Abstract
In plants and animals, the microRNA (miRNA) class of small regulatory RNA plays an essential role in controlling gene expression in all aspects of development, to respond to environmental stress, or to defend against pathogen attack. This well-established master regulatory role for miRNAs has led to each protein-mediated step of both the plant and animal miRNA pathways being thoroughly characterized. Furthermore, this degree of characterization has led to the development of a suite of miRNA-based technologies for gene expression manipulation for fundamental research or for use in industrial or medical applications. In direct contrast, molecular research on the miRNA pathway of macroalgae, specifically seaweeds (marine macroalgae), remains in its infancy. However, the molecular research conducted to date on the seaweed miRNA pathway has shown that it shares functional features specific to either the plant or animal miRNA pathway. In addition, of the small number of seaweed species where miRNA data is available, little sequence conservation of individual miRNAs exists. These preliminary findings show the pressing need for substantive research into the seaweed miRNA pathway to advance our current understanding of this essential gene expression regulatory process. Such research will also generate the knowledge required to develop novel miRNA-based technologies for use in seaweeds. In this review, we compare and contrast the seaweed miRNA pathway to those well-characterized pathways of plants and animals and outline the low degree of miRNA sequence conservation across the polyphyletic group known as the seaweeds.
1. The microRNA Pathway and Its Evolution in Eukaryotes
In eukaryotes, an expansive population of small regulatory RNAs (sRNAs) have been demonstrated to act as master controllers of gene expression functioning at either the transcriptional or posttranscriptional level. Moreover, sRNA-directed gene expression regulation has been shown to be essential for the normal progression of development, to respond to environmental stress, to defend against invading pathogens, and to maintain genome integrity via inhibiting the activity of mobile genetic elements [1,2,3,4,5,6,7]. The critical importance of sRNA-directed gene expression regulation as part of eukaryotic development is best evidenced when dysfunction of this regulatory mechanism occurs, namely: loss of control of sRNA-directed gene expression regulation has been associated with the onset of several disease states in numerous higher eukaryotes. For example, defective sRNA-directed gene expression regulation has been linked to the onset of specific types of cancer in humans [8] and for other mammalian species [9], chronic kidney disease in domesticated dogs [10], and mastitis in dairy cows [11]. Although an array of functionally distinct species of sRNA have been identified across evolutionarily diverse eukaryotes, sRNAs can typically be grouped into two primary classes, including the (1) microRNA (miRNA), and (2) small-interfering RNA (siRNA) classes [12,13,14]. Grouping sRNAs into these two classes is based on the structure of the double-stranded RNA (dsRNA) precursor transcript from which the sRNA is liberated during the production stage of its functional maturation into a sRNA silencing signal [12,15,16,17]. More specifically, a single or predominant miRNA silencing signal is liberated from a stem-loop structured precursor composed of paired stem arms (i.e., nucleotides in the 5′ arm base pair with nucleotides in the 3′ arm) and an unpaired loop region: a dsRNA folding structure that is the result of incomplete complementarity of the non-coding RNA (ncRNA) precursor [12,15]. In contrast, multiple siRNA silencing signals, sometimes numbering in the hundreds, are processed from molecules of perfectly dsRNA, with such precursors being naturally generated in eukaryote nuclear genomes by the transcription of unique DNA structures, such as the convergent bidirectional transcription of repetitive DNA elements [16,17].
Post liberation from the dsRNA precursor, and their subsequent functional maturation, miRNAs and siRNAs form single-stranded ncRNAs of a predominant length of 20 to 24 nucleotides (20–24-nt) [12,13,14,15,16,17]. This size, together with the structure and genomic origin of the precursor, determines the loading of each sRNA species into a specific protein effector complex, termed the RNA-induced silencing complex (RISC), which facilitates the regulatory mechanism of gene expression control directed by the loaded sRNA [18,19,20,21,22]. For example, in plants, miRNAs are primarily in the form of a 21-nt silencing signal which directs their preferential loading into a protein effector complex that mediates target gene expression regulation via a messenger RNA (mRNA) cleavage mode of RNA silencing [18,19,20]. In animal species however, miRNA silencing signals are primarily 22-nt in length upon their functional maturation, a size which guides their directed loading into a protein effector complex that controls the expression of target gene transcripts via a translational repression mode of RNA silencing [21,22]. Protein effector complex loading of miRNAs in both the plant and animal systems, activates the complex to form, miRNA-loaded RISC (miRISC) [19,22]. Plant miRNAs display a high degree of complementarity to the target gene transcripts whose expression is regulated by miRISC. Therefore, each plant miRNA only directs expression regulation of a small number of closely related target genes which encode proteins of highly similar molecular function [23,24]. In direct contrast, in the animal system, the miRNA silencing signal only has limited complementarity at its 5′ end, termed the ‘seed sequence’ to its target gene transcripts, thereby facilitating the ability of each miRISC-loaded miRNA to influence the expression of a considerably higher number of target genes, target genes that encode protein products of completely unrelated molecular function [25,26].
Since their initial discovery in Caenorhabditis elegans (roundworm) in 1993 [27], miRNAs have been identified and experimentally validated across the eukaryotes, from the unicellular green microalgae, Chlamydomonas reinhardtii (Chlamydomonas) [28] to the genetic model plant species Arabidopsis thaliana (Arabidopsis) [29], the widely used species for developmental biology studies Drosophila melanogaster (fruit fly) [30] and Mus musculus (mouse) [31], through to humans (Homo sapiens) [32]. Profiling of the miRNA landscape of a wide breadth of eukaryotes has shown a comprehensive lack of sequence homology between the predominant miRNA families encoded by plant and animal nuclear genomes. This, in addition to the mechanistic differences in the production and action stages of the plant and animal miRNA pathways, strongly suggests that miRNAs have evolved independently from an ancient sRNA pathway, most likely a siRNA-like pathway, that already existed in the last common ancestor of these two predominant eukaryote kingdoms [27,28,29,30,31].
The notion that the miRNA pathway has evolved largely independently in individual eukaryotic lineages is further supported by the only recent identification of shared miRNA species between members of the two main divisions of the Viridiplantae, algae and plants. Namely, three miRNAs of similar sequence identity were discovered in the green microalgae C. reinhardtii, and the thalloid liverwort species, Pellia endiviifolia [32,33]. This recent identification of miRNAs shared in algae and plants emphasizes the importance of sampling, at an adequate depth of coverage, the miRNA landscape of many species from each phylum to confidently identify the degree of miRNA conservation across the eukaryotes [32,33,34,35]. Sampling each species at an adequate depth of coverage, as well as sampling multiple developmental stages and different growth environment conditions of the species under assessment, not only allows for the identification of the full miRNA repertoire of a species, but aids in improving the possibility of uncovering the full degree of miRNA conservation in the eukaryotes [36,37,38]. Such an approach also helps to address the inherent difficulty in confidently annotating both conserved and species-specific miRNAs (also termed ‘newly evolved miRNAs’) in each species under analysis [39,40,41,42] with the adoption of this cautious approach to miRNA pathway analysis adding further weight to the prevailing view that the miRNA pathway has evolved convergently in plants and animals.
In the eukaryotes, the extensive miRNA pathway data available today strongly suggests that the miRNA pathway has likely evolved independently at least nine times in the excavates, including in slime molds, brown algae, green algae, land plants, twice in the sponges (e.g., demosponge—Amphimedon queenslandica and calcareous sponge Sycon ciliatum), cnidarians, and the bilaterians [28,33,35,42,43,44,45,46,47,48]. Alternatively, the lack of miRNA sequence conservation between plants and animals could be accounted for by high sequence turnover rates which would have removed any trace of miRNA conservation in these two contemporary lineages [35,42,44,45,48]. As example, only a single conserved miRNA has been identified between C. reinhardtii (unicellular) and Volvox carteri (multicellular), two species of green microalgae that diverged from each other approximately (~) 200 million years ago (Mya) [28,33,49]. Similarly, albeit more surprising, profiling of the miRNA landscapes of A. thaliana and A. lyrata revealed that only 66% of MICRORNA (MIR) gene families are conserved between these two closely related species of Arabidopsis which only diverged from each other ~10 Mya [13,29,36,41].
In spite of the lack of miRNA sequence conservation, many studies have shown that the genomes of species capable of producing miRNAs encode (1) Dicer (Dcr) or DICER-LIKE (DCL), the RNase III-like endonuclease required to process the miRNA from its dsRNA precursor, and (2) ARGONAUTE (AGO), an endonuclease which forms the catalytic core of miRISC to mediate miRNA-directed target gene expression regulation, post miRNA maturation [16,17,18,19,20,21,22]. Therefore, for eukaryote species with available nuclear genome sequence data, but where miRNA profiling has not yet been performed, the first step in miRNA biology research should be the bioinformatic identification of genomic loci that encode for the Dcr/DCL and AGO endonucleases. Such an approach has formed the basis for construction of a detailed molecular understanding of the miRNA pathways of plants, animals and microalgae via the use of experimental model species such as Arabidopsis, Drosophila and Chlamydomonas, respectively [16,17,18,19,50,51,52,53]. Furthermore, detailed functional characterization of the miRNA pathway in plants, animals and microalgae has enabled the development of novel gene expression manipulation technologies such as the artificial miRNA (amiRNA), molecular sponge, and target mimic approaches: molecular tools which can be used in either the research setting to further characterize miRNA function, or in industry to manipulate the biosynthesis pathways of consumer market desired natural products. In stark contrast to the plant, animal and microalgal systems, research on miRNA biology in macroalgae, specifically the seaweeds (marine macroalgae), included within the green (Chlorophyta), brown (Phaeophyceae), and red (Rhodophyta) divisions of algae, remains in its infancy. In this review, we describe the well characterized plant, animal and microalgae miRNA pathways using Arabidopsis, Drosophila, and Chlamydomonas as our respective model species. We then detail the scarce findings reported to date on either the global miRNA populations or the core protein machinery of the miRNA pathways of green, brown, and red seaweeds and compare these preliminary findings with those reported in plants, animals, and microalgae. Further, we go on to outline the requirement to develop miRNA-based technologies for further functional characterization of the seaweed miRNA pathway and for use as a biotechnological tool to manipulate the biosynthesis pathways of consumer-desired natural products specific to the seaweeds.
2. The miRNA Pathway of Arabidopsis thaliana
The majority of Arabidopsis MIR genes occupy intergenic regions of the Arabidopsis nuclear genome, and which share structural features identical to those of protein-coding genes, including housing both a promoter region and terminator sequence within the gene body [54,55]. Due to these shared structural features, RNA polymerase II (Pol II; the same RNA polymerase responsible for protein-coding gene transcription in Arabidopsis) transcribes a ncRNA from the MIR gene, however, the nascent transcript does not contain either a translation start or stop codon, and due to these missing signals, the ncRNA will not be used as a translation template [54,55]. Pol II catalyzed transcription of the MIR gene derived ncRNA does nonetheless identify the ncRNA for posttranscriptional modifications, including the addition of a 7-methylguanosine capping structure at the 5′ terminus (5′ cap) and a polyadenylated tail at the 3′ terminus (3′ poly(A) tail) of the transcript [56,57]. In addition, due to the long length of Arabidopsis MIR gene transcription products, some also contain intron sequences, which identifies these miRNA precursors for further posttranscriptional modification via intron splicing [58,59]. Each MIR gene transcription product contains a region of partial sequence self-complementarity which directs this region of the ncRNA to fold back onto itself to form a stem-loop structured imperfectly dsRNA termed the primary-miRNA (pri-miRNA) [54,55]. In the Arabidopsis cell nucleus, the zinc-finger protein with RNA-binding capacity, SERRATE1 (Ath-SE1), recognizes and binds the stem-loop structured pri-miRNA and transports the bound molecule to specialized nuclear bodies, termed D-bodies (Dicing-bodies), where the Dicing complex (D-complex; also called the microprocessor complex) proteins localize to [60,61] (Figure 1).
In the D-body, the Ath-SE1 bound pri-miRNA undergoes a two-step processing event catalyzed by the core D-complex proteins, DICER-LIKE1 (Ath-DCL1) and dsRNA BINDING1 (Ath-DRB1) [60,61,62,63]. Initially, the Ath-DCL1 endonuclease cleaves off the unpaired, single-stranded RNA regions (i.e., removal of ncRNA sequences which flank the dsRNA stem-loop structure) of the pri-miRNA to produce the smaller sized processing intermediate, the precursor-miRNA (pre-miRNA). Next, Ath-DCL1 cleaves the pre-miRNA at specific positions within the molecule to remove a section of the paired stem arms, and the unpaired loop region of the pre-miRNA to generate the much smaller sized dsRNA molecule, the miRNA/miRNA* duplex [64,65,66,67] (Figure 1). RNase IIIa and RNase IIIb, the Dicing domains of Ath-DCL1, are positioned 21-nt apart from each other on DCL1-bound dsRNA substrates, and accordingly, most Arabidopsis miRNA silencing signals are 21-nt in length post precursor transcript processing by the D-complex. Arabidopsis DCL1 possesses two dsRNA binding domains (dsRBDs) at its carboxyl terminus (C-terminus), and therefore, can bind and process miRNA precursors on its own. However, both the accuracy (i.e., the position of cleavage) and efficiency (i.e., the rate of cleavage) of Ath-DCL1-catalyzed pri-miRNA and pre-miRNA processing is enhanced via Ath-DCL1 functionally interacting with the other D-complex core proteins, Ath-SE1 and Ath-DRB1 [60,61,64,66,68]. Moreover, during the precursor processing phases of the production stage of the Arabidopsis miRNA pathway, the primary role conducted by Ath-DRB1 is to accurately position Ath-DCL1 on the precursor transcript to ensure that just a single miRNA/miRNA* duplex is liberated from the much longer length pri-miRNA and pre-miRNA precursors by Ath-DCL1 dicing [64,65,68]. Ath-DCL1 cleavage generates a 2-nt overhang at the 3′ terminus of both strands of the miRNA/miRNA* duplex, and this dicing signature is recognized by the sRNA-specific methyltransferase, HUA ENHANCER1 (Ath-HEN1) (Figure 1). Ath-HEN1 adds a methyl group to the 2′ hydroxyl group (2′-O-methylation) of the 3′ terminal nucleotide of both the miRNA guide strand and miRNA* passenger strand while the two duplex strands are still hybridized to each other [69,70]. Ath-HEN1 similarly modifies the 3′ terminal nucleotide of each duplex strand of each class of siRNA which also accumulates in Arabidopsis cells. This universal sRNA modification is thought to protect the 3′ end of sRNAs post duplex strand separation from uridylation, and therefore degradation, to thus ensure that the sRNA retains its ‘functionality’ prior to its loading into its specific effector complex (i.e., miRISC and siRISC) [69,70].
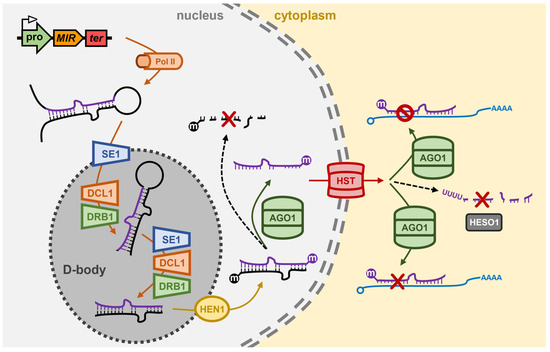
Figure 1.
The miRNA pathway of the model plant Arabidopsis thaliana. In the nucleus of the Arabidopsis cell, Pol II transcribes the pri-miRNA from a MIR gene. The pri-miRNA is bound by SE1 and is transported to the D-body where it is processed by DCL1/DRB1 into a pre-miRNA and then a miRNA/miRNA* duplex. HEN1 methylates the 3′ terminus of both duplex strands and AGO1 retains the miRNA guide strand and discards the miRNA* passenger strand. HST is involved in the nucleus-to-cytoplasm export of some miRNAs, and in the cytoplasm of the Arabidopsis cell, AGO1 uses its loaded miRNA to guide the silencing of target gene transcripts predominantly via mRNA cleavage but also via translational repression. HESO1 controls the steady-state levels of Arabidopsis miRNAs to add an additional layer of complexity to miRNA-directed gene expression regulation. AGO1, ARGONAUTE1; D-body, nuclear Dicing body; DCL1, DICER-LIKE1; DRB1, dsRNA BINDING1; HEN1, HUA ENHANCER1; HESO1, HEN1 SUPPRESSOR1; MIR, MICRORNA gene; Pol II, RNA polymerase II; pro, MIR gene promoter; SE1, SERRATE1; ter, MIR gene terminator.
