Abstract
Background/Objectives: This review examines the role of pharmacogenomics in individual responses to the pharmacotherapy of various drugs of abuse, including alcohol, cocaine, and opioids, to identify genetic variants that contribute to variability in substance use disorder treatment outcomes. In addition, it explores the pharmacomicrobiomic aspects of substance use, highlighting the impact of the gut microbiome on bioavailability, drug metabolism, pharmacodynamics, and pharmacokinetics. Results: Research on pharmacogenetics has identified several promising genetic variants that may contribute to the individual variability in responses to existing pharmacotherapies for substance addiction. However, the interpretation of these findings remains limited. It is estimated that genetic factors may account for 20–95% of the variability in individual drug responses. Therefore, genetic factors alone cannot fully explain the differences in drug responses, and factors such as gut microbiome diversity may also play a significant role. Drug microbial biotransformation is produced by microbial exoenzymes that convert low molecular weight organic compounds into analogous compounds by oxidation, reduction, hydrolysis, condensation, isomerization, unsaturation, or by the introduction of heteroatoms. Despite significant advances in pharmacomicrobiomics, challenges persist including the lack of standardized methodologies, inter-individual variability, limited understanding of drug biotransformation mechanisms, and the need for large-scale validation studies to develop microbiota-based biomarkers for clinical use. Conclusions: Progress in the pharmacogenomics of substance use disorders has provided biological insights into the pharmacological needs associated with common genetic variants in drug-metabolizing enzymes. The gut microbiome and its metabolites play a pivotal role in various stages of drug addiction including seeking, reward, and biotransformation. Therefore, integrating pharmacogenomics with pharmacomicrobiomics will form a crucial foundation for significant advances in precision and personalized medicine.
1. Introduction
Initial substance use is often driven by curiosity, but with reinforcement, such behavior can evolve into a habitual pattern, ultimately leading to addiction []. This progression is of particular public health concern, as evidenced by the recent fentanyl crisis []. Substance addiction is characterized by specific psychological symptoms, such as intense cravings or repeated failed attempts to regulate consumption, and by physiological manifestations including tolerance and withdrawal syndrome []. The regular consumption of addictive substances can exert a profound impact on both physical well-being and socio-emotional stability, heightening the risk for polysubstance dependence, medical emergencies, and dysfunction in family dynamics []. Furthermore, substance use has been linked to various psychiatric conditions and their associated symptoms including major depressive disorder, suicidal thoughts and behaviors, schizophrenia, bipolar disorder, borderline personality disorder, anxiety disorders, and antisocial personality disorder []. Thus, the potential individual, familial, and societal consequences of substance abuse underscore the critical need to deepen our understanding of the pharmacogenetic mechanisms involved in addiction. Moreover, developing novel strategies to enhance the metabolism and clearance of these substances is essential for mitigating their harmful effects [].
Pharmacogenetics constitutes the study of how genetic variability influences drug responses and adverse reactions, aiming to optimize individualized treatment approaches. Understanding how genetic differences impact drug responses can also shed light on fundamental pharmacological and pathological mechanisms []. Early pharmacogenetic research primarily targeted candidate genes, chosen based on prior knowledge of the pharmacokinetic and pharmacodynamic properties of psychotropic medications []. Genetic diversity in humans is largely attributed to single nucleotide polymorphisms (SNPs), which involve single-base alterations in the DNA sequence including insertions, deletions, and copy number variations [].
The development of chip-based microarray technology, capable of analyzing thousands of genetic polymorphisms across the genome, has significantly advanced genetic association studies and facilitated the emergence of genome-wide association studies (GWASs). This technological progress has led to the evolution of pharmacogenomics, a field that extends beyond single-gene analyses to encompass the entire genome, enabling the accurate genotyping of between 500,000 and over 4 million SNPs in a cost-efficient manner. As a rapidly expanding discipline, pharmacogenomics aims to identify genetic markers that can guide clinicians in selecting safe and effective treatments customized to individual patients []. Consequently, pharmacogenomic testing holds great promise for optimizing drug therapy across a broad spectrum of medical conditions [].
The combined genetic material of a host organism and its associated microbiota has led to the introduction of the hologenome concept []. According to the holobiont theory of evolution, natural selection can act not only on the host genome, but also on the metagenome of the microbiota, shaping them into a cohesive functional unit []. Stilling et al. [] introduced the term holoepigenome to describe the epigenetic characteristics and heritability of the gut microbiome (GM). More recently, Pepke et al. [] expanded this definition, describing the holoepigenome as the complete set of epigenetic modifications and their complex interactions within the host genome.
The GM constitutes a highly diverse and complex biological ecosystem, which is estimated to encompass over 5 million distinct genes and approximately 100 trillion cells [,]. Established at birth, the GM undergoes continuous modifications throughout the lifespan of an individual, dynamically reflecting the health status at different life stages [,]. These fluctuations can be transient or permanent and are influenced by factors such as diet, stress, hormonal changes, and environmental exposures [,]. Beyond its crucial role in metabolic processes, the GM also contributes to immune regulation and behavioral traits, while significantly impacting drug metabolism through biotransformation mechanisms including hydrolysis, demethylation, deamination, and various reactive transformations []. Since the 20th century, research has explored the influence of the GM on drug metabolism and efficacy, revealing its capacity to activate, inactivate, or modify the toxicity of drugs and xenobiotics [].
Pharmacomicrobiomics examines how variations in the microbiome influence the disposition, efficacy, and response to drugs and xenobiotics []. More specifically, this field explores the ways in which intra- and inter-individual differences in microbial composition affect drug action, pharmacokinetics, pharmacodynamics, metabolism, bioavailability, therapeutic outcomes, and potential toxicity, rather than drug–drug interactions with specific microorganisms [,,]. Research has identified several mechanisms underlying drug–microbiota interactions including biotransformation (where microbes metabolize drugs), bioaccumulation (where drugs are stored within microbial cells), and the direct modulation of microbial growth by pharmacological compounds []. In this regard, several chemical compounds have been reported to be degraded by bacterial species. For example, the degradation of digoxin, a cardiac glycoside, by Eggerthella lenta and Lactiplantibacillus pentosus [,], the degradation of salicin, a dietary plant β-glucoside, by Lactobacillus acidophilus [], or prontosil by Clostridium and Eubacterium species [], which uses several bacterial enzymes such as β-glucuronidase [], phospho-β-glucosidase [], and nitroreductase []. Table 1 illustrates the key differences between pharmacogenomics and pharmacomicrobiomics (according to [,]).

Table 1.
Differences between pharmacogenomics and pharmacomicrobiomics.
In this narrative review, we examined the role of pharmacogenomics in modulating individual responses to the pharmacotherapy of various drugs of abuse including alcohol, cocaine, and opioids. By identifying promising genetic variants, we have highlighted potential contributors to individual variability in treatment outcomes for substance use disorder (SUD). However, additional factors such as GM diversity may also play an important role in pharmacotherapy efficacy. For this reason, we explored the pharmacomicrobiomic aspects related to substance use as a means to understand the complex interactions between the GM, the host, and drugs, which possess important implications for bioavailability, drug metabolism, pharmacodynamics, pharmacokinetics, and the risk of drug-induced toxicity.
2. The Role of Pharmacogenomics in Drug Response
Significant inter-individual differences in the efficacy and safety of numerous therapeutic agents arise from genetically regulated polymorphisms affecting drug-metabolizing enzymes, transport proteins, and receptor targets. The emergence of pharmacogenomics as a distinct field has promoted advancements in pharmacogenetics by uncovering the genetic foundations of these inherited differences at the genomic level, providing deeper insights into the mechanisms underlying drug response variability [,]. Pharmacogenomics has given rise to the practice of “precision medicine”, which combines pharmacology (the study of drugs) and genomics (the study of genes and their functions) to offer a more customized approach []. In the context of drug addiction, pharmacogenetics studies the genetic variation that affects treatment response, defined as treatment outcomes or adverse effects []. However, pharmacogenetics has only recently been applied to the study of addiction treatment for alcoholism and opioid addiction. In contrast, there are currently no approved therapies for psychostimulants (e.g., cocaine and methamphetamine addiction). Figure 1 presents several factors affecting the individual variability in drug response and personalized treatment based on pharmacogenomics.
