Schmidt, W.E. Red Algae eLS; Wiley: Chichester, UK, 2010; pp. 1–7. [Google Scholar]
- Brawley, S.H.; Blouin, N.A.; Ficko-Blean, E.; Wheeler, G.L.; Lohr, M.; Goodson, H.V.; Jenkins, J.W.; Blaby-Haas, C.E.; Helliwell, K.E.; Chan, C.X.; et al. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc. Natl. Acad. Sci. USA 2017, 114, E6361–E6370. [Google Scholar] [CrossRef]
- Shainker-Connelly, S.J.; Crowell, R.M.; Stoeckel, S.; Vis, M.L.; Krueger-Hadfield, S.A. Population genetics of the freshwater red alga Batrachospermum gelatinosum (Rhodophyta) I: Frequent intragametophytic selfing in a monoicous, haploid-diploid species. J. Phycol. 2024, 60, 1420–1436. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Mohammadi, M.; Alian, M.; Muralitharan, G.; Chauhan, V.S.; Balan, V. Exploring the versatility of Porphyridium sp.: A comprehensive review of cultivation, bio-product extraction, purification, and characterization techniques. Biotechnol Adv. 2024, 77, 108471. [Google Scholar] [CrossRef]
- Vieira, C.; Brooks, C.M.; Akita, S.; Kim, M.S.; Saunders, G.W. Of sea, rivers and symbiosis: Diversity, systematics, biogeography and evolution of the deeply diverging florideophycean order Hildenbrandiales (Rhodophyta). Mol. Phylogenet. Evol. 2024, 197, 108106. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Derbel, H.; Duchez, D.; Gardarin, C.; Dubessay, P.; Drira, M.; Fendri, I.; Michaud, P.; Abdelkafi, S. Enhanced growth and metabolite production from a novel strain of Porphyridium sp. Bioengineered 2024, 15, 2294160. [Google Scholar] [CrossRef]
- Gao, F.; Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Xie, S. Identification of conserved and novel microRNAs in Porphyridium purpureum via deep sequencing and bioinformatics. BMC Genom. 2016, 17, 612. [Google Scholar] [CrossRef]
- Barozai, M.Y.K.; Qasim, M.; Din, M.; Achakzai, A.K.K. An update on the microRNAs and their targets in unicellular red alga Porphyridium cruentum. Pak. J. Bot. 2018, 50, 817–825. [Google Scholar]
- Gao, F.; Nan, F.; Feng, J.; Xie, S. Characterization and Comparative Analysis of MicroRNAs in 3 Representative Red Algae. Iran J. Biotechnol. 2021, 19, e2868. [Google Scholar]
- Thangaraj, B.; Jolley, C.C.; Sarrou, I.; Bultema, J.B.; Greyslak, J.; Whitelegge, J.P.; Lin, S.; Kouřil, R.; Subramanyam, R.; Boekema, E.J.; et al. Efficient light harvesting in a dark, hot, acidic environment: The structure and function of PSI-LHCI from Galdieria sulphuraria. Biophys. J. 2011, 100, 135–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Saga, N.; Kitade, Y. Porphyra: A model plant in marine sciences. Fish. Sci. 2002, 68, 1075–1078. [Google Scholar] [CrossRef]
- Lembi, C.A.; Waaland, J.R. Algae and Human Affairs; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Shen, S.; Zhang, G.; Li, Y.; Wang, L.; Xu, P.; Yi, L. Comparison of RNA expression profiles on generations of Porphyra yezoensis (Rhodophyta), based on suppression subtractive hybridization (SSH). BMC Res. Notes 2011, 4, 428. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zou, J.; Xu, D.; Su, F.; Ye, N. Identification of miRNA from Porphyra yezoensis by high-throughput sequencing and bioinformatics analysis. PLoS ONE 2010, 5, e10698. [Google Scholar] [CrossRef]
- He, L.; Huang, A.; Shen, S.; Niu, J.; Wang, G. Comparative Analysis of MicroRNAs between Sporophyte and Gametophyte of Porphyra yezoensis. Comp. Funct. Genom. 2012, 2012, 912843. [Google Scholar] [CrossRef]
- Popper, Z.A. Extraction and detection of arabinogalactan proteins. Methods Mol. Biol. 2011, 715, 245–254. [Google Scholar]