Post 2′-O-methylation of both duplex strands, Ath-DRB1 orientates the loading of the miRNA/miRNA* duplex into the ARGONAUTE1 (Ath-AGO1) endonuclease to ensure that Ath-AGO1 retains the miRNA guide strand and ‘slices’ the miRNA* sequence to remove the passenger strand from miRISC [18,19,71] (Figure 1). The now single-stranded and functionally mature miRNA, either on its own, or in complex with Ath-AGO1, is then exported out of the nucleus and into the cytoplasm of the Arabidopsis cell. HASTY (Ath-HST), the Arabidopsis ortholog of the animal RanGTP-dependent dsRNA-binding protein, Exportin-5 (XPO5), was originally thought to be required for the export of all Arabidopsis miRNAs from the nucleus to the cytoplasm [72,73] (Figure 1). However, more recent research has questioned the exact degree of requirement of Ath-HST for the nucleus export of mature miRNA silencing signals with the level of only some miRNAs reduced in the Arabidopsis hst mutant [72,73]. In the cytoplasm, Ath-AGO1 forms the catalytic core of miRISC for almost all Arabidopsis miRNAs (Figure 1), with each miRNA silencing signal loaded by Ath-AGO1 (and by the AGO1 homologs of other plant species) displaying a high degree of sequence complementarity to its target genes [18,19]. Furthermore, the target sites of miRISC-loaded miRNAs are predominantly located within the coding sequence of target genes, and together the (1) high degree of sequence complementarity between the miRNA and its targeted gene(s), and (2) coding sequence location of miRNA target sites, results in mRNA cleavage forming the primary mechanism of target gene expression regulation directed by an Arabidopsis miRNA [18,19]. Although much less prevalent than the target mRNA cleavage mechanism of miRNA-directed RNA silencing, more recent research has shown that many Arabidopsis miRNAs also mediate a translational repression mode of gene expression repression [74,75]: a process facilitated by protein machinery additional to the core machinery proteins described above, and which therefore sits outside of the scope of this review.
3. The miRNA Pathway of Drosophila melanogaster
Like in Arabidopsis, Pol II appears to be the primary DNA-dependent RNA polymerase responsible for MIR gene transcription in Drosophila [76,77,78]. However, in contrast to Arabidopsis where MIR genes are predominantly positioned between protein-coding genes, ~35% of Drosophila MIR genes are located proximal to one another on Drosophila chromosomes to form MIR gene clusters, and as such, are co-transcribed by Pol II to form polycistronic pri-miRNAs [79,80] (Figure 2). A further difference between the MIR gene genomic landscapes of Arabidopsis and Drosophila is that an additional ~30% of Drosophila miRNA precursor transcripts overlap with the sequences of protein-coding genes, with the precursors of this subclass of miRNAs, termed mirtrons, only forming post their release from the precursor-mRNA transcribed from the host gene following intron splicing [81,82] (Figure 2). Their positioning within protein-coding sequences results in mirtron expression being reliant on the expression of the host gene, and not on the unique composition of transcription regulating DNA-based elements of the MIR genes which occupy a position distinct to other gene loci on Drosophila chromosomes [78,83]. Post splicing, a mirtron containing intron folds to form a stem-loop structured dsRNA which is recognized for processing as a miRNA precursor transcript (i.e., recognized as a pri-miRNA) by the nucleus-localized RNase III-like endonuclease, Dme-Drosha [84,85] (Figure 2). As for Ath-DCL1, Dme-Drosha cleaves the single-stranded regions of the RNA transcript that flank the stem-loop folding structure of the pri-miRNA to form the pre-miRNA, which in Drosophila and other animal species, are dsRNA molecules of a uniform size of 60–70-nt and which harbor shared structural features of a stem-loop, an exposed phosphate group at the 5′ terminus, and a 2-nt overhang at the 3′ terminal end [84,85]. Dme-Drosha functionally interacts with the dsRNA binding protein, Dme-Pasha (also named DiGeorge syndrome critical region 8 (DGCR8)) to form the microprocessor complex in the nucleus of the Drosophila cell, with Dme-Pasha promoting both the accuracy and efficiency of Dme-Drosha catalyzed dicing of monocistronic, polycistronic and mirtronic pri-miRNAs [84,86,87] (Figure 2). During the nucleus-localized precursor processing stage of the Drosophila miRNA pathway, the RNA-binding protein Arsenic resistance 2 (Dme-Ars2) directs an analogous role to that of Ath-SE1, via Dme-Ars2 binding and stabilizing pri-miRNA precursors and promoting their interaction with the core protein machinery of the microprocessor complex, Dme-Drosha and Dme-Pasha, to ensure efficient pre-miRNA production [84,85,86,87,88] (Figure 2).
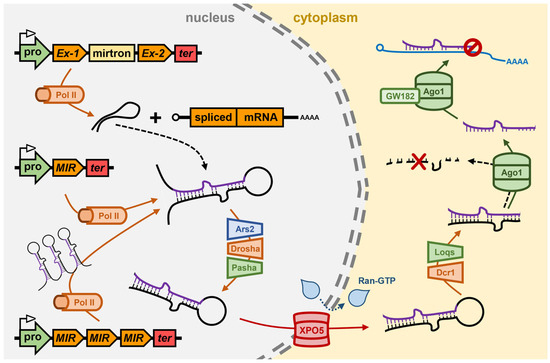
Figure 2.
The miRNA pathway of the model animal Drosophila melanogaster. In the nucleus of the Drosophila cell, Pol II transcribes the pri-miRNA from a MIR gene. Pol II is also responsible for the transcription of mirtrons from host genes or for the transcription of MIR gene clusters. After folding into a stem-loop dsRNA structure, the pri-miRNA is bound by Ars2 and is then processed into a pre-miRNA by Drosha/Pasha. The pre-miRNA is exported from the nucleus to the cytoplasm by the RanGTP-dependent exportin, XPO5. In the cytoplasm of the Drosophila cell, the pre-miRNA is further processed into the miRNA/miRNA* duplex by Dcr1/Loqs. After duplex strand separation and degradation of the miRNA* passenger strand, Ago1 uses the loaded miRNA to guide the silencing of target gene transcripts via translational repression, a mode of miRNA-directed gene expression regulation that also requires GW182. Ago1, Argonaute1; Ars2, Arsenite resistance protein2; Dcr1, Dicer1; GW182, glycine/tryptophan repeat protein 182; Loqs, Loquacious; MIR, microRNA gene; mirtron, miRNA precursor-containing intron; Pol II, RNA polymerase II; pro, MIR gene promoter; Ran-GTP, RAS-related nuclear protein GTPase; ter, MIR gene terminator; XPO5, Exportin-5.
The pre-miRNA is next exported out of the nucleus and released into the cytoplasm by the action of the Ran-GTP-dependent dsRNA binding protein, Dme-XPO5 [89,90] (Figure 2). In the cytoplasm of the Drosophila cell, and once released by the Dme-Ran-GTP/Dme-XPO5 complex, the pre-miRNA is further processed by the cytoplasm-localized Dicer class of RNase III-like endonuclease, Dicer1 (Dme-Dcr1) [91,92,93]. Like Dme-Drosha, both the accuracy and efficiency of Dme-Dcr1 processing of pre-miRNA is enhanced via its interaction with its functional partner, the dsRNA binding protein, Loquacious (Loqs) (also called Transactivation response element RNA-binding protein (TRBP)) [92,94]. Dme-Dcr1/Dme-Loqs-directed processing of the pre-miRNA consists of removal of an unrequired section of the stem arms and the loop region of the stem-loop structure of the pre-miRNA, to generate a predominantly 22-nt miRNA/miRNA* duplex with 2-nt overhangs at the 3′ end of each duplex strand [91,92,93,94] (Figure 2). Typically, sequencing of the miRNA repertoire of an animal species has revealed that the abundance of the miRNA* strand is much lower than the level of the corresponding miRNA strand, usually at least ~100-fold lower, to suggest that preferential duplex strand selection is also a predominant biogenesis mechanism in animals, as it is in the plant model Arabidopsis. However, in contrast to Arabidopsis where Ath-DRB1 is responsible for orientating the miRNA/miRNA* duplex into Ath-AGO1 for miRNA guide strand selection, the Dme-Dcr1 functional partner protein of the cytoplasmic microprocessor complex, Dme-Loqs, appears not to function in an analogous manner at this stage in the Drosophila miRNA production pathway [71,92,94]. It is apparent however that the thermodynamic properties at the terminal base pair of the 5′ end of each duplex strand guides the orientation of the miRNA/miRNA* duplex into Dme-Ago1 for miRNA strand selection via unwinding of the duplex strands, retainment of the miRNA guide strand by Dme-Ago1, and removal of the miRNA* passenger strand for its subsequent degradation [95,96,97] (Figure 2).
A member of the AGO protein family has also been shown to form the catalytic core of miRISC in other animal species, including C. elegans, mouse and humans, however, animal AGOs which are preferentially loaded with miRNA silencing signals are not cleavage competent, lacking the ‘Slicer’ activity of Ath-AGO1 [18,19,95,96]. Therefore, due to the lack of slicing ability by miRNA-loaded animal AGOs, translational repression forms the predominant mechanism of miRISC-mediated, miRNA-directed target gene expression regulation in animals [98,99,100]. Translational repression is a mild form of RNA silencing compared to the target transcript cleavage mode of RNA silencing directed by plant miRNAs. However, in the animal system, miRNA-guided target recognition is based on a low degree of miRNA/target mRNA complementarity with only miRNA nucleotide positions 2 to 8 required for an animal miRISC to use its loaded miRNA cargo as an identity guide to target mRNAs for expression regulation [101,102]. In addition to this, the target sites of animal miRNAs are almost exclusively positioned in the 3′ UTRs of miRISC-regulated mRNAs [103,104]. Therefore, these two parameters specific to the animal miRNA pathway results in (1) each miRNA identifying numerous unrelated transcripts for expression regulation, and (2) many of the targeted mRNAs being under co-regulation by two or more miRNAs, which would together, enhance or strengthen the overall degree of control on target gene expression regulation by the translational repression mode of RNA silencing. The PIWI domain of Dme-Ago1 mediates its interaction with the scaffolding protein GW182 via binding to the glycine/tryptophan (GW) repeats housed in the amino terminal (N-terminal) region of Dme-GW182 [105,106,107] (Figure 2). Interaction with Dme-GW182 provides a link between Dme-Ago1-mediated translational repression and de-adenylation complexes which in turn direct the removal of 3′ poly(A) tails and decay of miRISC bound mRNAs [105,106,107].
4. The miRNA Pathway of Chlamydomonas reinhardtii
The presence of miRNA silencing signals was first reported in Drosophila and Arabidopsis in 2001 [30] and 2002 [29], respectively, yet this class of regulatory RNA was not described in the unicellular green microalgae C. reinhardtii until 2007 following the publication of two studies [28,33], with some of the identified miRNAs reported by both studies. The [33] study identified 68 candidate miRNAs, of which 21 were further shown to originate from precursor molecules which could form stem-loop folding structures like those adopted by plant and animal miRNA precursors. Further confidence that a bona fide set of Chlamydomonas miRNAs had been identified was provided by the authors reporting that a miRNA* passenger strand in addition to a miRNA guide sequence was detected for 12 of the 21 miRNAs where a precursor molecule had also been identified [33]. The [28] study revealed that the predominant size of Chlamydomonas miRNAs was 21-nt like Arabidopsis miRNAs, however, genome mapping of the identified miRNAs showed that these sequences aligned to both the introns of protein-coding genes (~30%) and intergenic regions (~70%) of Chlamydomonas chromosomes, similar to the composition of the MIR gene landscape in Drosophila [28]. The authors also reported that the expression of some of the identified miRNAs was different between the vegetative and gametic cell forms of Chlamydomonas, and via interpretation of their in vitro analyses, this early report proposed that target transcript cleavage was the likely mode of RNA silencing directed by Chlamydomonas miRNAs [28]. Interestingly, although both reports [28,33] revealed that the Chlamydomonas miRNA pathway shared features specific to either the plant or animal miRNA pathway, no sequence similarity was found for the newly identified Chlamydomonas miRNAs to those previously identified for any plant or animal species whose miRNA landscape had been profiled and deposited into the miRNA Registry, miRBase (https://www.mirbase.org/) [108].
Chlamydomonas reinhardtii encodes three DCL genes, Cre-DCL1 to Cre-DCL3, and considering that Chlamydomonas diverged from the last common ancestor of land plants over 1 billion years ago [109], it is unsurprising that the three Chlamydomonas DCLs are not direct orthologues of the Ath-DCL1 to Ath-DCL3 genes [110]. Via the use of amiRNA technology, and the mapping of the causative mutation of transformant lines defective in amiRNA-directed silencing of the endogenous gene PHYTOENE SYNTHASE (PSY), ref. [111] identified Cre-DCL3, a Dcr more structurally similar to the animal Drosha enzyme than to Dcr, as the DCL family member most likely responsible for miRNA production in Chlamydomonas [111]. More specifically, in the Chlamydomonas dcl3 mutant, the amiRNA did not accumulate to detectable levels as it failed to be processed from the precursor which was developed to deliver the PSY-targeting amiRNA. Accordingly, amiRNA-specific cleavage products could not be detected in the dcl3 mutant and PSY expression remained at its wild-type level [111]. In addition to the amiRNA failing to accumulate to detectable levels in the Chlamydomonas dcl3 mutant, the clear 21-nt peak of the global sRNA population observed in wild-type cells by sRNA sequencing was absent in the mutant background with endogenous miRNA accumulation shown to be globally reduced in the dcl3 mutant. Moreover, miRNA accumulation was reduced in the Chlamydomonas dcl3 mutant regardless of the genomic origin of the endogenous miRNAs, with the levels of MIR gene-derived miRNAs, mirtron miRNAs, and miRNAs derived from the 3′ UTRs of host genes, all reduced in their abundance [111]. Further evidence that Cre-DCL3 is the DCL family member responsible for miRNA production in Chlamydomonas was provided by the authors’ demonstration that the dcl3 mutation could be complemented via the introduction of a Chlamydomonas chromosome fragment which encoded a wild-type copy of the Cre-DCL3 gene. In the complemented transformant lines, endogenous miRNAs accumulated to their wild-type levels, miRNA-specific target transcript cleavage products were readily detectable, and the expression of known miRNA target genes returned to wild-type equivalent levels [111]
A second transgene-based screen for mutants defective in miRNA-directed RNA silencing of a reporter gene, identified the dull slicer-16 (dus16) mutant [112]. Mapping of the causative mutation of the dus16 mutant revealed that the disrupted gene, Cre05.g232004, encoded an RNA binding protein which harbors both an RNA-binding motif (RBM) and a dsRBD: a functional domain configuration which would enable the encoded DUS16 protein to recognize and bind both single-stranded and double-stranded forms of RNA [112]. Moreover, and as shown for the Chlamydomonas dcl3 mutant [111], pri-miRNA transcripts generated by the activity of Pol II over-accumulated, and 21-nt miRNA silencing signals were reduced to almost undetectable levels, in the dus16 mutant background [112]. The construction of reporter tagged versions of Cre-DUS16 revealed that Cre-DUS16 predominantly localized to the nucleus with a smaller abundance of the tagged protein version localizing to cytoplasmic bodies. In addition, mass spectrometry analysis revealed that Cre-DUS16 was associated with Cre-DCL3, the predominant DCL family member responsible for miRNA production in Chlamydomonas [112]. Further characterization of Cre-DUS16 showed that it not only interacts with Cre-DCL3, but that Cre-DUS16 is required by Cre-DCL3 to enhance both the accuracy and efficiency of Cre-DCL3-catalyzed processing of miRNA precursor transcripts in Chlamydomonas [113] (Figure 3). This finding provided the first definitive evidence that Cre-DCL3/Cre-DUS16 form the microprocessor complex in the nucleus of Chlamydomonas cells and which functions in an analogous manner to the nucleus-localized microprocessor complexes DCL1/DRB1 in Arabidopsis [64,65], and Drosha/Pasha in Drosophila [84,85,86,87]. It is important to note here that, to date, no Ath-SE1 homolog has been described for Chlamydomonas, however, the identification of a RBM and a dsRBD in the Cre-DUS16 protein raises the intriguing proposal that Cre-DUS16 may function as both Ath-SE1 and Ath-DRB1 of the Arabidopsis microprocessor complex in the production stage of the Chlamydomonas miRNA pathway [112].