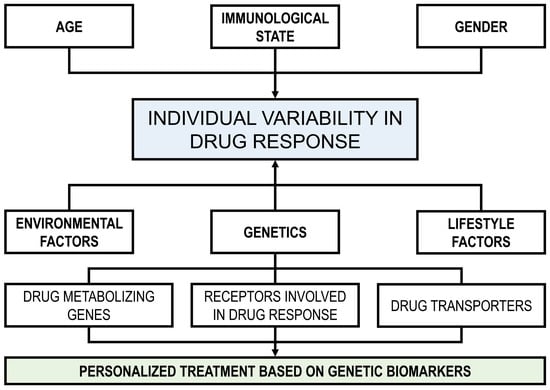
Figure 1.
Factors affecting individual variability in drug response and personalized treatment based on pharmacogenomics.
Genetic variation can affect gene expression at multiple levels including transcriptional regulation, mRNA splicing and stability, and protein translation, stability, and function. These variations can subsequently impact protein folding, structure, and biological processes such as enzymatic activity, binding affinity, and stability [,]. Polymorphisms in genes that encode key components of pathways related to SUDs may contribute to individual differences in response to addiction treatments. These variations are typically polygenic, involving multiple genes, but could also be oligogenic, involving a small number of genes that have a dominant influence on the response [,].
Research has shown that genetic factors are critical in determining individual variations in drug therapy outcomes and significantly contribute to adverse drug reactions [,,]. SNPs refer to variations in DNA sequences caused by the alteration of a single nucleotide at the genomic level []. These genetic variations can influence drug metabolism and responses, making SNPs a key focus in pharmacogenomic research. For example, Salvador-Martín et al. [] identified that HLA DNA variants (rs2395185 and rs2097432) were linked to long-term responses to anti-tumor necrosis factor therapy in Spanish children with inflammatory bowel disease, highlighting the important role of SNPs in drug response.
The Pharmacogenomics Knowledge Base (PharmGKB: http://www.pharmgkb.org accessed on 17 February 2025), established in 2000, serves as a comprehensive resource for pharmacogenomics data []. As of September 2023, PharmGKB had annotated 766 drugs, 201 clinical guidelines, 933 drug labels, and 428 FDA drug labels. It provides detailed information on the relationships between drugs, genes, and diseases, offering researchers valuable insights into genetic variation annotations, drug-centric pathways, key pharmacogenes, and various diseases []. Table 2 shows the genetics of several approved and investigational pharmacotherapies for addictions and the genetic variants.

Table 2.
Drug therapies used in pharmacogenetic studies and genetic variations.
Drug addiction is a prevalent, polygenic, chronic, and relapsing brain disorder that could benefit from pharmacotherapies more precisely defined through molecular-genetic approaches. The pharmacogenetic studies reviewed identified several promising genetic variants that may contribute to the individual variability in responses to existing pharmacotherapies for drug addiction. However, the interpretation of these findings remains limited. It is estimated that genetic factors may account for 20–95% of the variability in individual drug responses []. Therefore, genetic factors alone cannot fully explain the differences in drug responses, and other factors such as GM diversity may also be important [].
3. Influence of Drugs of Abuse on the Gut Microbiome
An expanding body of research has revealed that various drugs of abuse affect the GM, both in animal models and in humans. The bidirectional drug–microbe interaction may be pivotal for future drug development and clinical application. In this section, we review the major studies regarding the influence of drugs of abuse, such as alcohol, psychostimulants, opioids, cannabinoids, and nicotine, on the composition of the GM.
3.1. Alcohol
Alcohol is a depressant and is one of the oldest and most widely used recreational substances. Its primary mechanism of action is to increase the activity of γ-aminobutyric acid (GABA), which is a major inhibitory neurotransmitter in the brain, and this increased activity results in central nervous system depression []. Another primary target of alcohol in the neural system is the N-methyl-D-aspartate (NMDA) receptor, a key component of the glutamate receptor family that plays a central role in several brain functions including learning, memory, and synaptic plasticity [].
In a mouse model of alcoholic liver disease, members of the phyla Bacteroidota and Verrucomicrobiota were increased in alcohol-fed mice compared with a relative predominance of members of the phylum Bacillota in the control mice []. Exposure of mice to 4 weeks of chronic intermittent vaporized ethanol significantly altered the GM, increasing the levels of Alistipes and decreasing Clostridium IV, Dorea, and Coprococcus [].
Several human studies have shown that alcohol consumption leads to an increase in the abundance of the members of the phylum Pseudomonadota and to a decrease in the members of the phylum Bacteroidota [,]. At the genus level, increases in the abundance of Bacteroides, Clostridium, Holdemania, Sutterella, and Streptococcus have been reported as well as decreases in the genera Akkermansia and Faecalibacterium [,,]. Recently, Du et al. [] found that the microbial diversity and composition of the GM were dysregulated in patients with alcohol use disorder (AUD), with a decrease in α-diversity and an increase in β-diversity indices including a decreased abundance in the members of the family Lachnospiracea and in the genera Bacteroides, Dialister, Faecalibacterium, and Gemmiger, and increased levels in the genera Coprobacillus, Clostridium, Escherichia, Fusobacterium, Gemella, Megamonas, Prevotella, and Rothia. In contrast, patients with alcoholic cirrhosis showed an increase in the abundance of Escherichia/Shigella and Prevotella, and a decrease in the genera Blautia and Faecalibacterium [].
3.2. Psychostimulants
Psychostimulants are indirect sympathomimetic substances with a high potential for misuse and addiction, which increase the release of dopamine and enhance synaptic transmission as well as the availability of monoamine neurotransmitters at the synapse such as dopamine, norepinephrine, and serotonin []. These pharmacological compounds are recognized for their invigorating properties and extensive global utilization including both in medical and non-medical contexts for performance enhancement and recreational purposes []. Commonly abused psychostimulants include amphetamine, methamphetamine, MDMA (ecstasy), methylphenidate, and cocaine [].
The results regarding GM composition in relation to psychostimulant use are diverse due to the varying chemical properties and receptor interactions of these substances. In preclinical studies, the fecal microbial diversity is slightly higher in methamphetamine-treated rats, with a decrease in the propionate-producing genus Phascolarctobacterium and an increase in members of the family Ruminococcaceae []. In humans, the methamphetamine use was associated with increases in the genera Finegoldia, Fusobacterium, Parvimonas, Peptoniphilus, Peptostreptococcus, Porphyromonas, Streptobacillus, and Streptococcus, and with decreases in Bacteroides, Butyricicoccus, Faecalibacterium, and Succinivibrio []. Furthermore, in the GM of methamphetamine users, Yang et al. [] reported significant decreases in the abundance of members of the phylum Myxococcota (formerly class Deltaproteobacteria) and the family Bacteroidaceae as well as increases in the abundance of Sphingomonadales, Xanthomonadales, Romboutsia, and Lachnospiraceae. In a recent study, He et al. [] showed significant changes in the GM composition of methamphetamine use disorder (MUD) and methamphetamine causal use (MCU) individuals compared with the controls, with a dominance of Bacteroides, Blautia, Coprococcus, Dialister, Faecalibacterium, Lachnospira, Megamonas, Prevotella, Roseburia, and Ruminococcus. These authors also reported a higher abundance of the genera Clostridium, Devosia, Dorea, and Halomonas in the GM of the MUD group compared with the MCU group.