- Krueger-Hadfield, S.A.; Roze, D.; Mauger, S.; Valero, M. Intergametophytic selfing and microgeographic genetic structure shape populations of the intertidal red seaweed Chondrus crispus. Mol. Ecol. 2013, 22, 3242–3260. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2010, 23, 321–335. [Google Scholar] [CrossRef]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2010, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Nan, F.; Song, W.; Feng, J.; Lv, J.; Xie, S. Identification and Characterization of miRNAs in Chondrus crispus by High-Throughput Sequencing and Bioinformatics Analysis. Sci. Rep. 2016, 6, 26397. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.M.; Zhang, J.P. Records of Chinese Seaweeds; Science Press: Beijing, China, 1999; pp. 128–132. [Google Scholar]
- Ask, E.I.; Azanza, R.V. Advances in cultivation technology of commercial eucheumatoid species: A review with suggestions for future research. Aquaculture 2002, 206, 257–277. [Google Scholar] [CrossRef]
- Li, L.H.; Qi, B.; Yang, S.L. Functional effect of dietary fiber from Eucheuma on reducing serum lipids. J. Fish. Sci. China 2008, 15, 943–949. [Google Scholar]
- Gao, F.; Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Xie, S. Identification and characterization of microRNAs in Eucheuma denticulatum by high-throughput sequencing and bioinformatics analysis. RNA Biol. 2016, 13, 343–352. [Google Scholar] [CrossRef]
- Troell, M.; Rönnbäck, P.; Halling, C.; Kautsky, N.; Buschmann, A. Ecological engineering in aquaculture: Use of seaweeds for removing nutrients from intensive mariculture. J. Appl. Phycol. 1999, 11, 89–97. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.Y.; Yin, H.; Sun, X.; Zhang, X.; Xu, N. A survey of the full-length transcriptome of Gracilariopsis lemaneiformis using single-molecule long-read sequencing. BMC Plant Biol. 2022, 22, 597. [Google Scholar] [CrossRef]
- Zhang, X.C.; Fei, X.G. The national aquatic product original variety approval committee-Gracilaria lemaneiformis 981 and an introduction to its cultivation techniques. Sci Fish Farm 2008, 6, 21–22. [Google Scholar]
- Wang, Z.; Wang, X.; Ke, C. Bioaccumulation of trace metals by the live macroalga Gracilaria lemaneiformis. J. Appl. Phycol. 2014, 26, 1889–1897. [Google Scholar] [CrossRef]
- Xiang, J.X.; Saha, M.; Zhong, K.L.; Zhang, Q.S.; Zhang, D.; Jueterbock, A.; Krueger-Hadfield, S.A.; Wang, G.G.; Weinberger, F.; Hu, Z.M. Genome-scale signatures of adaptive gene expression changes in an invasive seaweed Gracilaria vermiculophylla. Mol. Ecol. 2023, 32, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Krueger-Hadfield, S.A.; Blakeslee, A.M.H.; Fowler, A.E. Incorporating ploidy diversity into ecological and community genetics. J. Phycol. 2019, 55, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Hammann, M.; Wang, G.G.; Boo, S.M.; Aguilar-Rosas, L.E.; Weinberger, F. Selection of heat-shock resistance traits during the invasion of the seaweed Gracilaria vermiculophylla. Marine Biol. 2016, 163, 104. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Nyberg, C.D.; Wuff, A. UVR defense mechanisms in eurytopic and invasive Gracilaria vermiculophylla (Gracilariales, Rhodophyta). Physiol. Plant. 2012, 146, 205–216. [Google Scholar] [CrossRef]
- Rueness, J. Life history and molecular sequences of Gracilaria vermiculophylla (Gracilariales, Rhodophyta), a new introduction to European waters. Phycologia 2005, 44, 120–128. [Google Scholar] [CrossRef]
- Andreakis, N.; Procaccini, G.; Maggs, C.; Kooistra, W.H. Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 2007, 16, 2285–2299. [Google Scholar] [CrossRef]
- Andreakis, N.; Kooistra, W.H.; Procaccini, G. Microsatellite markers in an invasive strain of Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta): Insights in ploidy level and sexual reproduction. Gene 2007, 406, 144–151. [Google Scholar] [CrossRef]