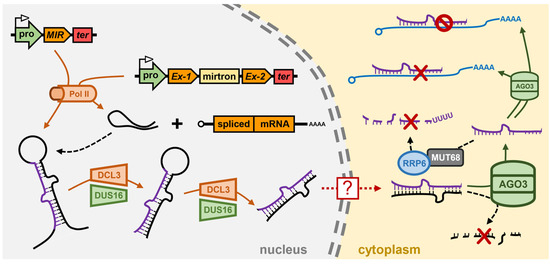
Figure 3.
The miRNA pathway of the model green microalgae Chlamydomonas reinhardtii. In the nucleus of a Chlamydomonas cell, Pol II transcribes pri-miRNAs directly from MIR genes, or from protein-coding host genes with the mirtron pri-miRNA forming post intron splicing. Post folding into a stem-loop dsRNA structure, the pri-miRNA is processed into a pre-miRNA by the DCL3/DUS16 partnership. DCL3/DUS16 are also required for further processing of the pre-miRNA into the miRNA/miRNA* duplex to indicate that all processing steps of the production stage of the Chlamydomonas miRNA pathway occur in the nucleus. The protein mediator of the export of the miRNA/miRNA* duplex out of the nucleus and into the cytoplasm of a Chlamydomonas cell remains unknown (red boxed question mark). In the cytoplasm, the miRNA/miRNA* duplex strands are separated from each other by an unknown mechanism, and then the miRNA* passenger strand is degraded. AGO3 retains the miRNA guide strand and uses it to direct expression regulation of target gene transcripts via either a mRNA cleavage or translational repression mode of RNA silencing. The steady state levels of Chlamydomonas miRNAs are controlled by MUT68, together with RRP6, to add an additional layer of regulatory complexity to miRNA-directed gene expression control in Chlamydomonas. AGO3, Argonaute3; DCL3, Dicer-like3; DUS16, Dull slicer-16; MIR, MICRORNA gene; mirtron, miRNA precursor containing intron; MUT68, mutant 68 (terminal nucleotidyltransferase); Pol II, RNA polymerase II; pro, MIR gene promoter; RRP6, Ribosomal RNA processing6; ter, MIR gene terminator; ?, unknown protein factor.
In addition to encoding three DCLs, Chlamydomonas encodes three AGO proteins, Cre-AGO1 to Cre-AGO3 [110], and in the dcl3 and dus16 mutants where miRNA production is largely defective [111,112], the abundance of the cytoplasmic AGO, Cre-AGO3, was shown to be almost undetectable. This finding indicated that in the absence of loading of its preferred cargo, miRNA silencing signals, the Cre-AGO3 protein was destabilized, and therefore likely to be rapidly turned over in the cell [114]. Similarly, in the ago3-1 mutant, global miRNA abundance is reduced [114] to (1) indicate that mature miRNA silencing signals are rapidly turned over in the Chlamydomonas cell in the absence of loading into Cre-AGO3, and (2) further identify Cre-AGO3 as the primary AGO protein family member required to mediate miRNA-directed RNA silencing in Chlamydomonas. Protein pull-down experimentation and subsequent sequencing of the low molecular weight fraction to profile the sRNA landscape of Cre-AGO3, further showed that the majority of loaded sRNAs were between 20- to 22-nt in length, with most of these being 21-nt in length and which harbored a 5′ terminal uracil (U) residue [114]. This analysis indicated that like Ath-AGO1 [18,19], Cre-AGO3 has a strong loading preference based on sRNA size and starting nucleotide composition [114].
The initial characterization of miRNA-directed RNA silencing in Chlamydomonas, including the use of amiRNAs designed to have an extremely high degree of complementarity to their targeted genes, indicated that target transcript cleavage formed the predominant mode of miRNA-directed RNA silencing in this microalga [115,116,117]. However, subsequent reassessment attempts to confidently identify target genes for Chlamydomonas miRNAs using the well-established and stringent criteria for miRNA target gene identification in plants, failed to convincingly identify strong candidates as target genes whose expression is regulated by miRNA-directed transcript cleavage [114,118]. What the experimental analysis associated with this bioinformatic assessment did demonstrate though was that for the few miRNAs with a high degree of complementarity to their target transcript(s), mRNA cleavage indeed formed the primary mode of RNA silencing directed by this small subclass of Chlamydomonas miRNAs to control target gene expression [114,115,116,117,118]. Furthermore, this revised analysis to target gene identification showed that most Chlamydomonas miRNAs only have limited complementarity to their target genes across the seed region of the miRNA, and that putative target sites are frequently positioned in, or proximal to, the 3′ UTR of the target transcript. Given these characteristics shared with animal miRNAs, it is unsurprising that translational repression appears to be the predominant form of RNA silencing directed by this subgroup of miRNAs with only ‘seed region’ matches to their target gene transcripts [114,118,119,120,121] (Figure 3). Interestingly, seed region complementarity between a miRNA and its putative targets, led [120] to predict that each Chlamydomonas miRNA returned a high enough degree of seed region complementarity to putatively target ~10 protein-coding sequences for expression regulation. Together, these works [114,118,119,120,121] have shown that the cytoplasm localized AGO, Cre-AGO3, forms the catalytic core of miRISC in Chlamydomonas, and interestingly that Cre-AGO3 can direct both a mRNA cleavage and a translational repression mode of RNA silencing to control target gene expression: distinct mechanisms of target gene expression regulation that appear to be determined based on the degree of complementarity of the Cre-AGO3-loaded miRNA, to its target gene transcripts.
The terminal nucleotidyltransferase, Cre-MUT68, has also been shown to play a core functional role in the Chlamydomonas miRNA pathway via controlling the steady state levels of miRNAs and siRNAs (Figure 3). The Cre-MUT68 nucleotidyltransferase was originally shown to add untemplated nucleotides to the 5′ cleavage fragments generated by RISC-catalyzed slicing of sRNA target transcripts to identify such cleavage products for decay [122]. Subsequent to the identification of a RISC-generated cleavage fragment clearance role for Cre-MUT68, characterization of the Chlamydomonas mut68 mutant revealed that the levels of numerous miRNAs were elevated in this mutant background along with an elevated abundance of stabilized RISC generated 5′ cleavage fragments [122]. In addition to elevated miRNA levels, Cre-AGO3 protein abundance was also elevated in the mut68 mutant background, which was likely to be the result of a feedback mechanism to attempt to accommodate the increased abundance of the global miRNA population in the mut68 mutant. Further molecular analysis of the mut68 mutant, and of the wild-type Cre-MUT68 protein itself, showed that with respect to miRNAs, Cre-MUT68 functions to preferentially add a tail of U residues to the 3′ terminal nucleotide of miRNAs (i.e., 3′ terminal uridylation), a sequence modification which identifies the U-tailed miRNA for its degradation by the exosome [122,123]. Using an in vitro system, the authors [122] went on to demonstrate that Cre-MUT68 could form a functional interaction with the 3′ to 5′ exoribonuclease and exosome subunit protein, Ribosomal RNA processing6 (Cre-RRP6) (Figure 3), which in the in vivo setting, would ensure tight regulation of maintenance of miRNA steady state levels via the efficient removal of damaged or unrequired miRNAs in Chlamydomonas cells [122]. The quality control surveillance mechanism performed by Cre-MUT68 in the Chlamydomonas miRNA pathway is highly similar to that performed by the nucleotidyltransferase HEN1 SUPPRESSOR1 (Ath-HESO1) in Arabidopsis which uridylates unmethylated miRNAs (Figure 1) and siRNAs to tag these RNA fragments for removal from the Arabidopsis cell [124]. The terminal uridyltransferase, Dme-Tailor, has also been shown to involved in the modification of mirtron pre-miRNAs to control the degree of contribution that the mirtron subclass of miRNAs makes to the global miRNA population of Drosophila [125]. Therefore, uridylation of miRNAs, or their precursor transcripts, appears to form a conserved mechanism to add an additional layer of complexity to the miRNA pathways of plants, animals and microalgae [122,123,124,125].
5. The microRNA Pathway of Green, Brown and Red Seaweed
5.1. microRNAs in the Chlorophyta
Of the Chlorophyta (green algae), the majority of knowledge gained to date on the miRNA pathway has been generated from the molecular characterization of C. reinhardtii. The initial miRNA studies in Chlamydomonas indicated that well over 100 miRNAs were present in this unicellular green microalga [28,33], with a subsequent study [126] suggesting that over 300 miRNAs were present in C. reinhardtii. The actual number of miRNAs in Chlamydomonas remains to be experimentally validated, however an excellent bioinformatic study which reassessed the initial predictions for C. reinhardtii indicated that the number of bona fide miRNAs in Chlamydomonas is likely to be much reduced with many candidates failing to meet the more stringent identification/annotation criteria established for plant miRNAs [35]. Moreover, the [35] reassessment of the [28,33] datasets more accurately reassigned many of the initially identified miRNAs to have originated from a siRNA-generating precursor transcript, and not from a precursor which would be capable of folding to form the dsRNA structure required to be recognized as a processing substrate by Cre-DCL3 for miRNA production.
In addition to C. reinhardtii, the miRNA profile of the multicellular green microalga, Volvox carteri has also been constructed with V. carteri traditionally serving as an important species to study the mechanistic basis of the developmental processes of embryonic cleavage, morphogenesis, and cell differentiation [127,128]. Profiling of both the gonidia and somatic cells of V. carteri identified ~170 putative miRNA candidates with the abundance of 16 miRNAs experimentally confirmed via the northern blot nucleic acid hybridization approach [129]. Of particular interest was the identification of cell type-specific enrichment of some of the identified V. carteri miRNAs in gonidia and somatic cells, and furthermore, the predicted target genes of these cell type-enriched miRNA populations were shown to have an opposing expression trend to the abundance of their potential miRNA regulators in these two developmentally distinct cell types [129]. This finding suggested, that like plant and animal miRNAs, green algae miRNAs potentially mediate key roles in regulating the expression of genes crucial for driving the development of V. carteri, and potentially of other microalga species.
In the green microalgae species Chlorella sorokiniana, Coccomyxa subellipsoidea and Botryococcus braunii, 98, 124, and 14 miRNAs, respectively, have been identified [130,131,132]. Interestingly, these analyses have generated differing results with respect to the composition of the individual miRNA populations identified in each microalga, with an abundance of species-specific miRNAs reported in C. sorokiniana (i.e., 60 out of 98 miRNAs were unique to C. sorokiniana) [130] versus a very high degree of conservation for the miRNAs identified in C. subellipsoidea with 118 out of 124 miRNAs detected in C. subellipsoidea previously reported for other organisms [131]. Such differences are likely accounted for by (1) the algorithmic basis of the approach used for the initial miRNA identification in each species, together with (2) the degree of stringency of the annotation process post miRNA identification via sequencing. Another interesting finding which stems from the miRNA profiling of these green microalgae is that aside from the V. carteri findings [129], target gene assessment in these Chlorophyta microalgae has inferred that the regulatory role directed by miRNAs is highly focused on controlling the expression of genes involved in biological processes such stress responses, chlorophyll production and photosynthesis, lipid, sucrose and starch metabolism, and the biosynthesis of secondary metabolites [130,131,132]. This is in direct contrast to those findings previously reported in both the plant and animal systems where miRNA-directed gene expression regulation has been demonstrated to be an essential molecular component of development. Specifically, a less essential role in controlling developmental gene expression in the green microalgae is most readily demonstrated by the mutational work conducted in C. reinhardtii with the Chlamydomonas dcl3, dus16 and ago3 mutants all displaying largely wild-type-like phenotypes irrespective of the extent of dysfunction of the miRNA pathway [112,113,114].
Acting as regulators of gene expression outside of development in algae is further evidenced by miRNAs studies in two other green microalgal species, namely Dunaliella salina and Haematococcus pluvialis [133,134,135,136]. Due to its high salinity tolerance, D. salina is widely distributed globally in salt lakes with D. salina accumulating high amounts of β-carotene in plastid-localized lipid globules, which together marks D. salina as an ideal model to study the molecular basis of salt tolerance in plants [133], and as a bioresource to produce β-carotene for use in industry or for pharmaceutical applications [134]. A large collection of known and novel miRNAs has been reported in D. salina [133,134]. However, although the morphology of D. salina changes considerably in an altered growth environment (i.e., high salt or intense light), the majority of the predicted target genes for the miRNAs which changed in their abundance in an altered growth environment where categorized by Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to function in metabolic pathways, including the metabolism of amino acids, nucleotides, energy, cofactors and vitamins, and to not be involved in regulating cell growth or development [133,134]. In suitable growth environments, the unicellular alga H. pluvialis exists in its motile green vegetative form, however, in a nutrient poor environment, H. pluvialis transitions into the chlamydospore stage of its lifecycle [135]. In this stage of its lifecycle, H. pluvialis losses its flagellum to become nonmotile, its cell wall thickens, and it turns red in color via accumulating a high abundance of the carotenoid molecule, astaxanthin, an important additive to both human and animal feed [135]. Assessment of the miRNA profile of H. pluvialis identified 405 miRNAs with homology to known plant miRNAs [135,136], and these were further grouped into 75 MIR gene families, including grouping into the highly conserved plant MIR156, MIR159, MIR160, MIR164 and MIR167 gene families [135]. As reported for D. salina miRNAs, Gene Ontology (GO) and KEGG analyses showed that the putative target genes of H. pluvialis miRNAs function in cellular processes such as fatty acid biosynthesis, regulation of ATP and transporter activity, high light and iron stress responses, and most interestingly, regulation of the astaxanthin biosynthesis pathway [135]. The authors [136] went on to show that specific miRNAs are preferentially loaded into the extracellular vesicles (EVs) released by both the motile and nonmotile forms of H. pluvialis. Furthermore, many of the EV-enriched miRNAs were subsequently shown to putatively regulate the expression of target genes involved in biological processes relating to EV formation and transport, such as being grouped into the functional categories; cell junction, membrane-enclosed lumen, membrane-part, membrane, organelle part, and organelle [136]. This forms a highly interestingly result as it not only demonstrates the ability of microalgae to form EVs which load specific miRNA cargo, but this finding additionally infers that miRNA-loaded EVs may play a role in cross-kingdom communication between microalgae and their interacting species. Surprisingly, despite the growing volume of work on miRNA biology in Chlorophyta microalgae, to date, no characterization of the miRNA population of a green seaweed species has been reported.