In the case of cocaine, Volpe et al. [] found that the use of this substance was associated with an increased and decreased abundance of members of the phyla Bacteroidota and Bacillota, respectively. Gerace et al. [] reported that the dominant genera in stool samples from cocaine users were Bacteroides, Bifidobacterium, Blautia, Collinsella, and Faecalibacterium. Comparing the GM of cocaine users to non-users, these authors revealed a significant reduction in the α-diversity of cocaine users. Moreover, they also identified higher fecal abundances of Erysipelotrichaceae, [Clostridium]_innocuum_group spp., [Ruminococcus]_torques_group spp., Blautia spp., Clostridium_sensu_stricto spp., Collinsella spp., Dorea. spp., Escherichia-Shigella spp., Eubacterium spp., Holdemanella spp., Megamonas spp., Peptococcus spp., Romboutsia spp., Rothia spp., Senegalimassilia spp., Streptococcus spp., and Turicibacter spp., in the drug-use group. In contrast, these authors found reduced levels of Christensenellaceae, Desulfovibrionaceae, Lachnospiraceae, [Eubacterium]_ventriosum_group spp., [Eubacterium]_xylanophilum_group spp., Alistipes. spp., Bacteroides. spp., Barnesiella spp., Coprobacter spp., GCA-900066575 spp., Odoribacter spp., Oscillospira spp., Paraprevotella spp., Parasutterella spp., Prevotellaceae_NK3B31_group spp., Sutterella spp., and UCG-002 spp. [].
3.3. Opioids
Opioids are a group of powerful, medically important substances that are chemically related to the natural compounds found in opium, which is derived from the opium poppy plant (Papaver somniferum). These substances are recognized for their dual effects regarding strong pain relief and sedation []. However, they also carry a significant risk of addiction and abuse []. Opioids work by binding to specific receptors on the surface of various cells, known as opiate receptors (μ, κ, and δ).
Opioids used as analgesics diminish the intensity and unpleasant perception of pain through the activation of specific G protein-coupled receptors in the brain, spinal cord, and peripheral nervous system []. Acting as agonists at the opioid receptors, these compounds reduce neuronal excitability and inhibit the release of pain neurotransmitters []. In vitro studies have shown that opioids present conflicting results regarding their antimicrobial activity. On the other hand, morphine does not appear to have an inhibitory effect on microbial strains []. Other opioids, such as bupivacaine, pethidine, tramadol, fentanyl, and methadone, have shown potent bactericidal activity in vitro against Bacillus cereus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, S. epidermidis, Streptococcus pneumoniae, and S. pyogenes [,,,,].
One of the many debilitating side effects of chronic opioid use is opioid-induced intestinal dysfunction and bacterial translocation, both in the bacterial metabolite profile and in the intestinal barrier integrity [,]. In preclinical studies, morphine induced changes in the GM composition of a mouse model associated with a significant increase in pathogenic bacteria such as Clostridium, Enterococcus, Flavobacterium, Fusobacterium, and Sutterella []. In another study in mice, subcutaneous injections of tramadol inhibited the growth of S. aureus but did not eliminate P. aeruginosa []. However, the relationship between GM dysbiosis and opioid use in humans has not been well-studied []. In a cohort of cirrhotic patients, multiple chronic opioid use (e.g., hydromorphone, fentanyl, methadone, morphine, oxycodone, percocet, tramadol, and combinations of these drugs) was associated with significant changes in GM composition, with a lower relative abundance of members of the families Bacteroidaceae and Ruminococcaceae, and of the genus Bacteroides [].
3.4. Cannabinoids
Cannabinoids are compounds derived from the cannabis plant (Cannabis sativa and C. indica), which is widely used worldwide for both cultural and medical purposes. These substances have been the subject of extensive research due to their low potential to induce dependence, their relatively mild adverse health effects, and numerous potential therapeutic benefits [,]. In vitro studies have shown that cannabis exerts an antimicrobial activity against a broad range of microorganisms including Bacillus subtilis, S. aureus, E. coli, and P. aeruginosa []; methicillin-resistant S. aureus []; and Clostridium spp., Enterococcus spp., Pseudomonas spp., and Pectobacterium spp. [].
Given that the main method of cannabis use in humans is via smoking, few studies have been conducted on the effect of this substance on the GM. Mehrpouya-Bahrami et al. [] found that CB1 antagonism reduced intestinal permeability and altered the GM including an increase in the abundance of Akkermansia muciniphila and a decrease in the members of the families Lachnospiraceae and Erysipelotrichaceae. In a cohort study comparing cannabis users and non-users, Panee et al. [] reported that the abundance of Prevotella and Bacteroides was inversely correlated among participants. In this regard, the ratio of Prevotella/Bacteroides was 13 times higher in the non-users. Vijay et al. [] reported a positive association of endocannabinoids with bacterial α-diversity as well as with short-chain fatty acid (SCFA)-producers such as Bifidobacterium, Coprococcus, and Faecalibacterium while noting a negative association with Collinsella and Escherichia-Shigella.
3.5. Nicotine
Nicotine, which is one of the primary components of tobacco, has all the features of an addictive substance []. It modulates dopamine activity in the midbrain, particularly in the mesolimbic system, which promotes the development and maintenance of rewarding behaviors []. Two in vitro studies have investigated the antimicrobial activity of nicotine. The psychotropic compound was active against Listeria monocytogenes and viridans streptococci (S. viridans) [] as well as against E. faecalis, E. coli, and P. aeruginosa [].
Several clinical studies have been conducted on the effect of tobacco on the GM. For instance, smoking cessation determines profound changes in the GM, with an increase in microbial diversity and the abundance of the Bacillota and Actinomycetota phyla and a decrease in members of the Bacteroidota and Pseudomonadota phyla []. In addition, Vogtmann et al. [], investigating the effect of smoking on the upper gastrointestinal microbiome, reported that smoking was associated with an increase in both α- and β-diversity, and the species Dialister invisus and Megasphaera micronuciformis were the most frequently detected in smokers. Moreover, a decrease in microbial population diversity, with a reduction in the genera Collinsella, Enterorhabdus, Faecalibacterium, and Gordonibacter, was found in Crohn’s disease patients who smoked [].
In a study including never, former, and current smokers, Lee et al. [] found no difference in α-diversity among the three groups. However, bacterial β-diversity exhibited significant differences. In current smokers, it increased the abundance of the phylum Bacteroidota while it decreased Bacillota and Pseudomonadota compared with those who never smoked. Conversely, there were no differences between the former and those who had never smoked. In a review study, Savin et al. [] reported increases in members of the phyla Pseudomonadota and Bacteroidota, and in the genera Bacteroides, Clostridium, and Prevotella. They also reported a decrease in the abundance of Actinomycetota and Bacillota phyla, and in the genera Bifidobacterium and Lactococcus.
Shanahan et al. [] reported that smokers had lower bacterial diversity in the upper small intestinal mucosa compared with non-smokers. The GM in smokers showed a higher relative abundance of members of the phylum Bacillota (genera Streptococcus and Veillonella) and Actinomycetota (genus Rothia) as well as lower levels of Bacteroidota (genus Prevotella) and Pseudomonadota (genus Neisseria). These results are in contrast to those of Stewart et al. [], who reported an increase in the abundance of the genera Clostridium and Prevotella and a decrease in the genus Bacteroides. In addition, Nolan-Kenney et al. [] found a relatively higher abundance of the Catenibacterium genus and of members belonging to the family Erysipelotrichaceae in current smokers. On the other hand, Lin et al. [] reported that the relative abundance of the phyla Bacteroidota and Bacillota, and of more than 40 genera, were altered with cigarette and alcohol consumption, with the most significant decrease in the abundance of members of the family Ruminococcaceae. More recently, Antinozzi et al. [] reviewed the influence of traditional and electronic cigarette smoking on the human gut microbiota. The genus Prevotella appeared to be significantly increased in current and former cigarette smokers, but not in electronic cigarette users. Furthermore, the genus Desulfovibrio (phylum Thermodesulfobacteriota) showed a progressive increase correlated with cigarette consumption (measured in packs per year). Moreover, Alphaproteobacteria levels were higher in the current smokers compared with those who had never smoked.