- Andreakis, N.; Costello, P.; Zanolla, M.; Saunders, G.W.; Mata, L. Endemic or introduced? Phylogeography of Asparagopsis (Florideophyceae) in Australia reveals multiple introductions and a new mitochondrial lineage. J. Phycol. 2016, 52, 141–147. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spanò, A.; Morabito, M.; Maugeri, T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the Straits of Messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Pirozzi, I.; de Nys, R.; Paul, N.A. Feeding preferences and the nutritional value of tropical algae for the abalone Haliotis asinina. PLoS ONE 2012, 7, e38857. [Google Scholar] [CrossRef]
- Neethu, P.V.; Suthindhiran, K.; Jayasri, M.A. Antioxidant and Antiproliferative Activity of Asparagopsis taxiformis. Pharmacogn. Res. 2017, 9, 238–246. [Google Scholar]
- Thépot, V.; Campbell, A.H.; Paul, N.A.; Rimmer, M.A. Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish Shellfish Immunol. 2021, 113, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mol. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Machado, L.; Tomkins, N.; Magnusson, M.; Midgley, D.J.; de Nys, R.; Rosewarne, C.P. In Vitro Response of Rumen Microbiota to the Antimethanogenic Red Macroalga Asparagopsis taxiformis. Microb. Ecol. 2018, 75, 811–818. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Park, T.; Seo, J.; Lee, Y.; Bae, D.; et al. Red seaweed extracts reduce methane production by altering rumen fermentation and microbial composition in vitro. Front. Vet. Sci. 2022, 9, 985824. [Google Scholar] [CrossRef]
- Patwary, Z.P.; Zhao, M.; Wang, T.; Paul, N.A.; Cummins, S.F. A Proteomic Analysis for the Red Seaweed Asparagopsis taxiformis. Biology 2023, 12, 167. [Google Scholar] [CrossRef]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Viron, N.; Alhagdow, M.; Karimi, M.; Jones, M.; Amsellem, Z.; Sicard, A.; Czerednik, A.; Angenent, G.; Grierson, D.; et al. Flexible tools for gene expression and silencing in tomato. Plant Physiol. 2009, 151, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Eamens, A.L.; Agius, C.; Smith, N.A.; Waterhouse, P.M.; Wang, M.B. Efficient silencing of endogenous microRNAs using artificial microRNAs in Arabidopsis thaliana. Mol. Plant. 2011, 4, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Zhang, L.; Gao, Z.; Zhu, C.X.; Guo, X. Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol. 2011, 13, 304–316. [Google Scholar] [CrossRef]
- Hauser, F.; Chen, W.; Deinlein, U.; Chang, K.; Ossowski, S.; Fitz, J.; Hannon, G.J.; Schroeder, J.I. A genomic-scale artificial microRNA library as a tool to investigate the functionally redundant gene space in Arabidopsis. Plant Cell 2013, 25, 2848–2863. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Webb, J.; Zhao, M.; Campbell, A.H.; Paul, N.A.; Cummins, S.F.; Eamens, A.L. The microRNA Pathway of Macroalgae: Its Similarities and Differences to the Plant and Animal microRNA Pathways. Genes 2025, 16, 442. https://doi.org/10.3390/genes16040442
Webb J, Zhao M, Campbell AH, Paul NA, Cummins SF, Eamens AL. The microRNA Pathway of Macroalgae: Its Similarities and Differences to the Plant and Animal microRNA Pathways. Genes. 2025; 16(4):442. https://doi.org/10.3390/genes16040442
Chicago/Turabian StyleWebb, Jessica, Min Zhao, Alexandra H. Campbell, Nicholas A. Paul, Scott F. Cummins, and Andrew L. Eamens. 2025. "The microRNA Pathway of Macroalgae: Its Similarities and Differences to the Plant and Animal microRNA Pathways" Genes 16, no. 4: 442. https://doi.org/10.3390/genes16040442
APA StyleWebb, J., Zhao, M., Campbell, A. H., Paul, N. A., Cummins, S. F., & Eamens, A. L. (2025). The microRNA Pathway of Macroalgae: Its Similarities and Differences to the Plant and Animal microRNA Pathways. Genes, 16(4), 442. https://doi.org/10.3390/genes16040442