5.2. microRNAs in the Phaeophyceae
Like the Chlorophyta, the Phaeophyceae (brown algae) form a large group of photosynthetic organisms, however, via comparison, the brown algae have undergone a much longer and largely independent evolution from their last common ancestor of land plants [137]. Due to its wide global distribution and perceived importance for the study of the evolution of multicellularity in the Phaeophyceae and other seaweeds, the genome of Ectocarpus siliculosus was published in 2010 [138], a considerable undertaking which also detailed the presence of homologs of the core pathway machinery proteins Esi-DCL1 and Esi-AGO1, as well as to report on the identification of 26 miRNAs. The identified miRNA sRNAs, displayed features similar to those of their plant counterparts, with 24/26 Esi-miRNAs expressing a 5′ terminal U residue and being 21-nt in length at maturity. At the genome level, the Esi-miRNAs also displayed similarities to animal miRNAs, with 17 out of the 26 identified miRNAs determined to have been processed from the intron of a protein-coding host gene [138]. In a subsequent miRNA study of E. siliculosus, an extremely large set of over 500 putative miRNA candidates were reported [139], although many of the proposed candidates were shown to vary in length compared to the expected predominant size of mature plant and animal miRNA silencing signals at 21-nt and 22-nt, respectively. However, via quantitative reverse transcriptase polymerase chain reaction (RT-qPCR)-based approaches, the authors did experimentally validate the expression of 22 mature miRNA candidates as well as to confirm the presence of a precursor transcript for 8 of the 22 experimentally validated Esi-miRNAs. The RT-qPCR approach was also used to show that the expression of Esi-DCL1 and Sci-AGO1 was altered by the cultivation of E. siliculosus in a high salt environment, and further, that the abundance of some of the characterized Esi-miRNA candidates was also altered accordingly [139]. Via the use of stringent selection criteria, the [138,139] E. siliculosus miRNA datasets were reanalyzed by [43], with this analysis firmly establishing that the size of the total miRNA population of E. siliculosus is likely composed of ~63 MIR gene families. Findings stemming from this study of particular interest included (1) the discovery that only one of the 63 identified MIR gene families contained multiple members, (2) the identification of only two MIR gene clusters (i.e., individual MIR genes within five kilobases (kb) of each other), (3) mapping of the origin of 75% of the miRNAs to protein-coding host gene sequences (44 mapped to introns and 3 mapped to UTRs), and (4) that all experimentally validated miRNAs were at an equivalent level of abundance in both the sporophyte and gametophyte generations of the E. siliculosus lifecycle [43]. This final finding adds further weight to the suggestion that miRNAs are not important regulators of the expression of genes crucial to the development of algae.
Saccharina japonica (previously Laminaria japonica) is a cold temperature species of brown seaweed which is extensively cultivated on ropes for use as a human feed source in the eastern coastal regions of China and Korea as well as western Japan. In the approximate 100-year period that S. japonica has been grown in this region its area of cultivation has expanded greatly, including expansion into cultivation zones of temperate and even subtropical waters [140]. The expansion of the growing region of cultivated S. japonica cultivars infers that these cultivars have been adapted to growth in warmer waters, a suggestion which led [141] to investigate the contribution which miRNAs may have had at the molecular level to the adaptability of cultivated S. japonica to grow at higher temperatures [141]. This research identified 49 conserved and 75 novel miRNAs in S. japonica, with the majority of both miRNA classes identified in the control and elevated temperature samples of S. japonica. Moreover, 7 conserved and 25 novel Sja-miRNAs were determined to significantly differentially accumulate when S. japonica was cultivated at a higher growth temperature, suggesting that these 32 miRNAs might represent heat stress responsive miRNAs [141]. A second S. japonica study [142] identified a highly similar number of miRNA candidates (n = 117) to the 124 miRNAs identified by [141], and went on to group the 117 miRNAs identified into 98 MIR gene families. Of particular surprise was the finding that none of the miRNAs identified in S. japonica shared any sequence similarity to the miRNAs previously catalogued for E. siliculosus [43,142]. Saccharina and Ectocarpus separated from their last common ancestor ~95 Mya to show that miRNA populations of brown seaweeds evolve very rapidly. Therefore, this finding added additional weight to the indication that in direct contrast to the plant and animal miRNA systems where core sets of MIR gene families are strictly conserved, such an evolutionary requirement is not observed in algae, including both microalgae and the seaweeds. Furthermore, in animal lineages, miRNA evolution from genomic hairpins appears to be the most predominant mechanism of new miRNA generation [143], and considering that none of the S. japonica or E. siliculosus miRNAs showed any real degree of sequence similarity to repeat sequences or protein-coding genes as has been repeatedly reported in plants [43,142], this also may form the driving mechanism of rapid MIR gene evolution in seaweeds.
5.3. microRNAs in the Rhodophyta
5.3.1. microRNAs in Red Microalgae
The Rhodophyta (red algae) belongs to the Plantae supergroup and as such forms a large group of micro- and macroalgal photosynthetic organisms due to their common ancestor having hosted a cyanobacterium which subsequently evolved into a plastid via the process of primary endosymbiosis [144,145]. The light-harvesting pigment molecules phycoerythrin and phycocyanin accumulate to high abundance in red algae, with their levels masking the other photosynthesis-related molecules such as the chlorophylls and other predominant carotenoids, a piment molecule profile which gives the Rhodophyta their distinct red color as well as to enable numerous species within this phylum to inhabit diverse environments [146,147,148,149]. Further, numerous Rhodophyta serve as important sources of industrial or medical products due to their biosynthesis of unique biomolecules such as agar and carrageenan gels [146,147,148,149], and this has led to the profiling of the sRNA populations of a restricted number of red micro- and macroalgae to determine the composition of the miRNA landscape in this unique and highly important algae phylum.
For red microalgae, the miRNA populations of the abundant antioxidant producing species Porphyridium purpureum, the industrially promising species Porphyridium cruentum, and the extremophilic heterotrophic species Galdieria sulphuraria have been reported [150,151,152]. Via a deep sRNA sequencing approach, ref. [150] bioinformatically identified an extensive population of 254 P. purpureum miRNAs composed of 203 known and 51 novel miRNAs. Northern blotting and RT-qPCR were used to experimentally validate a select cohort of Ppu-miRNAs to provide confidence that a biologically relevant population of miRNAs had been identified for P. purpureum [150]. Bioinformatic predictions via GO analysis of Ppu-miRNA target genes suggested that most of the putative candidates encode proteins involved in protein transport, signal transduction, and photosynthesis-related processes, including chloroplast membrane production and maintenance, chloroplast relocation, and photoreceptor activity [150]. In the closely related Porphyridium spp., P. cruentum, 49 miRNAs were identified that were grouped into 46 MIR gene families. Interestingly, only two of the identified miRNAs (i.e., Pcr-miR156 and Pcr-miR419) were reported previously in P. purpureum [150], a finding which once again indicates negligeable MIR gene conservation in the algae, even between very closely related species [150,151]. However, in spite of the lack of miRNA conservation between these two Porphyridium spp., proteins such as Ribulose-1,5-bisphosphate carboxylase oxygenase (RuBisCO: rate limiting enzyme of photosynthesis), light harvesting complex subunits, phycobiliproteins, oxygen-evolving enhancer proteins, and granule-bound starch synthase, all of which perform essential roles in photosynthesis, were also identified in P. cruentum as putative miRNA target genes [151], as where many of those putative target genes identified for P. purpureum miRNAs [150].
Galdieria sulphuraria has evolved to acquire unique biological properties which allow the species to thrive in extreme environments including hot springs, acidic soils and volcanic rocks [153,154]. The miRNA population of G. sulphuraria was constructed as a first step towards uncovering a role for miRNA-directed gene expression regulation in the abiotic stress adaptability of this extremophile red microalgae [152]. This endeavor uncovered the presence of 134 Gsu-miRNAs, including 120 known and 14 novel miRNAs, with a small subset of those identified experimentally validated via sRNA northern blotting and RT-PCR. Curiously, 21 miRNAs returned homology to members of highly conserved plant MIR gene families, whereas no sequence homology was found between G. sulphuraria and C. reinhardtii miRNAs. Additional characteristics for the G. sulphararia miRNA population included that ~92% formed single member MIR gene families, a U residue was the predominant 5′ terminal nucleotide, and the miRNAs were predominantly 21-nt in length [152]. The authors went on to reveal that organic substance metabolic processes, chloroplast and thylakoids, electron transport chain, and electron transport, formed the major functional groupings for most putative target genes identified for both the known and novel Gsu-miRNA populations [152].
5.3.2. microRNAs in Red Macroalgae
Porphyra yezoensis (syn. Pyropia yezoensis) is a red seaweed which inhabits the intertidal zone of shallow cold seawaters and is widely cultivated in China, Japan and Korea where it is used to produce sea vegetable-based products, as well as forming a traditional source of edible seaweed in Ireland and Britain [155,156]. Of the ~70 species which belong to the Porphyra/Pyropia complex worldwide, P. yezoenesis is viewed as the best model species owing to its long-established use in biological research and for its agronomic importance [155,156]. P. yezoensis has a dimorphic lifecycle composed of two highly morphologically distinct generations, the sporophyte (microscopic diploid form) and gametophyte (macroscopic haploid form) generations [157]. At the molecular level, gene expression analysis via the use of available, yet limited expressed sequence tag (EST) data, showed that less than a quarter of P. yezoensis genes are active across its two lifecycle generations [157]. This finding prompted [158,159] to profile the miRNA population of P. yezoensis to determine the contribution of miRNA-directed gene expression regulation to the development of two such morphologically distinct forms of this red seaweed. The initial miRNA profiling exercise in 2010 [158] identified 224 candidate Pye-miRNAs, including 53 Pye-miRNAs which only showed a low level of sequence conservation to known plant miRNAs (and which were subsequently redescribed as P. yezoensis-specific miRNAs), 41 Pye-miRNAs which matched previously identified C. reinhardtii miRNAs (with 17 of these returning high identity scores), 54 Pye-miRNAs which are conserved in the ground moss Physcomitrella patens, and 16 Pye-miRNAs with no sequence identity to any other previously reported miRNA isolated in either plants or algae (i.e., classed as novel or species-specific Pye-miRNAs). The target sequences of the identified Pye-miRNAs were found to be primary located in the coding region of putative target genes with identified targets assigned to a wide range of biological functions, including chromatin structure and dynamics, translation – ribosome structure and biogenesis, and signal transduction. Interestingly, the genes putatively targeted for expression regulation by the 16 novel miRNAs identified were determined to largely encode proteins of no known molecular function, a finding that tentatively suggests that these target genes may perform functions specific to the red seaweeds, or even to P. yezoensis itself [158].
In direct contrast to this initial study, a subsequent P. yezoensis miRNA study, which applied the much more stringent rules used for miRNA identification in plants, only identified 14 Pye-miRNA candidates [159]. Further, the authors [159] showed that the pre-miRNA precursor transcripts of the 14 identified Pye-miRNAs shared structural properties highly similar to those previously reported for well-characterized plant miRNA precursor transcripts. In addition, this work revealed that only a single Pye-miRNA was expressed during both the sporophyte and gametophyte generations of the P. yezoensis lifecycle, while seven and six Pye-miRNAs were exclusively expressed in the sporophyte and gametophyte generation, respectively [159]. This finding infers that these 13 Pye-miRNAs may mediate generation-specific molecular processes in P. yezoensis. Unfortunately, the absence of genome sequence data, and molecular resources (e.g., the availability of extensive EST libraries), resulted in the failure of [159] to confidently predict the likely biological functions of most identified putative target genes for the 14 Pye-miRNAs isolated in their study.
Chondrus crispus is an intertidal red seaweed which naturally inhabits the rocky shorelines of the north Atlantic coast, and like other red seaweeds, C. crispus has a complex cell wall composed of cellulose, hemicelluloses, and unique sulphated galactans such as carrageenan [160,161]. Such red algal-specific molecules have rocketed numerous Rhodophyta species into the research spotlight, including C. crispus, considering that they have wide industrial applications as well as to serve as an important food source for humans and livestock: uses worth hundreds of millions of dollars annually [162,163]. Sequencing and annotation of the ~105 megabase (Mb) C. crispus genome revealed that most genes are of small size being composed of a single exon (monoexonic), and of those protein-coding sequences which harbor introns, the introns are widely dispersed and are of a short length [164]. It is interesting to note that those genes with introns are expressed at a higher level than monoexonic genes, a finding which indicates an important regulatory function for intronic sequences in Chondrus. Although most genes are of small size and lack introns, C. crispus was revealed to encode both a Ccr-DCL and Ccr-AGO endonuclease to tentatively suggest that sRNA silencing pathways are functional in this red seaweed [164]. In addition to encoding these core machinery proteins of RNA silencing, the C. crispus nuclear genome was revealed to harbor a large, complex, and functionally diverse set of genes involved in halogen metabolism related processes, including heme peroxidases, phosphatidic acid phosphatases, halo peroxidases, haloalkane dehalogenases, and haloacid dehalogenases [164]. These genome-based findings identified C. crispus as an excellent model to study miRNA-directed molecular control of halide chemistry and halogen metabolism.
The combined use of sRNA sequencing together with the bioinformatic analysis of available C. crispus EST data, enabled the identification of 117 Ccr-miRNAs, including 108 known miRNAs and nine novel Ccr-miRNAs [165]. These 117 miRNAs were subsequently grouped into 110 MIR gene families, including 103 single member families and five, one and one MIR gene families which included two, three and four members, respectively. In addition, the most frequent length of C. crispus miRNAs upon maturity was 19-nt, and not 21-nt like plant miRNAs to which many of the known Ccr-miRNA displayed sequence homology [165]. Northern blot hybridization (n = 3) and RT-qPCR (n = 18) were also used to experimentally validate 21 randomly selected Ccr-miRNA sRNAs. The authors used plant miRNA targeting requirements of near perfect complementarity between the targeting miRNA and the target site of the regulated mRNA to identify 160 putative target genes for the 117 Ccr-miRNAs. Target gene GO analysis indicated that most putative targets function in nucleotide binding, protein folding, and intracellular membrane-bound organelle processes. Of particular interest was the finding that via the use of KEGG mapping, 22 genes identified as putative targets of Ccr-miRNA-directed expression regulation, perform functional roles in peroxisome proliferator-activated receptor (PPAR) signaling pathways [165]. This finding strongly suggests that such signaling pathways require exquisite regulatory control in C. crispus, and potentially in other red seaweed species.
Together, Shulian Xie’s group have identified 469 MIR gene families, composed of 503 members, across three representative red algal species, including the red microalgae P. purpureum [150] and G. suphuraria [152], and the red seaweed C. crispus [165]. Via comparative analysis of the three miRNA datasets generated by this research group, 64 miRNA homologs were identified between P. purpureum and G. suphuraria. In addition, this analysis showed that 72 and 186 MIR gene family representatives were conserved between C. crispus and P. purpureum, and C. crispus and G. suphuraria, respectively [150,152]. Many of the miRNAs conserved in these red algal species were originally identified in plants, including some of the most highly conserved MIR gene families in plants, such as the MIR156, MIR159, MIR164, MIR165/166, MIR168, MIR169, MIR396 and MIR398 families. The authorship team then went on to use the miR156 sRNA sequence for further analysis and showed that the sequences of Ppu-miR156, Gsu-miR156 and Ccr-miR156, retained an approximate 90% identity to the 294 mature miR156 sequences entered into miRBase from 47 plant species. This analysis also showed that although the PRE-MIR156 precursor transcripts had diverged widely in plants, and the three assessed red algal species, the mature miR156 sequence has remained highly conserved to indicate that miR156 controls the expression of genes which perform an essential function in both plants and red algae [152]. In Arabidopsis, the MIR156 gene family contains ten members (Ath-miR156a to Ath-miR156j) with family members regulating the expression of a small subclade of the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factor gene family, including SPL9 and SPL10, which function together to initiate the transition of Arabidopsis from vegetative to reproductive development [1,2]. In C. crispus however, the predicted target gene of Ccr-miR156, CHC_T00006037001, encodes a putative arsenite/tail-anchored protein-transporting ATPase protein possibly involved in arsenite transport across cell membranes for detoxification [152]. This finding therefore raises the intriguing question of why would the mature miR156 sequence be so tightly conserved between plants and red algae when miR156 regulates the expression of such functionally distinct target genes?