4. Gut Microbiome and Substance Abuse
The initiation of drug use is strongly linked to environmental and social factors [], with curiosity and expansion motives being the primary intrinsic drivers []. The existence of a positive association between the frequency of addictive substance use and the level of sensation seeking has been established []. In this regard, the GM may play a role in the initial stage of SUDs, particularly in the search and preference for novel substances.
Individuals with high novelty-seeking tendencies are particularly sensitive to novel, packaged addictive drugs. Those with cocaine and methamphetamine use disorders exhibit significantly greater drug-seeking behavior compared with healthy controls, which may contribute to sustained drug use []. Moreover, novelty-seeking traits have been positively correlated with relapse rates among cocaine users [,]. Interestingly, alterations in the GM can significantly enhance novelty-seeking behavior in animal models. Studies have shown that modifying the GM, either by administering probiotics or suppressing bacterial populations with antibiotics, leads to a marked increase in cocaine preference. This suggests that the GM and its metabolites may influence an individual’s predisposition to substances such as cocaine and morphine [,,,]. In addition, the GM may indirectly increase the likelihood of addictive drug use through its interaction with ghrelin, a hormone produced in the gastrointestinal tract. Positive correlations have been observed between ghrelin levels and the abundance of Clostridium and Ruminococcus. On the other hand, an increased Bacteroidota/Bacillota ratio as well as higher levels of Faecalibacterium and Prevotellaceae have been negatively associated with ghrelin concentrations []. Rahat-Rozenbloom et al. [] reported that ghrelin secretion decreases as SCFA production in the GM increases, and this upregulation of the ghrelin system may further contribute to drug cravings []. In summary, the GM and its metabolites may influence the ghrelin secretion levels, potentially affecting external behaviors such as novelty seeking, which in turn could impact an individual’s susceptibility to addictive substances.
The GM and its metabolites play a direct role in the reward mechanisms of addictive substances. The rewarding effect is triggered by the release of dopamine and other neurotransmitters that induce euphoria []. Through the GM–brain axis, the GM can influence brain regions involved in dopaminergic neurotransmission such as the ventral tegmental area and nucleus accumbens []. Lee et al. [] demonstrated a causal relationship between GM alterations and impaired reward responses in antibiotic-treated mice. Notably, normal reward behavior was restored through fecal microbial transplantation, highlighting the role of the GM in modulating reward sensitivity. Similarly, GM and its metabolites have been shown to be essential for morphine-induced reward mechanisms []. In addition to direct effects on dopaminergic pathways, the GM and its metabolites may indirectly regulate drug-related reward circuits via glucagon-like peptide 1 (GLP-1). GLP-1 and its analogs modulate abnormal reward effects induced by substances such as cocaine and amphetamines, with GLP-1 receptors (GLP-1R) being highly expressed in reward-related brain regions []. Research indicates that GLP-1 and GLP-1R enhance behavioral responses to cocaine in mice, while the loss of GLP-1R influences anxiety-related behaviors []. Changes in GLP-1 levels have been positively correlated with the abundance of Actinomycetota and Bacillota members, whereas Bacteroidota and Blautia spp. show an inverse correlation with the GLP-1 levels []. Furthermore, GM-derived SCFAs stimulate GLP-1 release and elevate its plasma concentration via the phospholipase C signaling pathway []. Overall, during relapse, the GM contributes to the enhancement of drug reward effects both directly, through dopaminergic modulation, and indirectly, via GLP-1 regulation.
Drug-induced epigenetic modifications have been identified as key mediators of genetic changes in brain regions involved in reward processing and drug-seeking behavior. These modifications also contribute to the development of tolerance to drug use []. The GM and its metabolites are known to influence the epigenetic landscape under various physiological conditions [,]. Intestinal microorganisms produce a diverse array of bioactive small molecules including SCFAs and other microbial by-products such as β-hydroxybutyrate, folate, and B vitamins. These compounds play crucial roles in both physiological regulation and epigenetic modifications [].
SCFAs are critical inhibitors of histone deacetylases (HDACs), enzymes that remove acetyl groups from histones, thereby leading to chromatin condensation and gene silencing [,]. Furthermore, SCFAs influence histone decrotonylation and acylation [] and act as donor substrates for histone acetyltransferases (HATs) []. Beyond histone modifications, SCFAs also modulate the activity of ten-eleven translocation (TET) methylcytosine dioxygenases, key enzymes involved in DNA demethylation. When targeted to gene promoters, TET enzymes generally enhance gene transcription, further highlighting the epigenetic regulatory role of SCFAs [].
Other microbial metabolites, such as B vitamins and methionine, serve as donors of methyl and acetyl groups by producing S-adenosylmethionine (SAM), which acts as a substrate for DNA methyltransferases (DNMTs) and histone methyltransferases (HMTs) []. While folate (vitamin B9) is primarily obtained through the diet, it can also be synthesized by various bacterial species including Bifidobacterium bifidum, B. longum, and Limosilactobacillus reuteri []. Most B vitamins, including biotin (vitamin B7), cobalamin (vitamin B12), niacin (vitamin B3), pantothenic acid (vitamin B5), and riboflavin (vitamin B2), are either produced by the microbiota or acquired through the diet. These vitamins are essential cofactors in the folate cycle, which is involved in key epigenetic processes such as DNA methylation, histone deacetylation, and histone acetylation [,,,]. All of these epigenetic modifications play a role in various processes related to the response to drugs of abuse [].
5. The Role of the Gut Microbiome in Drug Pharmacokinetics
Pharmacokinetics is a subfield of pharmacology focused on determining the fate of xenobiotics once introduced into a living organism. Drug pharmacokinetics encompass four key processes: absorption, distribution, metabolism, and excretion (ADME) []. Absorption refers to the transfer of a substance from its administration site into the bloodstream []. Currently, the influence of the GM on drug absorption remains largely unexplored, and the limited available studies did not focus on drugs of abuse [].
The GM can also impact the bioavailability of drugs without altering the drug chemical structure by modifying its absorption, solubility, and transport []. One key mechanism involves the production of secondary bile acids that can hydrolyze conjugated bile acids to their free form []. In particular, the GM can modulate the drug pharmacokinetics by regulating P-glycoprotein (MDR1 or ABCB1), a pivotal barrier to cellular drug uptake []. In addition, the GM can also affect OATP2B1, a transporter that mediates the absorption of various drugs []. Furthermore, SCFAs produced by the GM [] have a potential role in restoring intestinal permeability and influencing the passive absorption of drugs in intestinal epithelial cells [,].
Drugs undergo metabolism through various processes, including conjugation, reduction, oxidation, isomerization, hydrolysis, and condensation, with the liver serving as the primary site of drug metabolism or biotransformation in humans []. According to Lakshmanan [], most drugs are metabolized in two phases. In the first, the non-synthetic reactions introduce a hydrophilic group into the drug molecule, primarily through oxidation, reduction, and hydrolysis. Oxidation is catalyzed by a group of liver enzymes known collectively as cytochrome P450 (Cyp-450). Reduction occurs in drug molecules containing reducible nitro, azo, alkene, aldehyde, or ketone groups, and is catalyzed by specific enzymes called reductases. In turn, hydrolysis takes place in drug molecules that contain ester or amide linkages in their structure. In the second phase, metabolism primarily involves conjugation reactions in which the drug or its first phase metabolites are coupled with endogenous substances such as glucuronic acid, sulfate, or glycine. These synthetic reactions yield water-soluble metabolites that are more readily excreted via the kidneys in urine and the liver in bile compared with those produced in non-synthetic reactions.
However, the majority of orally administered hydrophilic drugs migrate from the upper to the lower intestinal tract, where the GM metabolizes them into hydrophobic metabolites. This transformation facilitates their absorption from the gastrointestinal tract into the bloodstream []. Figure 2 presents the influence of the GM on drug metabolism (modified from []).
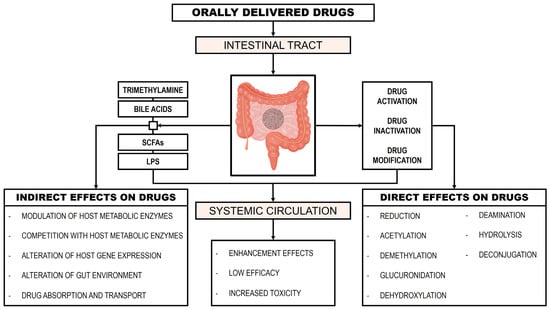
Figure 2.
Influence of the gut microbiome on drug metabolism.
Drug Biotransformation by the Gut Microbiome
Drug metabolism depends not only on the host, but also on the GM, and this specific field has been termed pharmacomicrobiomics []. Biotransformation consists of the conversion of chemical substances within a biological system including microorganisms present in several human microbiomes [,]. This microbial biotransformation is produced by microbial exoenzymes that convert low molecular weight organic compounds into analogous compounds by oxidation, reduction, hydrolysis, condensation, isomerization, unsaturation, or by the introduction of heteroatoms [].
In the case of psychostimulants, the presence of microbial esterases is involved in the biotransformation of cocaine. For example, the Delftia (formerly Comamonas) acidovorans strain MBLF, Pseudomonas fluorescens strain MBER, Rhodococcus spp. strain MB1, and Stenotrophomonas (formerly Pseudomonas) maltophilia strain MB11L can use cocaine as a carbon and nitrogen source and convert this substance to ecgonine methyl ester and benzoic acid, catalyzed by the enzyme cocaine esterase [,,]. In addition, bacterial carboxylesterases were isolated from cultures of Bacillus licheniformis strain ATCC14580 and B. subtilis subsp. subtilis strain 168 []. These enzymes catalyzed the hydrolysis of cocaine to benzoylecgonine and methanol, similar to human liver carboxylesterase hCE1 and hCE2. These enzymes have been used to treat acute cocaine toxicity [,,,,]. The microbial biotransformation of amphetamines and related compounds has also been explored in several studies. Amphetamine has been shown to be biotransformed by Mycobacterium smegmatis []. Additionally, strains of the fungus Cunninghamella echinulata were able to metabolize 3,4-methylenedioxy-N-methylarnphetamine (MDMA) and 3,4-methylenedioxyamphetamine (MDA) []. Moreover, amphetamine has been observed to bind to tyramine oxidase from the E. coli strain present in the human GM [].
Several opioids can undergo microbial biotransformation through a diverse range of microorganisms. Pseudomonas putida strain M10 has been shown to metabolize morphine and codeine via the enzyme morphine dehydrogenase [], which catalyzes their oxidation into morphinone and codeinone, respectively []. These intermediates are further converted by the enzyme morphinone reductase, leading to the formation of hydromorphone and hydrocodone [,,], with hydromorphone also being converted into dihydromorphine [,]. The enzyme (3-17)-hydroxysteroid dehydrogenase from Comamonas (formerly Pseudomonas) testosteroni has also been reported to be an effective catalyst in the bacterial biotransformation of morphine to morphinone, a key intermediate in hydromorphone biosynthesis []. Another microbial biotransformation described for morphine is the oxidation of this substance by the enzyme laccase, which is present in Ascomycetes, Deuteromycetes, and Basidiomycetes fungi [] as well as in the endospore of B. subtilis []. Codeine undergoes biotransformation by P. putida strain M10, resulting in the production of codeinone, hydrocodone, dihydrocodeine, and 14β-hydroxycodeine []. In addition, Streptomyces griseus has been reported to metabolize codeine into norcodeine []. Rhizobium radiobacter strain R89-1 also facilitates the biotransformation of codeine, yielding hydroxylated metabolites such as 14-hydroxycodeine, 14-hydroxycodeinone, 14-hydroxy-7,8-dihydrocodeine, and 14-hydroxy-7,8-dihydrocodeinone []. Moreover, the cyanobacterium Nostoc muscorum can metabolize codeine to 6-acetylcodeine, oxycodone, norcodeine, and morphine []. The microbial degradation of heroin has been demonstrated in Rhodococcus sp. strain H1, which converts the drug to 6-monoacetylmorphine (6-MAM), followed by further metabolism into morphine []. Fungi belonging to the genus Cylindrocarpon didymium produce biotransformation mediated by the oxidation of morphine to 2,2′-bimorphine []. Other fungal species of the genus Cunninghamella, such as C. bainieri, C. bertholletiae, C. blakesleeana and C. echinulata [,], produce the N-demethylation of codeine to norcodeine. In contrast, morphine and oxymorphone showed no biotransformation after incubation with C. echinulata, Curvularia lunata, Helicostylum piriforme, Sporotrichum sulfurescens, Trametes cinnabarina, and T. sanguinea [].
Akhtar et al. [] reported that a large number of microbial species can biotransform the 9-tetrahydrocannabinol (Δ9-THC) molecule present in cannabinoids by microbial hydroxylation, leading to the formation of a range of mono- and dihydroxylated derivatives [] such as the fungi Fusarium nivale, Gibberella fujikuroi, and Thamnidium elegans []. The biotransformation of Δ9-THC, Δ8-tetrahydrocannabinol (Δ8-THC), cannabidiol, and cannabinol through the partial oxidation of the n-pentyl side chain has been demonstrated in the filamentous fungus Syncephalastrum racemosum strain ATCC18192 and the bacterium Rhodococcus (formerly Mycobacterium) rhodochrous strain ATCC 19067 [,]. Table 3 presents a list of the major microbial enzymes and the corresponding genes involved in the transformation processes of various drugs.

Table 3.
Microbial enzymes and genes involved in the biotransformation of drugs of abuse.
6. Discussion
SUDs significantly affect the physical and mental health of individuals, making it a global public health issue that affected more than 284 million people aged 15–64 years old worldwide in 2020 []. At present, the treatment of SUDs mainly depends on psychological withdrawal and drug substitution, but the low efficacy and side effects of these therapies have led to the search for new treatments. The response to addiction pharmacotherapy is complex and depends on genetic, biological, environmental, and social factors []. Based on the fact that the dopaminergic system plays a critical role in the pharmacotherapy for addictions, an understanding of the role of the variation in genes involved in this system can be used as a personalized therapy for SUDs, which is one of the main focus of pharmacogenetics. Polymorphisms of several genes have been found to moderate the effects of the pharmacotherapy of alcohol, opioid, and cocaine SUDs (Table 2). However, the review of the development of pharmacogenetic biomarkers for pharmacotherapy personalization shows that the evidence for these biomarkers is still exiguous []. In this regard, despite the promising potential of these approaches, only a few treatments have been approved by the FDA and EMA for SUDs including acamprosate, disulfiram, methadone, and naltrexone [].