Eucheuma denticulatum, commonly known as “Spinosum”, is an important cultivated red seaweed species in warmer tropical waters forming an important source of carrageenan in the food industry and dietary fiber for blood health [166,167,168]. In another study from Shulian Xie’s group, 133 miRNAs were identified for E. denticulatum, which included homologs of members of 21 highly conserved plant MIR gene families such as the MIR164, MIR166, MIR169, MIR397 and MIR398 gene families [169]. The authors went on to experimentally validate three and 13 Ede-miRNAs via sRNA-specific northern blot hybridization and RT-qPCR, respectively. Considering the high degree of conservation of the identified Ede-miRNAs with plant miRNAs, plant parameters were applied to putatively identify 871 targets for the 133 Ede-miRNAs with ~70% of identified targets determined to likely be under transcript cleavage-based expression regulation, and the remaining ~30% possibly forming targets for the translation repression mode of miRNA-directed RNA silencing [169]. Furthermore, KEGG analysis revealed that the most significantly enriched pathways for the identified putative target genes were biosynthesis of secondary metabolites and metabolic pathways, including the enrichment of both the porphyrin and chlorophyll metabolism pathways.
Gracilaria lemaneiformis (syn. Gracilariopsis lemaneiformis) is also a widely cultivated red seaweed due to its high yield potential and is used to produce a considerable volume of agar and other commercially important polysaccharides and as a feed source in aquaculture [170,171]. As part of a large-scale transcriptome analysis of G. lemaneiformis, ref. [172] identified 91 miRNAs with 23, 28 and 40 Gla-miRNAs returning homology to known plant, microorganism and animal miRNAs, respectively. The growth and development, and therefore yield potential of G. lemaneiformis is adversely affected by higher seawater temperatures, and by heavy metal pollutants [173,174]. The expression of the precursors of three Gla-miRNAs with homology to mammalian miRNAs was therefore assessed in G. lemaneiformis samples cultivated at either a high temperature or in the presence of heavy metals. However, the expression of the pre-miRNAs of the three analyzed miRNAs showed little variation across the samples cultivated under different growth conditions [172]. Therefore, the extent of the role of miRNAs in the regulating the growth and development of G. lemaneiformis in less favorable environmental conditions could not be ascertained by this work.
In addition to G. lemaneiformis, the miRNA profile of a second Gracilaria species., G. vermiculophylla has been established [175]. Gracilaria vermiculophylla (syn. Gracilariopsis vermiculophylla) is a red seaweed species native to the coasts of the northwest Pacific Ocean, but over the last approximate 100 years, this seaweed has spread (via its unintentional introduction) to growing regions in the northwestern coastlines of the African continent, as well as to European coasts and North American shores [176,177] where it has thrived due to its enhanced tolerance to environmental stress and being less palatable to the herbivores native to its introduced areas of growth [178,179]. Profiling of the miRNA populations of G. vermiculophylla tetrasporophytes sampled from geographically distinct locations (i.e., the east coasts of North America and Japan and the northwest coast of Germany), established a large population of both known and novel miRNAs, many of which were also shown to have differing abundance levels in each assessed G. vermiculophylla tetrasporophyte sample [175]. In addition, for a number of the differentially accumulating miRNAs, some of the predicted gene targets displayed expression trends which opposed those of their potential miRNA regulator, sRNA and mRNA abundance trends which tentatively indicate that miRNA-directed target gene expression regulation may play a role in the adaptability of G. vermiculophylla to flourish in diverse aquatic environments.
5.3.3. The miRNA Pathway of the Red Seaweed Asparagopsis taxiformis
Asparagopsis taxiformis is a red seaweed species which is widely distributed globally in warm to tropical seawaters [180,181,182] and has garnered considerable attention in recent years stemming from its synthesis of biomolecules of unique chemical composition for use in agriculture, or the food technology and pharmaceutical industries [183,184,185,186,187]. Namely, A. taxiformis is unique in its ability to not only synthesize the halogenated organic compound bromoform, but to store bromoform at high quantities in specialized gland cells which can constitute up to 4.0% of the total dry weight of A. taxiformis [188]. Bromoform has antimicrobial activity and can alter the gut microbiome of ruminant livestock [189,190,191]. Specifically, when A. taxiformis is included in ruminant feedstock at levels as low as 2.0% of feed dry matter, the bromoform provided by A. taxiformis supplementation can reduce methane production by ruminants to almost undetectable levels [189,190,191]. Considering their demonstrated role as master regulators of gene expression, miRNA research should form a central component of the future molecular characterization of A. taxiformis, for this emerging commercial crop with industry investments across the Indo-Pacific region. A draft genome is available for A. taxiformis, and therefore, miRNA investigations would provide researchers and industry with an additional and novel avenue to gain a full understanding of all genetic components of the bromoform and other natural product biosynthesis pathways of this red seaweed species. As a first step towards achieving this goal, we have recently started to interrogate our genomic [192] resources to attempt to establish the extent to which the miRNA biosynthesis pathway exists in A. taxiformis. Via this approach, we have identified at high confidence, direct homologs of the core protein machinery of the Arabidopsis, Drosophila and Chlamydomonas miRNA pathways. Specifically, Figure 4A provides a schematic representation of the proposed miRNA pathway for A. taxiformis based on the pieces of homologous protein machinery uncovered by the bioinformatic interrogation of our A. taxiformis genomic data. In addition, Figure 4B details the functional domain landscape of each piece of miRNA pathway protein machinery identified as part of our bioinformatic analyses. The full amino acid sequence of the coding sequences of each piece of protein machinery core to the A. taxiformis miRNA pathway is also provided in Table S1.
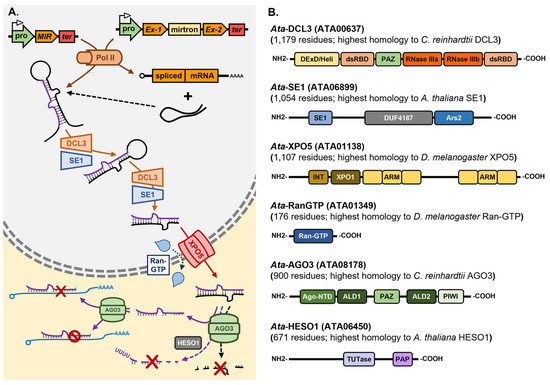
Figure 4.
Proposed miRNA pathway for the red seaweed Asparagopsis taxiformis. (A) Pol II transcribes pri-miRNAs from MIR genes or mirtron precursors from protein-coding host genes. DCL3, with the assistance of the RNA-binding protein SE1, processes pri-miRNAs into pre-miRNAs and then pre-miRNAs into miRNA/miRNA* duplexes in the nucleus of A. taxiformis cells. The miRNA/miRNA* duplex is exported from the nucleus to the cytoplasm of the A. taxiformis cell by a Ran-GTP-dependent XPO5-mediated process and is loaded into AGO3. After the removal and degradation of the miRNA* passenger strand, AGO3 uses the loaded miRNA as a sequence specificity guide to direct the silencing of miRNA target genes via either mRNA cleavage or the translational repression mode of gene expression control. The steady-state levels of miRNAs are controlled in the A. taxiformis cell cytoplasm by the activity of the nucleotidyltransferase, HESO1. AGO3, ARGONAUTE3; DCL3, DICER-LIKE3; HESO1, HEN1 SUPPRESSOR1; MIR, MICRORNA gene; mirtron, miRNA precursor-containing intron; Pol II, RNA polymerase II; pro, MIR gene promoter; ter, MIR gene terminator; Ran-GTP, RAS-related nuclear protein GTPase; SE1, SERRATE1; XPO5, EXPORTIN-5. (B) Schematic representation of the functional domain landscape of Asparagopsis homologs of the core machinery proteins of the Arabidopsis, Drosophila, and Chlamydomonas miRNA pathways including Ata-DCL3, Ata-SE1, Ata-XPO5, Ata-RanGTP, Ata-AGO3, and Ata-HESO1. Ago-NTD, Argonaute N-terminal domain; ALD, Argonaute linker domain; ARM, Armadillo repeat domain; Ars2, Arsenite-resistance2 domain; DExD/Hel, DExD/H-box helicase domain; DUF4187, domain of unknown function 4187; dsRBD, dsRNA-binding domain; INT, Importin-β N-terminal domain; PAP, nucleotide poly A polymerase domain; PAZ, Piwi, Argonaute, and Zwille domain; PIWI, Piwi domain; Ran-GTP, Ran small GTPase domain; RNase III, Ribonuclease III domain; SE1, Serrate1/Ars2 N-terminal domain; TUTase, nucleotidyltransferase domain; XPO1, Exportin1-like domain.
Interestingly, although this approach readily identified direct A. taxiformis homologs of the protein machinery essential for miRNA precursor transcript processing in the nucleus of eukaryotic cells, Ata-DCL3 and Ata-SE1 (homologs of Ath-DCL1/Dme-Drosha/Cre-DCL3 and Ath-SE1/Dme-Ars2, respectively), we failed to identify any candidate gene which could encode for a homolog of the DRB proteins, Ath-DRB1, Dme-Pasha and Cre-DUS16 [64,86,87,112,113]. In Arabidopsis, Drosophila and Chlamydomonas, Ath-DRB1, Dme-Pasha and Cre-DUS16, have been demonstrated to form functional partnerships with the Dcr endonucleases Ath-DCL1, Dme-Drosha and Cre-DCL3, respectively [65,86,113]. Once formed, these protein partnerships ensure accurate and efficient miRNA production right at the very commencement of their respective miRNA biogenesis pathways. The absence of a DRB homolog suggests that at this stage of the A. taxiformis miRNA pathway, it operates most analogously to the Chlamydomonas miRNA pathway where Cre-DUS16 appears to solely provide functional support to Cre-DCL3 for miRNA precursor transcript processing [112,113]. This would be in direct contrast to the functional processes operating at this stage of the plant and animal miRNA pathways with Ath-DCL1 requiring the assistance of both Ath-SE1 and Ath-DRB1 for miRNA precursor transcript processing in Arabidopsis [61,62,63,64,65,68,70], and the functional interaction requirement of Dme-Drosha with Dme-Ars2 and Dme-Pasha for pri-miRNA processing in Drosophila [84,85,86,87,88]. We also failed to uncover an Ath-HEN1 homolog in our datasets. However, this was not unexpected as the 2′-O-methylation marking of sRNAs as part of their functional maturation is largely plant-specific [69,70], with neither Drosophila nor Chlamydomonas miRNAs being modified at their 3′ terminal nucleotide during their production.
In Drosophila, Dme-Drosha/Dme-Pasha processed pre-miRNAs are exported from the nucleus to the cytoplasm via the action of Dme-XPO5, a Ran-GTP-dependent dsRNA-binding protein [89,90], with Ath-HST performing a similar nucleus exportation process of mature miRNA sRNAs in Arabidopsis post processing from their precursors by the microprocessor complex (i.e., Ath-DCL1/Ath-DRB1/Ath-SE1) [72,73]. A. taxiformis was determined to encode both a Dme-Ran-GTP and a Dme-XPO5 homolog. Interestingly, no direct Dme-XPO5/Ath-HST homolog has been reported for Chlamydomonas to suggest that in the A. taxiformis miRNA pathway, the nucleus to cytoplasm shuttling of pre-miRNAs or mature miRNAs operates similarly to that of the Drosophila and Arabidopsis pathways. Our analyses also uncovered Ata-AGO3, an AGO protein with close homology to Chlamydomonas AGO3 [114,120,121]. However, to date, due to a lack of miRNA data for A. taxiformis, together with considerable conjecture as to whether AGO-loaded miRNAs direct either a transcript cleavage or translational repression mode of RNA silencing for target gene expression control in the algae [114,115,116,117,118,119,120,121], future detailed molecular assessment is required to determine whether Ata-AGO3 is a cleavage-competent AGO like Ath-AGO1. Finally, our bioinformatic interrogation of our A. taxiformis genomic data identified an A. taxiformis protein with extensive homology to Ath-HESO1 and Cre-MUT68 [122,123,124]. Moreover, the identified candidate protein, ATA06450, was named Ata-HESO1 considering that it returned a much higher degree of identity to Ath-HESO1 than to Cre-MUT68 [122,123,124]. Identification of Ata-HESO1 indicates that this A. taxiformis candidate protein putatively functions as a nucleotidyltransferase to uridylate either unmethylated miRNAs, or their precursor transcripts (like the action of Dme-Tailor as part of the production of mirtrons in Drosophila) to fine tune the rate of miRNA turnover. Considering this promising start to characterization of the A. taxiformis miRNA pathway, the next step must be to construct the profile of the global miRNA population of A. taxiformis to further compare and/or contrast this essential gene regulatory pathway in this rapidly emerging model red seaweed species to those well-established miRNA pathways in plants, animals and microalgae.
6. Conclusions and Future Perspectives
Profiling of the global miRNA populations of micro- and macroalgal species has revealed a considerable lack of MIR gene conservation with members of the same MIR gene family failing to be isolated in even closely related species which belong to the same phylum [43,142,150,151]. A comprehensive lack of MIR gene conservation in the algae is further evidenced via the repeated reporting that most algal MIR gene families are single member families [28,33,43,152,165], a finding that is indicative of rapid MIR gene evolution in the algae. There is also the requirement to reassess the miRNA profiles of those macroalgal species where considerable conservation to plant and microalgal miRNAs has been reported, and especially for those species where conservation to animal miRNA sequences has also been reported [150,152,158,165,192]. This will ensure that only bona fide miRNAs are identified. The [35] study provides an excellent example of this requirement. Namely, the initial assessment of Chlamydomonas indicated that the miRNA profile of this green unicellular alga was composed of well over 100 individual miRNAs [28,33,126]. However, the reanalysis of these datasets via the use of the more stringent criteria established for plant miRNA identification and/or annotation [35], greatly reduced the total number of Chlamydomonas miRNAs to a population of just over 60 MIR gene families. Similarly, the initial analysis of E. siliculosus suggested a miRNA population of over 500 unique sRNAs [139], however, the reanalysis of this data where the highly stringent set of criteria established for plant miRNA identification was applied, a global miRNA population composed of ~63 MIR gene families was recalculated for E. siliculosus [43]. Due to the variable length of plant miRNA precursors, a defined set of required characteristics have been proposed [193] for the confident identification of miRNAs in plants, including (1) the miRNA guide strand must be processed from the opposite stem arm to the miRNA* passenger strand, (2) the miRNA and miRNA* sequences must hybridize to each other with 2-nt overhangs present at the 3′ terminus of each duplex strand, (3) four or less mismatched base pairings between the miRNA and miRNA* strand of the hybridized duplex, (4) a small number of asymmetric bulges to be present in the stem-loop structured precursor with each composed of one or two nucleotides only, and (5) a stem-loop structured dsRNA can readily fold to form between the miRNA guide strand and miRNA* passenger strand [193]. The application of these criteria will allow for the confident identification of likely miRNA candidates in macroalgal species such the red seaweed A. taxiformis for which miRNA data currently does not exist.
In contrast to plant and animal miRNAs, functional predictions for the putative target genes of algal miRNAs suggest that many targets direct roles outside of development [150,151,152,158,159,165]. A minor to negligible role in regulating developmental processes for algal miRNAs is best evidenced by the wild-type-like phenotypes displayed by the C. reinhardtii dcl3, dus16 and ago3 mutants: mutants defective in the functional activity of the core protein machinery of the Chlamydomonas miRNA pathway, Cre-DCL3, Cre-DUS16 and Cre-AGO3, respectively [112,113,114]. Similarly, in the brown seaweed E. siliculosus, all experimentally validated miRNAs were shown to accumulate to a similar degree of abundance in both the sporophyte and gametophyte generations of the E. siliculosus lifecycle, a finding which indicates that most Esi-miRNAs regulate the expression of target genes that perform biological functions distinct to development [43]. Together, these findings infer that the miRNA population of each algal species under analysis should be determined to uncover the species-specific functions controlled by miRNA-directed gene expression regulation. Uncovering the miRNA population of each specific species of seaweed under assessment is also required as the basis of development of highly efficient miRNA technologies such as the development of amiRNA technology. The amiRNA technology has proven to be a highly useful tool in Arabidopsis and in other plant species for the further characterization of miRNA function, to provide resistance to invading pathogens, or for the manipulation of the biosynthesis pathways of agronomically important biomolecules [194,195,196,197]. The molecular manipulation of components of the miRNA pathway for miRNA biology research or to alter biomolecule biosynthesis pathways has also been successfully achieved in Chlamydomonas via the use of amiRNA technology [111,115,116,117]. Therefore, for seaweed species such as A. taxiformis where biological research of the miRNA pathway is only in its infancy, the requirement to establish the profile of the miRNA population of A. taxiformis for the future development of miRNA-based technologies is readily apparent. Namely, a highly abundant endogenous Ata-miRNA, one which is processed from a structurally simple precursor transcript, could be used as the basis to develop amiRNA technology to manipulate key rate limiting steps of the bromoform biosynthesis pathway to alter the levels of this agriculturally important biomolecule as part of the development of A. taxiformis as a model species for future biotechnological research on red seaweeds.
Finally, based on the data discussed in this review, we propose a practical workflow for the reliable large-scale identification and functional characterization of miRNAs in seaweeds, as summarized in the flowchart presented in Figure 5. Given the unique biological and ecological characteristics of seaweeds, miRNA research in this group of species requires careful consideration of sample collection strategies, sequencing quality control, bioinformatics analysis, and functional validation. Our workflow begins with comprehensive sample collection, ensuring the inclusion of diverse developmental stages and environmental conditions to minimize sampling bias. For seaweed species where a nuclear genome sequence is available, or where transcriptomic data exists, a bioinformatic approach should next be applied to identify core miRNA pathway machinery proteins such as the Dcr/DCL and AGO endonucleases. This initial bioinformatic-based analysis can be extended to ascertain the presence of essential functional domains within the identified pieces of core protein machinery, as well as to determine domain structure and composition, with the collective results of such analyses used to predict whether the miRNA pathway of the specific seaweed species under analysis in more functionally similar to the well-characterized pathways of plants, animals or microalgae. Comparing seaweed miRNA pathways to plant- and animal-like mechanisms would provide insight into the mode of action directed by individual members of the miRNA population, such as whether miRNA target gene expression is likely controlled via either a mRNA cleavage or translational repression mode of RNA silencing.
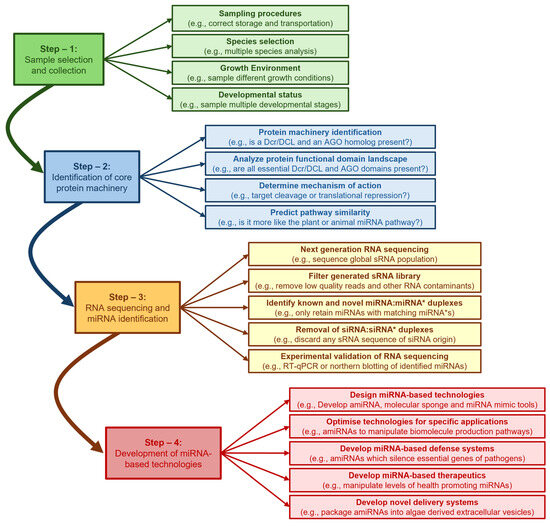
Figure 5.
Proposed best practice approach to miRNA identification, characterization, and technology development in seaweeds. The flowchart outlines the four key steps for identifying, analyzing and validating miRNAs in seaweeds. The process is divided into four major modules, which include (1) sample collection, (2) identification of core protein machinery and the inference of miRNA-directed regulatory mechanisms, (3) sRNA sequencing for sRNA library generation and miRNA identification and annotation via bioinformatics, together with experimental validation, and (4) application development. Quality control measures are emphasized at each step to ensure reliability and accuracy in miRNA discovery and functional validation.
Post core protein machinery identification, the generation of a high-quality sRNA sequencing library is essential. Then, bioinformatics is again required to apply rigorous filtering to remove low-quality reads and contaminants, as well as to ensure a sufficient sequencing depth to capture the typically low abundance of miRNAs. The bioinformatic miRNA identification process integrates structural predictions, conservation analysis, and verification of miRNA/miRNA* duplex formation to improve the accuracy of the process, while in parallel, accounting for the lower conservation levels observed for algal miRNAs. This step must also discard sRNAs originating from siRNA-generating loci, to reduce or even eliminate the likelihood of incorrectly identifying siRNAs as newly identified or species-specific miRNAs. Finally, the workflow extends to application development, including the design of amiRNA tools tailored to seaweed biology, with potential applications in aquaculture and biotechnology. This structured, stepwise approach offers a robust foundation for future research, facilitating high-confidence miRNA discovery and functional validation studies in non-model algal species. By establishing standardized methodologies, our proposed workflow enhances the reliability and reproducibility of miRNA research in seaweeds, paving the way for novel insights into regulatory networks and the potential development of novel biotechnological applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16040442/s1, Table S1: Amino acid sequences of candidate machinery proteins of the Asparagopsis taxiformis miRNA pathway.
Author Contributions
All authors were involved in the writing, reviewing, and editing of the original draft as part of preparing the submitted version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data discussed in this review are in the public domain.
Acknowledgments
The initial experimental characterization of the miRNA population of Asparagopsis taxiformis was made possible by an award from the University of the Sunshine Coast internal funding schemes SPARK and LAUNCH. The authors apologize for not being able to discuss or cite numerous studies in this review due to the specific focus of the article together with space restrictions. The authors also thank the Editor and two expert reviewers who provided insightful feedback to improve the article during the revision process.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AGO, Argonaute; amiRNA, artificial miRNA; Ars2, arsenic resistance protein2; Dcr, Dicer; DCL, Dicer-like; D-body, Dicing body; D-complex, Dicing complex; dsRNA, double-stranded RNA; dsRBD, double-stranded RNA binding domain; DRB1, dsRNA binding1; DUS16, Dull slicer-16; EST, expressed sequence tag; GO, Gene Ontology; GW182, glycine/tryptophan repeat protein 182; HEN1, Hua enhancer1; HESO1, HEN1 suppressor1; KEGG, Kyoto Encyclopedia of Genes and Genomes; Loqs, Loquacious; mRNA, messenger RNA; miRNA, microRNA guide strand; miRNA*, microRNA* passenger strand; MIR gene, miRNA gene; mirtron, miRNA containing intron; miRISC, miRNA-loaded RNA-induced silencing complex; MUT68, mutant 68; Mya, million years ago; ncRNA, non-coding RNA; nt, nucleotide; pol II, RNA polymerase II; pri-miRNA, primary-miRNA; pre-miRNA, precursor miRNA; Ran-GTP, RAS-related nuclear protein GTPase; RBM, RNA binding motif; RISC, RNA-induced silencing complex; RT-qPCR, quantitative reverse transcriptase polymerase chain reaction; SE1, Serrate1; spp., species; sRNA, small RNA; siRNA, small-interfering RNA; siRISC, siRNA-loaded RNA-induced silencing complex; UTR, untranslated region; U, uracil; XPO5, Exportin-5.
References
- Wang, J.W.; Czech, B.; Weigel, D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Park, M.Y.; Conway, S.R.; Wang, J.W.; Weigel, D.; Poethig, R.S. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 2009, 138, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Kapoor, A.; Zhu, J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 2006, 18, 2051–2065. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Chiou, T.J.; Lin, S.I.; Aung, K.; Zhu, J.K. A miRNA involved in phosphate-starvation response in Arabidopsis. Curr. Biol. 2005, 15, 2038–2043. [Google Scholar] [CrossRef]
- Boutet, S.; Vazquez, F.; Liu, J.; Béclin, C.; Fagard, M.; Gratias, A.; Morel, J.B.; Crété, P.; Chen, X.; Vaucheret, H. Arabidopsis HEN1: A genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. 2003, 13, 843–848. [Google Scholar] [CrossRef]
- Agorio, A.; Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 2007, 19, 3778–3790. [Google Scholar] [CrossRef]
- Eamens, A.; Vaistij, F.E.; Jones, L. NRPD1a and NRPD1b are required to maintain post-transcriptional RNA silencing and RNA-directed DNA methylation in Arabidopsis. Plant J. 2008, 55, 596–606. [Google Scholar] [CrossRef]
- Watahiki, A.; Wang, Y.; Morris, J.; Dennis, K.; O’Dwyer, H.M.; Gleave, M.; Gout, P.W.; Wang, Y. MicroRNAs associated with metastatic prostate cancer. PLoS ONE 2011, 6, e24950. [Google Scholar] [CrossRef]
- Pizzi, G.; Groppetti, D.; Brambilla, E.; Pecile, A.; Grieco, V.; Lecchi, C. MicroRNA as epigenetic regulators of canine cryptorchidism. Res. Vet. Sci. 2023, 162, 104961. [Google Scholar] [CrossRef]
- Clark, S.D.; Song, W.; Cianciolo, R.; Lees, G.; Nabity, M.; Liu, S. Abnormal Expression of miR-21 in Kidney Tissue of Dogs With X-Linked Hereditary Nephropathy: A Canine Model of Chronic Kidney Disease. Vet. Pathol. 2019, 56, 93–105. [Google Scholar] [CrossRef]
- Stefanon, B.; Cintio, M.; Sgorlon, S.; Scarsella, E.; Licastro, D.; Zecconi, A.; Colitti, M. Regulatory Role of microRNA of Milk Exosomes in Mastitis of Dairy Cows. Animals 2023, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Llave, C.; Kasschau, K.D.; Rector, M.A.; Carrington, J.C. Endogenous and silencing-associated small RNAs in plants. Plant Cell 2002, 14, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Krogh, A. Computational evidence for hundreds of non-conserved plant microRNAs. BMC Genom. 2005, 6, 119. [Google Scholar] [CrossRef]
- Talmor-Neiman, M.; Stav, R.; Klipcan, L.; Buxdorf, K.; Baulcombe, D.C.; Arazi, T. Identification of trans-acting siRNAs in moss and an RNA-dependent RNA polymerase required for their biogenesis. Plant J. 2006, 48, 511–521. [Google Scholar] [CrossRef]
- Jones, L. Revealing micro-RNAs in plants. Trends Plant Sci. 2002, 7, 473–475. [Google Scholar] [CrossRef]
- Gasciolli, V.; Mallory, A.C.; Bartel, D.P.; Vaucheret, H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 2005, 15, 1494–1500. [Google Scholar] [CrossRef]
- Xie, Z.; Johansen, L.K.; Gustafson, A.M.; Kasschau, K.D.; Lellis, A.D.; Zilberman, D.; Jacobsen, S.E.; Carrington, J.C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004, 2, E104. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef]
- Baumberger, N.; Baulcombe, D.C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl. Acad. Sci. USA 2005, 102, 11928–11933. [Google Scholar] [CrossRef]
- Ronemus, M.; Vaughn, M.W.; Martienssen, R.A. MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by argonaute, dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell 2006, 18, 1559–1574. [Google Scholar] [CrossRef]
- Banerjee, D.; Slack, F. Control of developmental timing by small temporal RNAs: A paradigm for RNA-mediated regulation of gene expression. Bioessays 2002, 24, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hutvágner, G.; Zamore, P.D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002, 297, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wang, L.J.; Mao, Y.B.; Cai, W.J.; Xue, H.W.; Chen, X.Y. Control of root cap formation by MicroRNA-targeted auxin response factors in Arabidopsis. Plant Cell 2005, 17, 2204–2216. [Google Scholar] [CrossRef]
- Nilsen, T.W. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet. 2007, 23, 243–249. [Google Scholar] [CrossRef]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Zhao, T.; Li, G.; Mi, S.; Li, S.; Hannon, G.J.; Wang, X.J.; Qi, Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007, 21, 1190–1203. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Weinstein, E.G.; Rhoades, M.W.; Bartel, B.; Bartel, D.P. MicroRNAs in plants. Genes Dev. 2002, 16, 1616–1626. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Chiang, H.R.; Schoenfeld, L.W.; Ruby, J.G.; Auyeung, V.C.; Spies, N.; Baek, D.; Johnston, W.K.; Russ, C.; Luo, S.; Babiarz, J.E.; et al. Mammalian microRNAs: Experimental evaluation of novel and previously annotated genes. Genes Dev. 2010, 24, 992–1009. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.K.; Griffiths-Jones, S.; Enright, A.J. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. USA 2007, 104, 17719–17724. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.; Schwach, F.; Studholme, D.J.; Thuenemann, E.C.; Baulcombe, D.C. miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 2007, 447, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Alaba, S.; Piszczalka, P.; Pietrykowska, H.; Pacak, A.M.; Sierocka, I.; Nuc, P.W.; Singh, K.; Plewka, P.; Sulkowska, A.; Jarmolowski, A.; et al. The liverwort Pellia endiviifolia shares microtranscriptomic traits that are common to green algae and land plants. New Phytol. 2015, 206, 352–367. [Google Scholar] [CrossRef]
- Tarver, J.E.; Donoghue, P.C.; Peterson, K.J. Do miRNAs have a deep evolutionary history? Bioessays 2012, 34, 857–866. [Google Scholar] [CrossRef]
- Sunkar, R.; Zhu, J.K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef]
- Jiang, D.; Yin, C.; Yu, A.; Zhou, X.; Liang, W.; Yuan, Z.; Xu, Y.; Yu, Q.; Wen, T.; Zhang, D. Duplication and expression analysis of multicopy miRNA gene family members in Arabidopsis and rice. Cell Res. 2006, 16, 507–518. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Yu, D. Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 2012, 7, e48951. [Google Scholar] [CrossRef]
- Ben Amor, B.; Wirth, S.; Merchan, F.; Laporte, P.; d’Aubenton-Carafa, Y.; Hirsch, J.; Maizel, A.; Mallory, A.; Lucas, A.; Deragon, J.M.; et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009, 19, 57–69. [Google Scholar] [CrossRef]
- Jian, X.; Zhang, L.; Li, G.; Zhang, L.; Wang, X.; Cao, X.; Fang, X.; Chen, F. Identification of novel stress-regulated microRNAs from Oryza sativa L. Genomics 2010, 95, 47–55. [Google Scholar] [CrossRef]
- Fahlgren, N.; Jogdeo, S.; Kasschau, K.D.; Sullivan, C.M.; Chapman, E.J.; Laubinger, S.; Smith, L.M.; Dasenko, M.; Givan, S.A.; Weigel, D.; et al. MicroRNA gene evolution in Arabidopsis lyrata and Arabidopsis thaliana. Plant Cell 2010, 22, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Fromm, B.; Billipp, T.; Peck, L.E.; Johansen, M.; Tarver, J.E.; King, B.L.; Newcomb, J.M.; Sempere, L.F.; Flatmark, K.; Hovig, E.; et al. A Uniform System for the Annotation of Vertebrate microRNA Genes and the Evolution of the Human microRNAome. Annu. Rev. Genet. 2015, 49, 213–242. [Google Scholar] [CrossRef] [PubMed]
- Tarver, J.E.; Cormier, A.; Pinzón, N.; Taylor, R.S.; Carré, W.; Strittmatter, M.; Seitz, H.; Coelho, S.M.; Cock, J.M. microRNAs and the evolution of complex multicellularity: Identification of a large, diverse complement of microRNAs in the brown alga Ectocarpus. Nucleic Acids Res. 2015, 43, 6384–6398. [Google Scholar] [CrossRef]
- Axtell, M.J.; Westholm, J.O.; Lai, E.C. Vive la différence: Biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 2011, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, H.; Casas-Mollano, J.A. On the origin and functions of RNA-mediated silencing: From protists to man. Curr. Genet. 2006, 50, 81–99. [Google Scholar] [CrossRef]
- Millar, A.A.; Waterhouse, P.M. Plant and animal microRNAs: Similarities and differences. Funct. Integr. Genomics 2005, 5, 129–135. [Google Scholar] [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef]
- Jones-Rhoades, M.W.; Bartel, D.P.; Bartel, B. MicroRNAs and their regulatory roles in plants. Annu. Rev. Plant Biol. 2006, 57, 19–53. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Qi, Y. MicroRNAs in a multicellular green alga Volvox carteri. Sci. China Life Sci. 2014, 57, 36–45. [Google Scholar] [CrossRef]