Genetic factors alone are not sufficient to explain the individual variability in drug response, and additional factors such as the role of the GM may also be pivotal []. Several GWASs have been conducted to investigate the interplay between human genetic variation and the composition of the GM as well as their potential influence on drug addiction []. As the field of genome–microbiome interactions is still in its early stages, metagenome-wide association studies (MGWASs) hold promise for further uncovering complex associations, supported by resources such as the Human Microbiome Project and advancements in bioinformatics tools []. However, GWASs have certain limitations: (i) microarrays contain a limited number of preselected SNPs []; (ii) statistical methods for analyzing multimarker genetic effects in large databases are not easy to develop and implement []; (iii) these types of analyses often suffer from small sample sizes combined with the need for significant multiple testing correction []; and (iv) the effect of rare and unknown genetic polymorphisms should not be overlooked, as different variants in specific pathways may be responsible for relevant effects in common diseases []. Furthermore, the requirement for genome-wide corrections in GWASs requires large sample sizes, often involving thousands of subjects, which may result in the inability to detect variants with small but genuine effects [].
In 2010, Rizkallah et al. [] outlined three potential implications of pharmacomicrobiomics for the decade from 2010 to 2019. These included the development of personalized probiotics and phage therapies, both of which are currently being applied to mitigate the adverse effects of chemotherapy and treat persistent infections. However, the proposed resistome scanning or tissue mapping has not yet been adopted as a clinical strategy. Aziz et al. [] identified three critical challenges in pharmacomicrobiomics that could drive clinical innovations: (i) the systematic implementation of high-throughput microbiome screening; (ii) the application of phage-based precision microbiome engineering and editing; and (iii) the integration of pharmacomicrobiomics into clinical practice. Moreover, these authors predict an increase in pharmacomicrobiomic and pharmacogenomic testing, along with the development of clinical guidelines for key drug classes. We argue that such advancements will mark a critical turning point in the progression of precision medicine.
Pharmacomicrobiomics is a rapidly evolving discipline that aims to understand the complex interactions between the GM, the host, and drugs, with implications for pharmacokinetics, pharmacodynamics, drug metabolism, bioavailability, and potential drug toxicity []. While significant advances have been made in this field, several challenges persist, particularly with regard to medical drugs, although they can also apply to drugs of abuse. These challenges include (i) the lack of standardized methodologies in sample collection, sequencing, and data analysis in pharmacomicrobiomics studies, which complicates the comparison of results across different studies and hinders the development of clinical guidelines; (ii) the GM is a complex and dynamic system influenced by intrinsic and extrinsic factors (e.g., diet, lifestyle, host genetics, and age) that may confound the interpretation of results; (iii) inter-individual variability in the effects of drug–GM interactions must be considered; (iv) there is a lack of a comprehensive understanding of the genetic and biochemical mechanisms underlying drug biotransformation; (v) although the potential clinical applications of pharmacomicrobiomics are promising, the development of microbiota-based biomarkers of drug response will require large-scale validation studies and regulatory approval; (vi) many pharmacomicrobiomics studies often have limited sample sizes, which may restrict their statistical power and generalizability; and (vii) there is considerable variability in sequencing methods and molecular tools, making it difficult to compare results across studies [].
To address these limitations, several strategies are expected to be implemented in the future including the use of: (i) new databases for pharmacomicrobiomics studies []; (ii) next-generation meta-omics analysis and high-throughput sequencing techniques such as 16S ribosomal RNA microbial profiling, DNA microarrays, metatranscriptomics, metabolomics, and shotgun metagenomics []; (iii) machine learning, a subfield of artificial intelligence that allows machines to acquire knowledge from data without explicit programming []; (iv) the development of predictive models and software to simulate drug–microbiota interactions, thereby reducing the research time and costs []; and (v) psychobiotics, including probiotics, prebiotics, synbiotics, and postbiotics, which may have the potential to modify the composition and activity of the GM, influencing its interaction with drugs and altering their effects on the host [].
Finally, in the context of this discussion, it is worth mentioning the emerging field of pharmacotranscriptomics, which explores the relationship between variations in the transcriptome and the pharmacokinetics and pharmacodynamics of drugs. More specifically, it aims to identify interindividual differences, facilitate biomarker discovery, and assess drug efficacy, expanding beyond the scope of traditional pharmacogenomics []. In fact, two main strategies are commonly employed and shared by both disciplines: one focuses on analyzing candidate pharmacogenes or pharmacotranscripts, while the other involves comprehensive GWASs or transcriptome-wide association studies (TWASs) to uncover new markers []. Although studies based on candidate genes or transcripts provide strong statistical power, they are limited in their ability to discover novel genes or transcripts. On the other hand, GWASs/TWASs are more effective for identifying new relevant markers, but they may present reduced statistical power due to the large number of independent tests. Recent applications of pharmacotranscriptomics include the profiling of resistant triple-negative breast cancer cells treated with lapatinib and berberine as well as analyzing gene networks involved in ferroptosis regulation in cancer [,]. Despite pharmacotranscriptomics showing substantial advancements, its direct clinical applications to SUDs are still limited, with the absence of studies on drugs of abuse underscoring this current gap within the field. Furthermore, the integration of gene expression profiling into clinical practice for personalized therapies remains in the developmental phase, lacking the widespread application seen in pharmacogenomics. Nevertheless, as research progresses, pharmacotranscriptomics holds significant potential for contributing to the personalized treatment of SUDs.
7. Conclusions
This review highlights the significant role of the GM and its metabolites in various stages of drug addiction including drug seeking, reward, and biotransformation. Identifying pharmacological targets for the treatment of SUDs remains challenging. Therefore, the advancement of precision medicine and the development of biomarkers capable of monitoring drug responses offer valuable opportunities for the development of pharmacotherapies for SUDs. Progress in the pharmacogenomics of SUDs has provided biological insights into the pharmacological needs associated with common genetic variants in drug-metabolizing enzymes.
We conclude that integrating pharmacogenomics with pharmacomicrobiomics will form a crucial foundation for significant advances in precision and personalized medicine, representing a promising field that requires further exploration and enhanced focus in both future research and clinical practice, with the potential to improve patient outcomes, optimize treatment strategies, reduce adverse drug reactions, and lower healthcare costs in the management of SUDs.