- Valli, A.A.; Santos, B.A.; Hnatova, S.; Bassett, A.R.; Molnar, A.; Chung, B.Y.; Baulcombe, D.C. Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs. Genome Res. 2016, 26, 519–529. [Google Scholar] [CrossRef]
- Voshall, A.; Kim, E.J.; Ma, X.; Moriyama, E.N.; Cerutti, H. Identification of AGO3-associated miRNAs and computational prediction of their targets in the green alga Chlamydomonas reinhardtii. Genetics 2015, 200, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Nakahara, K.; Pham, J.W.; Kim, K.; He, Z.; Sontheimer, E.J.; Carthew, R.W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 2004, 117, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004, 18, 1655–1666. [Google Scholar] [CrossRef]
- Meyers, B.C.; Green, P.J.; Lu, C. miRNAs in the plant genome: All things great and small. Genome Dyn. 2008, 4, 108–118. [Google Scholar]
- Xie, Z.; Khanna, K.; Ruan, S. Expression of microRNAs and its regulation in plants. Semin. Cell Dev. Biol. 2010, 21, 790–797. [Google Scholar] [CrossRef]
- Kim, S.; Yang, J.Y.; Xu, J.; Jang, I.C.; Prigge, M.J.; Chua, N.H. Two cap-binding proteins CBP20 and CBP80 are involved in processing primary MicroRNAs. Plant Cell Physiol. 2008, 49, 1634–1644. [Google Scholar] [CrossRef]
- Fang, X.; Cui, Y.; Li, Y.; Qi, Y. Transcription and processing of primary microRNAs are coupled by Elongator complex in Arabidopsis. Nat Plants. 2015, 1, 15075. [Google Scholar] [CrossRef]
- Bielewicz, D.; Kalak, M.; Kalyna, M.; Windels, D.; Barta, A.; Vazquez, F.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Introns of plant pri-miRNAs enhance miRNA biogenesis. EMBO Rep. 2013, 14, 622–628. [Google Scholar] [CrossRef]
- Knop, K.; Stepien, A.; Barciszewska-Pacak, M.; Taube, M.; Bielewicz, D.; Michalak, M.; Borst, J.W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. Active 5′ splice sites regulate the biogenesis efficiency of Arabidopsis microRNAs derived from intron-containing genes. Nucleic Acids Res. 2016, 45, 2757–2775. [Google Scholar] [CrossRef]
- Lobbes, D.; Rallapalli, G.; Schmidt, D.D.; Martin, C.; Clarke, J. SERRATE: A new player on the plant microRNA scene. EMBO Rep. 2006, 7, 1052–1058. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Lu, F.; Dong, A.; Huang, H. SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 2006, 47, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Spector, D.L. Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 2007, 17, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, Y.; Utsumi, M.; Ohba, Y.; Watanabe, Y. Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis. Plant Cell Physiol. 2007, 48, 1243–1453. [Google Scholar] [CrossRef]
- Vazquez, F.; Gasciolli, V.; Crété, P.; Vaucheret, H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 2004, 14, 346–351. [Google Scholar] [CrossRef]
- Kurihara, Y.; Takashi, Y.; Watanabe, Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA 2006, 12, 206–212. [Google Scholar] [CrossRef]
- Song, L.; Axtell, M.J.; Fedoroff, N.V. RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr. Biol. 2010, 20, 37–41. [Google Scholar] [CrossRef]
- Werner, S.; Wollmann, H.; Schneeberger, K.; Weigel, D. Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr. Biol. 2010, 20, 42–48. [Google Scholar] [CrossRef]
- Dong, Z.; Han, M.H.; Fedoroff, N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl. Acad. Sci. USA 2008, 105, 9970–9975. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Cheng, Y.; Jia, D. HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 2012, 129, 1085–1094. [Google Scholar] [CrossRef]
- Baranauskė, S.; Mickutė, M.; Plotnikova, A.; Finke, A.; Venclovas, Č.; Klimašauskas, S.; Vilkaitis, G. Functional mapping of the plant small RNA methyltransferase: HEN1 physically interacts with HYL1 and DICER-LIKE1 proteins. Nucleic Acids Res. 2015, 43, 2802–2812. [Google Scholar] [CrossRef]
- Eamens, A.L.; Smith, N.A.; Curtin, S.J.; Wang, M.B.; Waterhouse, P.M. The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA 2009, 15, 2219–2235. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Brioudes, F.; Jay, F.; Sarazin, A.; Grentzinger, T.; Devers, E.A.; Voinnet, O. HASTY, the Arabidopsis EXPORTIN-5 ortholog, regulates cell-to-cell and vascular microRNA movement. EMBO J. 2021, 40, e107455. [Google Scholar] [CrossRef] [PubMed]
- Lanet, E.; Delannoy, E.; Sormani, R.; Floris, M.; Brodersen, P.; Crété, P.; Voinnet, O.; Robaglia, C. Biochemical evidence for translational repression by Arabidopsis microRNAs. Plant Cell 2009, 21, 1762–1768. [Google Scholar] [CrossRef]
- Reis, R.S.; Hart-Smith, G.; Eamens, A.L.; Wilkins, M.R.; Waterhouse, P.M. Gene regulation by translational inhibition is determined by Dicer partnering proteins. Nat. Plants. 2015, 1, 1402. [Google Scholar] [CrossRef]
- Biemar, F.; Zinzen, R.; Ronshaugen, M.; Sementchenko, V.; Manak, J.R.; Levine, M.S. Spatial regulation of microRNA gene expression in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 2005, 102, 15907–15911. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, Z.; Liang, J.; Ge, Q.; Duan, X.; Ma, F.; Li, F. The full-length transcripts and promoter analysis of intergenic microRNAs in Drosophila melanogaster. Genomics 2011, 97, 294–303. [Google Scholar] [CrossRef][Green Version]
- Church, V.A.; Pressman, S.; Isaji, M.; Truscott, M.; Cizmecioglu, N.T.; Buratowski, S.; Frolov, M.V.; Carthew, R.W. Microprocessor Recruitment to Elongating RNA Polymerase II Is Required for Differential Expression of MicroRNAs. Cell Rep. 2017, 20, 3123–3134. [Google Scholar] [CrossRef][Green Version]
- Ryazansky, S.S.; Gvozdev, V.A.; Berezikov, E. Evidence for post-transcriptional regulation of clustered microRNAs in Drosophila. BMC Genomics 2011, 12, 371. [Google Scholar] [CrossRef]
- Vodala, S.; Pescatore, S.; Rodriguez, J.; Buescher, M.; Chen, Y.W.; Weng, R.; Cohen, S.M.; Rosbash, M. The oscillating miRNA 959-964 cluster impacts Drosophila feeding time and other circadian outputs. Cell Metab. 2012, 16, 601–612. [Google Scholar] [CrossRef]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Agius, P.; Westholm, J.O.; Chen, M.; Okamura, K.; Robine, N.; Leslie, C.S.; Lai, E.C. Computational and experimental identification of mirtrons in Drosophila melanogaster and Caenorhabditis elegans. Genome Res. 2011, 21, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Natalin, P.; Stark, A.; Brennecke, J.; Cohen, S.M.; Izaurralde, E. Genome-wide analysis of mRNAs regulated by Drosha and Argonaute proteins in Drosophila melanogaster. Mol. Cell Biol. 2006, 26, 2965–2975. [Google Scholar] [CrossRef]
- Landthaler, M.; Yalcin, A.; Tuschl, T. The human DiGeorge syndrome critical region gene 8 and its D. melanogaster homolog are required for miRNA biogenesis. Curr. Biol. 2004, 14, 2162–2167. [Google Scholar] [CrossRef]
- Martin, R.; Smibert, P.; Yalcin, A.; Tyler, D.M.; Schäfer, U.; Tuschl, T.; Lai, E.C. A Drosophila pasha mutant distinguishes the canonical microRNA and mirtron pathways. Mol. Cell Biol. 2009, 29, 861–870. [Google Scholar] [CrossRef]
- Sabin, L.R.; Zhou, R.; Gruber, J.J.; Lukinova, N.; Bambina, S.; Berman, A.; Lau, C.K.; Thompson, C.B.; Cherry, S. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell 2009, 138, 340–351. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Shibata, S.; Sasaki, M.; Miki, T.; Shimamoto, A.; Furuichi, Y.; Katahira, J.; Yoneda, Y. Exportin-5 orthologues are functionally divergent among species. Nucleic Acids Res. 2006, 34, 4711–4721. [Google Scholar] [CrossRef]
- Park, J.K.; Liu, X.; Strauss, T.J.; McKearin, D.M.; Liu, Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr. Biol. 2007, 17, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ishizuka, A.; Siomi, H.; Siomi, M.C. Processing of pre-microRNAs by the Dicer-1-Loquacious complex in Drosophila cells. PLoS Biol. 2005, 3, e235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ye, X.; Liu, X.; Fincher, L.; McKearin, D.; Liu, Q. Dicer-1 and R3D1-L catalyze microRNA maturation in Drosophila. Genes Dev. 2005, 19, 1674–1679. [Google Scholar] [CrossRef]
- Förstemann, K.; Tomari, Y.; Du, T.; Vagin, V.V.; Denli, A.M.; Bratu, D.P.; Klattenhoff, C.; Theurkauf, W.E.; Zamore, P.D. Normal microRNA maturation and germ-line stem cell maintenance requires Loquacious, a double-stranded RNA-binding domain protein. PLoS Biol. 2005, 3, e236. [Google Scholar] [CrossRef]
- Förstemann, K.; Horwich, M.D.; Wee, L.; Tomari, Y.; Zamore, P.D. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell 2007, 130, 287–297. [Google Scholar] [CrossRef]
- Kawamata, T.; Seitz, H.; Tomari, Y. Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nat. Struct. Mol. Biol. 2009, 16, 953–960. [Google Scholar] [CrossRef]
- Okamura, K.; Liu, N.; Lai, E.C. Distinct mechanisms for microRNA strand selection by Drosophila Argonautes. Mol. Cell 2009, 36, 431–444. [Google Scholar] [CrossRef]
- Khvorova, A.; Reynolds, A.; Jayasena, S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell 2003, 115, 209–216. [Google Scholar] [CrossRef]
- Iwasaki, S.; Kawamata, T.; Tomari, Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol. Cell 2009, 34, 58–67. [Google Scholar] [CrossRef]
- Iwasaki, S.; Tomari, Y. Argonaute-mediated translational repression (and activation). Fly 2009, 3, 204–206. [Google Scholar] [CrossRef]
- Parker, M.S.; Park, E.A.; Sallee, F.R.; Parker, S.L. Canonical Matches of Human MicroRNAs with mRNAs: A Broad Matrix of Position and Size. Microrna 2016, 5, 211–221. [Google Scholar] [CrossRef]
- Wee, L.M.; Flores-Jasso, C.F.; Salomon, W.E.; Zamore, P.D. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 2012, 151, 1055–1067. [Google Scholar] [CrossRef] [PubMed]
- Nahvi, A.; Shoemaker, C.J.; Green, R. An expanded seed sequence definition accounts for full regulation of the hid 3′ UTR by bantam miRNA. RNA 2009, 15, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fu, H.; Zhang, X.; Li, Y. Identifications of conserved 7-mers in 3′-UTRs and microRNAs in Drosophila. BMC Bioinform. 2007, 8, 432. [Google Scholar] [CrossRef]
- Matsuura-Suzuki, E.; Kiyokawa, K.; Iwasaki, S.; Tomari, Y. miRNA-mediated gene silencing in Drosophila larval development involves GW182-dependent and independent mechanisms. EMBO J. 2024, 43, 6161–6179. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Huntzinger, E.; Izaurralde, E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 2008, 15, 346–353. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Yoon, H.S.; Hackett, J.D.; Ciniglia, C.; Pinto, G.; Bhattacharya, D. A molecular timeline for the origin of photosynthetic eukaryotes. Mol. Biol. Evol. 2004, 21, 809–818. [Google Scholar] [CrossRef]
- Casas-Mollano, J.A.; Rohr, J.; Kim, E.J.; Balassa, E.; Van Dijk, K.; Cerutti, H. Diversification of the core RNA interference machinery in Chlamydomonas reinhardtii and the role of DCL1 in transposon silencing. Genetics 2008, 179, 69–81. [Google Scholar] [CrossRef]
- Sun, T.; Tao, M.; Di, Q.; Hu, Z.; Li, H.; Lou, S. Identification of CrDCL1-mediated microRNA biogenesis in green alga Chlamydomonas reinhardtii. Front Microbiol. 2025, 16, 1487584. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Onishi, M.; Kim, E.J.; Cerutti, H.; Ohama, T. RNA-binding protein DUS16 plays an essential role in primary miRNA processing in the unicellular alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 10720–10725. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Cerutti, H. Cooperative processing of primary miRNAs by DUS16 and DCL3 in the unicellular green alga Chlamydomonas reinhardtii. Commun. Integr. Biol. 2017, 10, e1280208. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kim, E.J.; Cerutti, H.; Ohama, T. Argonaute3 is a key player in miRNA-mediated target cleavage and translational repression in Chlamydomonas. Plant J. 2016, 85, 258–268. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, W.; Bai, X.; Qi, Y. Gene silencing by artificial microRNAs in Chlamydomonas. Plant J. 2009, 58, 157–164. [Google Scholar] [CrossRef]
- Molnar, A.; Bassett, A.; Thuenemann, E.; Schwach, F.; Karkare, S.; Ossowski, S.; Weigel, D.; Baulcombe, D. Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J. 2009, 58, 165–174. [Google Scholar] [CrossRef]
- Schmollinger, S.; Strenkert, D.; Schroda, M. An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Curr. Genet. 2010, 56, 383–389. [Google Scholar] [CrossRef]
- Yamasaki, T.; Voshall, A.; Kim, E.J.; Moriyama, E.; Cerutti, H.; Ohama, T. Complementarity to a miRNA seed region is sufficient to induce moderate repression of a target transcript in the unicellular green alga Chlamydomonas reinhardtii. Plant J. 2013, 76, 1045–1056. [Google Scholar] [CrossRef]
- Lenz, D.; May, P.; Walther, D. Comparative analysis of miRNAs and their targets across four plant species. BMC Res. Notes. 2011, 4, 483. [Google Scholar]
- Hu, J.; Deng, X.; Shao, N.; Wang, G.; Huang, K. Rapid construction and screening of artificial microRNA systems in Chlamydomonas reinhardtii. Plant J. 2014, 79, 1052–1064. [Google Scholar]
- Chung, B.Y.; Valli, A.; Deery, M.J.; Navarro, F.J.; Brown, K.; Hnatova, S.; Howard, J.; Molnar, A.; Baulcombe, D.C. Distinct roles of Argonaute in the green alga Chlamydomonas reveal evolutionary conserved mode of miRNA-mediated gene expression. Sci. Rep. 2019, 9, 11091. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Rymarquis, L.A.; Kim, E.J.; Becker, J.; Balassa, E.; Green, P.J.; Cerutti, H. Uridylation of mature miRNAs and siRNAs by the MUT68 nucleotidyltransferase promotes their degradation in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2010, 107, 3906–3911. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Rohr, J.; Jeong, W.J.; Hesson, J.; Cerutti, H. Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science 2006, 314, 1893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Zhai, J.; Ramachandran, V.; Dinh, T.T.; Meyers, B.C.; Mo, B.; Chen, X. The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr. Biol. 2012, 22, 689–694. [Google Scholar] [CrossRef]
- Bortolamiol-Becet, D.; Hu, F.; Jee, D.; Wen, J.; Okamura, K.; Lin, C.J.; Ameres, S.L.; Lai, E.C. Selective Suppression of the Splicing-Mediated MicroRNA Pathway by the Terminal Uridyltransferase Tailor. Mol. Cell. 2015, 59, 217–228. [Google Scholar] [CrossRef]