Author Contributions
Conceptualization, A.B.-R. and J.J.B.; Investigation, J.J.B.; Writing—original draft preparation, J.J.B.; Writing—review and editing, A.B.-R.; Supervision, J.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Borrego-Ruiz, A. Motivación intrínseca y consumo de drogas: Una revisión de estudios sobre los motivos de curiosidad y de expansión. Health Addict. 2024, 24, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. A holistic review of fentanyl use and its impact on public health. Curr. Addict. Res. 2024, 8, 23–33. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- McLellan, A.T. Substance misuse and substance use disorders: Why do they matter in healthcare? Trans. Am. Clin. Climatol. Assoc. 2017, 128, 112–130. [Google Scholar]
- Rasic, D.; Weerasinghe, S.; Asbridge, M.; Langille, D.B. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug Alcohol Depend. 2013, 129, 49–53. [Google Scholar] [CrossRef]
- Patriquin, M.A.; Bauer, I.E.; Soares, J.C.; Graham, D.P.; Nielsen, D.A. Addiction pharmacogenetics: A systematic review of the genetic variation of the dopaminergic system. Psychiatr. Genet. 2015, 25, 181–193. [Google Scholar]
- Corponi, F.; Fabbri, C.; Serretti, A. Pharmacogenetics in psychiatry. Adv. Pharmacol. 2018, 83, 297–331. [Google Scholar]
- Abad-Santos, F.; Aliño, S.F.; Borobia, A.M.; García-Martín, E.; Gassó, P.; Maroñas, O.; Agúndez, J.A.G. Developments in pharmacogenetics, pharmacogenomics, and personalized medicine. Pharmacol. Res. 2024, 200, 107061. [Google Scholar]
- Mills, R.E.; Pittard, W.S.; Mullaney, J.M.; Farooq, U.; Creasy, T.H.; Mahurkar, A.A.; Kemeza, D.M.; Strassler, D.S.; Ponting, C.P.; Webber, C.; et al. Natural genetic variation caused by small insertions and deletions in the human genome. Genome Res. 2011, 21, 830–839. [Google Scholar]
- Evans, W.E.; Johnson, J.A. Pharmacogenomics: The inherited basis for interindividual differences in drug response. Annu. Rev. Genom. Hum. Genet. 2001, 2, 9–39. [Google Scholar]
- Wiese, K.M.; Flowers, S.A.; Ellingrod, V.L. Pharmacogenomics. In Applied Clinical Pharmacokinetics and Pharmacodynamics of Psychopharmacological Agents; Jann, M., Penzak, S., Cohen, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 121–135. [Google Scholar]
- Morris, J.J. What is the hologenome concept of evolution? F1000Research 2018, 7, 1664. [Google Scholar]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [PubMed]
- Pepke, M.L.; Hansen, S.B.; Limborg, M.T. Unraveling host regulation of gut microbiota through the epigenome-microbiome axis. Trends Microbiol. 2024, 32, 1229–1240. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental disorders associated with gut microbiome dysbiosis in children. Children 2024, 11, 796. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 28, 1–15. [Google Scholar]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Miftahussurur, M.; Alshawsh, M.A. Pharmacomicrobiomics: Influence of gut microbiota on drug and xenobiotic metabolism. FASEB J. 2022, 36, e22350. [Google Scholar]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R. Rethinking pharmacogenomics in an ecosystem: Drug-microbiome interactions, pharmacomicrobiomics, and personalized medicine for the human supraorganism. Curr. Pharmacogenom. Pers. Med. 2012, 10, 258–261. [Google Scholar] [CrossRef]
- Saad, R.; Rizkallah, M.R.; Aziz, R.K. Gut pharmacomicrobiomics: The tip of an iceberg of complex interactions between drugs and gut-associated microbes. Gut Pathog. 2012, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.G.; Hume, N.E.; Smith, P.B.; Peters, J.M.; Patterson, A.D. Omics approaches to probe microbiota and drug metabolism interactions. Chem. Res. Toxicol. 2016, 29, 1987–1997. [Google Scholar] [CrossRef]
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature 2021, 597, 533–538. [Google Scholar] [CrossRef]
- Haiser, H.J.; Seim, K.L.; Balskus, E.P.; Turnbaugh, P.J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microb. 2014, 5, 233–238. [Google Scholar] [CrossRef]
- Tai, Y.H.; Kao, C.Y.; Zhang, Y.P.; Chiou, Y.J.; Chiu, H.H.; Thuy, T.T.D.; Liao, H.W. An integrated platform for investigating drug-microbial interactions to support pharmacomicrobiomics studies. Talanta 2025, 283, 127094. [Google Scholar] [CrossRef]
- Theilmann, M.C.; Goh, Y.J.; Nielsen, K.F.; Klaenhammer, T.R.; Barrangou, R.; Hachem, M. Lactobacillus acidophilus metabolizes dietary plant glucosides and externalizes their bioactive phytochemicals. mBio 2017, 8, e01421-17. [Google Scholar] [CrossRef]
- Gao, S.; Sun, R.; Singh, R.; Yu So, S.; Chan, C.T.Y.; Savidge, T.; Hu, M. The role of gut microbial β-glucuronidase in drug disposition and development. Drug Discov. Today 2022, 27, 103316. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Pillai, S. Microbial β-glucosidases: Recent advances and applications. Biochimie 2024, 225, 49–67. [Google Scholar] [CrossRef]
- Boddu, R.S.; Perumal, O.; Divakar, K. Microbial nitroreductases: A versatile tool for biomedical and environmental applications. Biotechnol. Appl. Biochem. 2021, 68, 1518–1530. [Google Scholar] [PubMed]
- Aziz, R.K.; Hegazy, S.M.; Yasser, R.; Rizkallah, M.R.; ElRakaiby, M.T. Drug pharmacomicrobiomics and toxicomicrobiomics: From scattered reports to systematic studies of drug-microbiome interactions. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1043–1055. [Google Scholar] [PubMed]
- Zhao, Q.; Chen, Y.; Huang, W.; Zhou, H.; Zhang, W. Drug-microbiota interactions: An emerging priority for precision medicine. Sig. Transduct. Target. Ther. 2023, 8, 386. [Google Scholar] [CrossRef]
- Vesell, E.S. Advances in pharmacogenetics and pharmacogenomics. J. Clin. Pharmacol. 2000, 40, 930–938. [Google Scholar]
- Hamilton, J.M. The role of pharmacogenomics in precision mental health care. J. Am. Assoc. Nurse Pract. 2024, 36, 143–146. [Google Scholar]
- Graham, D.P.; Harding, M.J.; Nielsen, D.A. Pharmacogenetics of addiction therapy. Methods Mol. Biol. 2022, 2547, 437–490. [Google Scholar]
- Cacabelos, R.; Cacabelos, N.; Carril, J.C. The role of pharmacogenomics in adverse drug reactions. Expert Rev. Clin. Pharmacol. 2019, 12, 407–442. [Google Scholar] [CrossRef]
- He, C.; Peng, L.; Xing, S.; Li, D.; Wang, L.; Jin, T. Population genetic difference of pharmacogenomic VIP variants in the Tibetan population. Pharmacogenom. Pers. Med. 2021, 14, 1027–1040. [Google Scholar]
- Malki, M.A.; Pearson, E.R. Drug-drug-gene interactions and adverse drug reactions. Pharmacogenom. J. 2020, 20, 355–366. [Google Scholar]
- Guo, J.; Zhou, W.; Ma, X.; Li, Y.; Zhang, H.; Wei, J.; Du, S.; Jin, T. Genetic variability of CYP4F2, CYP2D6, CYP2E1, and ACE in the Chinese Yi population. Biochem. Genet. 2024; Advance online publication. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; Zapata-Cobo, P.; Velasco, M.; Palomino, L.M.; Clemente, S.; Segarra, O.; Sánchez, C.; Tolín, M.; Moreno-Álvarez, A.; Fernández-Lorenzo, A.; et al. Association between HLA DNA variants and long-term response to anti-TNF drugs in a Spanish pediatric inflammatory bowel disease cohort. Int. J. Mol. Sci. 2023, 24, 1797. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Whirl-Carrillo, M.; Klein, T.E. PharmGKB, an integrated resource of pharmacogenomic knowledge. Curr. Protoc. 2021, 1, e226. [Google Scholar] [CrossRef]