- Shu, L.; Hu, Z. Characterization and differential expression of microRNAs elicited by sulfur deprivation in Chlamydomonas reinhardtii. BMC Genom. 2012, 13, 108. [Google Scholar] [CrossRef]
- Matt, G.; Umen, J. Volvox: A simple algal model for embryogenesis, morphogenesis and cellular differentiation. Dev. Biol. 2016, 419, 99–113. [Google Scholar] [CrossRef]
- Biswal, D.P.; Panigrahi, K.C.S. Light- and hormone-mediated development in non-flowering plants: An overview. Planta 2020, 253, 1. [Google Scholar] [CrossRef]
- Dueck, A.; Evers, M.; Henz, S.R.; Unger, K.; Eichner, N.; Merkl, R.; Berezikov, E.; Engelmann, J.C.; Weigel, D.; Wenzl, S.; et al. Gene silencing pathways found in the green alga Volvox carteri reveal insights into evolution and origins of small RNA systems in plants. BMC Genom. 2016, 17, 853. [Google Scholar] [CrossRef]
- Azaman, S.N.A.; Satharasinghe, D.A.; Tan, S.W.; Nagao, N.; Yusoff, F.M.; Yeap, S.K. Identification and Analysis of microRNAs in Chlorella sorokiniana Using High-Throughput Sequencing. Genes 2020, 11, 1131. [Google Scholar] [CrossRef]
- Yang, R.; Chen, G.; Peng, H.; Wei, D. Identification and Characterization of MiRNAs in Coccomyxa subellipsoidea C-169. Int. J. Mol. Sci. 2019, 20, 3448. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Y.; Hu, X.L.; Li, D.; Wang, L.; Cheng, J.; Gao, K. Identification and analysis of microRNAs in Botryococcus braunii using high-throughput sequencing. Aquat. Biol. 2017, 26, 41–48. [Google Scholar] [CrossRef]
- Gao, X.; Cong, Y.; Yue, J.; Xing, Z.; Wang, Y.; Chai, X. Small RNA, transcriptome, and degradome sequencing to identify salinity stress responsive miRNAs and target genes in Dunaliella salina. J. App. Phycol. 2019, 31, 1175–1183. [Google Scholar] [CrossRef]
- Lou, S.; Zhu, X.; Zeng, Z.; Wang, H.; Jia, B.; Li, H.; Hu, Z. Identification of microRNAs response to high light and salinity that involved in beta-carotene accumulation in microalga Dunaliella salina. Algal Res. 2020, 48, 101925. [Google Scholar] [CrossRef]
- Wang, X.; Miao, X.; Chen, G.; Cui, Y.; Sun, F.; Fan, J.; Gao, Z.; Meng, C. Identification of microRNAs involved in astaxanthin accumulation responding to high light and high sodium acetate (NaAC) stresses in Haematococcus pluvialis. Algal Res. 2021, 54, 102179. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, Z.; Yan, X.; Lu, J.; Wang, C. Extracellular vesicles involved in growth regulation and metabolic modulation in Haematococcus pluvialis. Biotechnol. Biofuels Bioprod. 2024, 17, 15. [Google Scholar] [CrossRef]
- Bogaert, K.A.; Arun, A.; Coelho, S.M.; De Clerck, O. Brown algae as a model for plant organogenesis. Methods Mol Biol. 2013, 959, 97–125. [Google Scholar]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef]
- Billoud, B.; Nehr, Z.; Le Bail, A.; Charrier, B. Computational prediction and experimental validation of microRNAs in the brown alga Ectocarpus siliculosus. Nucleic Acids Res. 2014, 42, 417–429. [Google Scholar] [CrossRef]
- Tseng, C.K. Algal biotechnology industries and research activities in China. J. Appl. Phycol. 2001, 13, 375–380. [Google Scholar] [CrossRef]
- Liu, F.; Wang, W.; Sun, X.; Liang, Z.; Wang, F. Conserved and novel heat stress-responsive microRNAs were identified by deep sequencing in Saccharina japonica (Laminariales, Phaeophyta). Plant Cell Environ. 2015, 38, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.M.; Liu, F.; Duan, D.; Bourdareau, S.; Lipinska, A.P.; Coelho, S.M.; Tarver, J.E. Rapid Evolution of microRNA Loci in the Brown Algae. Genome Biol. Evol. 2017, 9, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, M.; Miura, S.; Nei, M. Origins and evolution of microRNA genes in plant species. Genome Biol Evol. 2012, 4, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Fredericq, S.; Schmidt, W.E. Red Algae eLS; Wiley: Chichester, UK, 2010; pp. 1–7. [Google Scholar]
- Brawley, S.H.; Blouin, N.A.; Ficko-Blean, E.; Wheeler, G.L.; Lohr, M.; Goodson, H.V.; Jenkins, J.W.; Blaby-Haas, C.E.; Helliwell, K.E.; Chan, C.X.; et al. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc. Natl. Acad. Sci. USA 2017, 114, E6361–E6370. [Google Scholar] [CrossRef]
- Shainker-Connelly, S.J.; Crowell, R.M.; Stoeckel, S.; Vis, M.L.; Krueger-Hadfield, S.A. Population genetics of the freshwater red alga Batrachospermum gelatinosum (Rhodophyta) I: Frequent intragametophytic selfing in a monoicous, haploid-diploid species. J. Phycol. 2024, 60, 1420–1436. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Mohammadi, M.; Alian, M.; Muralitharan, G.; Chauhan, V.S.; Balan, V. Exploring the versatility of Porphyridium sp.: A comprehensive review of cultivation, bio-product extraction, purification, and characterization techniques. Biotechnol Adv. 2024, 77, 108471. [Google Scholar] [CrossRef]
- Vieira, C.; Brooks, C.M.; Akita, S.; Kim, M.S.; Saunders, G.W. Of sea, rivers and symbiosis: Diversity, systematics, biogeography and evolution of the deeply diverging florideophycean order Hildenbrandiales (Rhodophyta). Mol. Phylogenet. Evol. 2024, 197, 108106. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Derbel, H.; Duchez, D.; Gardarin, C.; Dubessay, P.; Drira, M.; Fendri, I.; Michaud, P.; Abdelkafi, S. Enhanced growth and metabolite production from a novel strain of Porphyridium sp. Bioengineered 2024, 15, 2294160. [Google Scholar] [CrossRef]
- Gao, F.; Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Xie, S. Identification of conserved and novel microRNAs in Porphyridium purpureum via deep sequencing and bioinformatics. BMC Genom. 2016, 17, 612. [Google Scholar] [CrossRef]
- Barozai, M.Y.K.; Qasim, M.; Din, M.; Achakzai, A.K.K. An update on the microRNAs and their targets in unicellular red alga Porphyridium cruentum. Pak. J. Bot. 2018, 50, 817–825. [Google Scholar]
- Gao, F.; Nan, F.; Feng, J.; Xie, S. Characterization and Comparative Analysis of MicroRNAs in 3 Representative Red Algae. Iran J. Biotechnol. 2021, 19, e2868. [Google Scholar]
- Thangaraj, B.; Jolley, C.C.; Sarrou, I.; Bultema, J.B.; Greyslak, J.; Whitelegge, J.P.; Lin, S.; Kouřil, R.; Subramanyam, R.; Boekema, E.J.; et al. Efficient light harvesting in a dark, hot, acidic environment: The structure and function of PSI-LHCI from Galdieria sulphuraria. Biophys. J. 2011, 100, 135–143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Saga, N.; Kitade, Y. Porphyra: A model plant in marine sciences. Fish. Sci. 2002, 68, 1075–1078. [Google Scholar] [CrossRef]
- Lembi, C.A.; Waaland, J.R. Algae and Human Affairs; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Shen, S.; Zhang, G.; Li, Y.; Wang, L.; Xu, P.; Yi, L. Comparison of RNA expression profiles on generations of Porphyra yezoensis (Rhodophyta), based on suppression subtractive hybridization (SSH). BMC Res. Notes 2011, 4, 428. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zou, J.; Xu, D.; Su, F.; Ye, N. Identification of miRNA from Porphyra yezoensis by high-throughput sequencing and bioinformatics analysis. PLoS ONE 2010, 5, e10698. [Google Scholar] [CrossRef]
- He, L.; Huang, A.; Shen, S.; Niu, J.; Wang, G. Comparative Analysis of MicroRNAs between Sporophyte and Gametophyte of Porphyra yezoensis. Comp. Funct. Genom. 2012, 2012, 912843. [Google Scholar] [CrossRef]
- Popper, Z.A. Extraction and detection of arabinogalactan proteins. Methods Mol. Biol. 2011, 715, 245–254. [Google Scholar]
- Krueger-Hadfield, S.A.; Roze, D.; Mauger, S.; Valero, M. Intergametophytic selfing and microgeographic genetic structure shape populations of the intertidal red seaweed Chondrus crispus. Mol. Ecol. 2013, 22, 3242–3260. [Google Scholar] [CrossRef]
- Bixler, H.J.; Porse, H. A decade of change in the seaweed hydrocolloids industry. J. Appl. Phycol. 2010, 23, 321–335. [Google Scholar] [CrossRef]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2010, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Nan, F.; Song, W.; Feng, J.; Lv, J.; Xie, S. Identification and Characterization of miRNAs in Chondrus crispus by High-Throughput Sequencing and Bioinformatics Analysis. Sci. Rep. 2016, 6, 26397. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.M.; Zhang, J.P. Records of Chinese Seaweeds; Science Press: Beijing, China, 1999; pp. 128–132. [Google Scholar]
- Ask, E.I.; Azanza, R.V. Advances in cultivation technology of commercial eucheumatoid species: A review with suggestions for future research. Aquaculture 2002, 206, 257–277. [Google Scholar] [CrossRef]
- Li, L.H.; Qi, B.; Yang, S.L. Functional effect of dietary fiber from Eucheuma on reducing serum lipids. J. Fish. Sci. China 2008, 15, 943–949. [Google Scholar]
- Gao, F.; Nan, F.; Feng, J.; Lv, J.; Liu, Q.; Xie, S. Identification and characterization of microRNAs in Eucheuma denticulatum by high-throughput sequencing and bioinformatics analysis. RNA Biol. 2016, 13, 343–352. [Google Scholar] [CrossRef]
- Troell, M.; Rönnbäck, P.; Halling, C.; Kautsky, N.; Buschmann, A. Ecological engineering in aquaculture: Use of seaweeds for removing nutrients from intensive mariculture. J. Appl. Phycol. 1999, 11, 89–97. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Y.Y.; Yin, H.; Sun, X.; Zhang, X.; Xu, N. A survey of the full-length transcriptome of Gracilariopsis lemaneiformis using single-molecule long-read sequencing. BMC Plant Biol. 2022, 22, 597. [Google Scholar] [CrossRef]
- Zhang, X.C.; Fei, X.G. The national aquatic product original variety approval committee-Gracilaria lemaneiformis 981 and an introduction to its cultivation techniques. Sci Fish Farm 2008, 6, 21–22. [Google Scholar]
- Wang, Z.; Wang, X.; Ke, C. Bioaccumulation of trace metals by the live macroalga Gracilaria lemaneiformis. J. Appl. Phycol. 2014, 26, 1889–1897. [Google Scholar] [CrossRef]
- Xiang, J.X.; Saha, M.; Zhong, K.L.; Zhang, Q.S.; Zhang, D.; Jueterbock, A.; Krueger-Hadfield, S.A.; Wang, G.G.; Weinberger, F.; Hu, Z.M. Genome-scale signatures of adaptive gene expression changes in an invasive seaweed Gracilaria vermiculophylla. Mol. Ecol. 2023, 32, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Krueger-Hadfield, S.A.; Blakeslee, A.M.H.; Fowler, A.E. Incorporating ploidy diversity into ecological and community genetics. J. Phycol. 2019, 55, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Hammann, M.; Wang, G.G.; Boo, S.M.; Aguilar-Rosas, L.E.; Weinberger, F. Selection of heat-shock resistance traits during the invasion of the seaweed Gracilaria vermiculophylla. Marine Biol. 2016, 163, 104. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Nyberg, C.D.; Wuff, A. UVR defense mechanisms in eurytopic and invasive Gracilaria vermiculophylla (Gracilariales, Rhodophyta). Physiol. Plant. 2012, 146, 205–216. [Google Scholar] [CrossRef]
- Rueness, J. Life history and molecular sequences of Gracilaria vermiculophylla (Gracilariales, Rhodophyta), a new introduction to European waters. Phycologia 2005, 44, 120–128. [Google Scholar] [CrossRef]
- Andreakis, N.; Procaccini, G.; Maggs, C.; Kooistra, W.H. Phylogeography of the invasive seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) reveals cryptic diversity. Mol. Ecol. 2007, 16, 2285–2299. [Google Scholar] [CrossRef]
- Andreakis, N.; Kooistra, W.H.; Procaccini, G. Microsatellite markers in an invasive strain of Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta): Insights in ploidy level and sexual reproduction. Gene 2007, 406, 144–151. [Google Scholar] [CrossRef]
- Andreakis, N.; Costello, P.; Zanolla, M.; Saunders, G.W.; Mata, L. Endemic or introduced? Phylogeography of Asparagopsis (Florideophyceae) in Australia reveals multiple introductions and a new mitochondrial lineage. J. Phycol. 2016, 52, 141–147. [Google Scholar] [CrossRef]
- Genovese, G.; Tedone, L.; Hamann, M.T.; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against Leishmania. Mar. Drugs 2009, 7, 361–366. [Google Scholar] [CrossRef]
- Genovese, G.; Faggio, C.; Gugliandolo, C.; Torre, A.; Spanò, A.; Morabito, M.; Maugeri, T.L. In vitro evaluation of antibacterial activity of Asparagopsis taxiformis from the Straits of Messina against pathogens relevant in aquaculture. Mar. Environ. Res. 2012, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Pirozzi, I.; de Nys, R.; Paul, N.A. Feeding preferences and the nutritional value of tropical algae for the abalone Haliotis asinina. PLoS ONE 2012, 7, e38857. [Google Scholar] [CrossRef]
- Neethu, P.V.; Suthindhiran, K.; Jayasri, M.A. Antioxidant and Antiproliferative Activity of Asparagopsis taxiformis. Pharmacogn. Res. 2017, 9, 238–246. [Google Scholar]
- Thépot, V.; Campbell, A.H.; Paul, N.A.; Rimmer, M.A. Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish Shellfish Immunol. 2021, 113, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mol. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar] [CrossRef]
- Machado, L.; Tomkins, N.; Magnusson, M.; Midgley, D.J.; de Nys, R.; Rosewarne, C.P. In Vitro Response of Rumen Microbiota to the Antimethanogenic Red Macroalga Asparagopsis taxiformis. Microb. Ecol. 2018, 75, 811–818. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Park, T.; Seo, J.; Lee, Y.; Bae, D.; et al. Red seaweed extracts reduce methane production by altering rumen fermentation and microbial composition in vitro. Front. Vet. Sci. 2022, 9, 985824. [Google Scholar] [CrossRef]
- Patwary, Z.P.; Zhao, M.; Wang, T.; Paul, N.A.; Cummins, S.F. A Proteomic Analysis for the Red Seaweed Asparagopsis taxiformis. Biology 2023, 12, 167. [Google Scholar] [CrossRef]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Viron, N.; Alhagdow, M.; Karimi, M.; Jones, M.; Amsellem, Z.; Sicard, A.; Czerednik, A.; Angenent, G.; Grierson, D.; et al. Flexible tools for gene expression and silencing in tomato. Plant Physiol. 2009, 151, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Eamens, A.L.; Agius, C.; Smith, N.A.; Waterhouse, P.M.; Wang, M.B. Efficient silencing of endogenous microRNAs using artificial microRNAs in Arabidopsis thaliana. Mol. Plant. 2011, 4, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Zhang, L.; Gao, Z.; Zhu, C.X.; Guo, X. Highly efficient virus resistance mediated by artificial microRNAs that target the suppressor of PVX and PVY in plants. Plant Biol. 2011, 13, 304–316. [Google Scholar] [CrossRef]
- Hauser, F.; Chen, W.; Deinlein, U.; Chang, K.; Ossowski, S.; Fitz, J.; Hannon, G.J.; Schroeder, J.I. A genomic-scale artificial microRNA library as a tool to investigate the functionally redundant gene space in Arabidopsis. Plant Cell 2013, 25, 2848–2863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).