- Li, D.; Peng, L.; Xing, S.; He, C.; Jin, T. Genetic analysis of pharmacogenomic VIP variants in the Wa population from Yunnan province of China. BMC Genom. Data 2021, 22, 51. [Google Scholar] [CrossRef]
- Diekhof, E.K.; Richter, A.; Brodmann, K.; Gruber, O. Dopamine multilocus genetic profiles predict sex differences in reactivity of the human reward system. Brain Struct. Funct. 2021, 226, 1099–1114. [Google Scholar] [CrossRef]
- Kiefer, F.; Witt, S.H.; Frank, J.; Richter, A.; Treutlein, J.; Lemenager, T.; Nothen, M.M.; Cichon, S.; Batra, A.; Berner, M.; et al. Involvement of the atrial natriuretic peptide transcription factor GATA4 in alcohol dependence, relapse risk and treatment response to acamprosate. Pharmacogenom. J. 2011, 11, 368–374. [Google Scholar] [CrossRef]
- Morley, K.C.; Luquin, N.; Baillie, A.; Fraser, I.; Trent, R.J.; Dore, G.; Phung, N.; Haber, P.S. Moderation of baclofen response by a GABAB receptor polymorphism: Results from the BacALD randomized controlled trial. Addiction 2018, 113, 2205–2213. [Google Scholar] [CrossRef]
- Guardia, J.; Caso, C.; Arias, F.; Gual, A.; Sanahuja, J.; Ramirez, M.; Mengual, I.; Gonzalvo, B.; Segura, L.; Trujols, J.; et al. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multicenter clinical trial. Alcohol Clin. Exp. Res. 2002, 26, 1381–1387. [Google Scholar] [CrossRef]
- Setiawan, E.; Pihl, R.O.; Cox, S.M.; Gianoulakis, C.; Palmour, R.M.; Benkelfat, C.; Leyton, M. The effect of naltrexone on alcohol’s stimulant properties and self-administration behavior in social drinkers: Influence of gender and genotype. Alcohol Clin. Exp. Res. 2011, 35, 1134–1141. [Google Scholar] [CrossRef]
- Hutchison, K.E.; McGeary, J.; Smolen, A.; Bryan, A.; Swift, R.M. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychol. 2002, 21, 139–146. [Google Scholar] [CrossRef]
- Johnson, B.A.; Roache, J.D.; Javors, M.A.; DiClemente, C.C.; Cloninger, C.R.; Prihoda, T.J.; Bordnick, P.S.; Ait-Daoud, N.; Hensler, J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA 2000, 284, 963–971. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Wetherill, R.; Feinn, R.; Pond, T.; Gelernter, J.; Covault, J. Posttreatment effects of topiramate treatment for heavy drinking. Alcohol Clin. Exp. Res. 2014, 38, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Preuss, U.W.; Wurst, F.M.; Ridinger, M.; Rujescu, D.; Fehr, C.; Koller, G.; Bondy, B.; Wodarz, N.; Soyka, M.; Zill, P. Association of functional DBH genetic variants with alcohol dependence risk and related depression and suicide attempt phenotypes: Results from a large multicenter association study. Drug Alcohol Depend. 2013, 133, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Kampangkaew, J.P.; Spellicy, C.J.; Nielsen, E.M.; Harding, M.J.; Ye, A.; Hamon, S.C.; Kosten, T.R.; Nielsen, D.A. Pharmacogenetic roles of dopamine transporter (SLC6A3) variation on response to disulfiram treatment for cocaine addiction. Am. J. Addict. 2019, 28, 311–317. [Google Scholar] [CrossRef]
- Kosten, T.R.; Wu, G.; Huang, W.; Harding, M.J.; Hamon, S.C.; Lappalainen, J.; Nielsen, D.A. Pharmacogenetic randomized trial for cocaine abuse: Disulfiram and dopamine β-hydroxylase. Biol. Psychiatry 2013, 73, 219–224. [Google Scholar] [CrossRef]
- Shorter, D.; Nielsen, D.A.; Huang, W.; Harding, M.J.; Hamon, S.C.; Kosten, T.R. Pharmacogenetic randomized trial for cocaine abuse: Disulfiram and α1A-adrenoceptor gene variation. Eur. Neuropsychopharmacol. 2013, 23, 1401–1407. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Harding, M.J.; Hamon, S.C.; Huang, W.; Kosten, T.R. Modifying the role of serotonergic 5-HTTLPR and TPH2 variants on disulfiram treatment of cocaine addiction: A preliminary study. Genes Brain Behav. 2012, 11, 1001–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Nielsen, D.A.; Domingo, C.B.; Shorter, D.I.; Nielsen, E.M.; Kosten, T.R. Pharmacogenetics of dopamine β-hydroxylase in cocaine dependence therapy with doxazosin. Addict. Biol. 2019, 24, 531–538. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Hamon, S.; Kosten, T.R. The κ-opioid receptor gene as a predictor of response in a cocaine vaccine trial. Psychiatr. Genet. 2013, 23, 225–232. [Google Scholar] [CrossRef]
- Kosten, T.R.; Domingo, C.B.; Hamon, S.C.; Nielsen, D.A. DBH gene as predictor of response in a cocaine vaccine clinical trial. Neurosci. Lett. 2013, 541, 29–33. [Google Scholar] [CrossRef]
- Zahari, Z.; Lee, C.S.; Ibrahim, M.A.; Musa, N.; Yasin, M.A.M.; Lee, Y.Y.; Tan, S.C.; Mohamad, N.; Ismail, R. Influence of DRD2 polymorphisms on the clinical outcomes of opioid-dependent patients on methadone maintenance therapy. J. Pharm. Bioallied Sci. 2020, 12, S787–S803. [Google Scholar] [CrossRef]
- Levran, O.; O’Hara, K.; Peles, E.; Li, D.; Barral, S.; Ray, B.; Borg, L.; Ott, J.; Adelson, M.; Kreek, M.J. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum. Mol. Genet. 2008, 17, 2219–2227. [Google Scholar] [PubMed]
- Cheng, C.Y.; Hong, C.J.; Yu, Y.W.Y.; Chen, T.J.; Wu, H.C.; Tsai, S.J. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Mol. Brain Res. 2005, 140, 86–90. [Google Scholar] [PubMed]
- Oneda, B.; Crettol, S.; Bochud, M.; Besson, J.; Croquette-Krokar, M.; Hammig, R.; Monnat, M.; Preisig, M.; Eap, C.B. β-Arrestin2 influences the response to methadone in opioid-dependent patients. Pharmacogenom. J. 2011, 11, 258–266. [Google Scholar]
- Levran, O.; Peles, E.; Hamon, S.; Randesi, M.; Adelson, M.; Kreek, M.J. CYP2B6 SNPs are associated with methadone dose required for effective treatment of opioid addiction. Addict. Biol. 2013, 18, 709–716. [Google Scholar]
- Dagostino, C.; Allegri, M.; Napolioni, V.; D’Agnelli, S.; Bignami, E.; Mutti, A.; van Schaik, R.H.N. CYP2D6 genotype can help to predict effectiveness and safety during opioid treatment for chronic low back pain: Results from a retrospective study in an Italian cohort. Pharmacogenom. Pers. Med. 2018, 11, 179–191. [Google Scholar]
- Fonseca, F.; Gratacos, M.; Escaramis, G.; De Cid, R.; Martin-Santos, R.; Fernandez-Espejo, E.; Estivill, X.; Torrens, M. Response to methadone maintenance treatment is associated with the MYOCD and GRM6 genes. Mol. Diagn. Ther. 2010, 14, 171–178. [Google Scholar]
- Fang, C.P.; Liu, T.H.; Chung, R.H.; Tsou, H.H.; Kuo, H.W.; Wang, S.C.; Liu, C.C.; Liu, S.C.; Chen, A.C.H.; Liu, Y.L. Genetic variants in NECTIN4 encoding an adhesion molecule are associated with continued opioid use. PLoS ONE 2020, 15, e0234549. [Google Scholar] [CrossRef]
- Kamarajan, C.; Pandey, A.K.; Chorlian, D.B.; Manz, N.; Stimus, A.T.; Edenberg, H.J.; Wetherill, L.; Schuckit, M.; Wang, J.C.; Kuperman, S.; et al. AKCNJ6 gene polymorphism modulates theta oscillations during reward processing. Int. J. Psychol. 2017, 115, 13–23. [Google Scholar]
- Burns, J.A.; Kroll, D.S.; Feldman, D.E.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Molecular imaging of opioid and dopamine systems: Insights into the pharmacogenetics of opioid use disorders. Front. Psych. 2019, 10, 626. [Google Scholar]
- Levran, O.; Peles, E.; Randesi, M.; Correa da Rosa, J.; Ott, J.; Rotrosen, J.; Adelson, M.; Kreek, M.J. Glutamatergic and GABAergic susceptibility loci for heroin and cocaine addiction in subjects of African and European ancestry. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 118–123. [Google Scholar]
- Nelson, E.C.; Lynskey, M.T.; Heath, A.C.; Wray, N.; Agrawal, A.; Shand, F.L.; Henders, A.K.; Wallace, L.; Todorov, A.A.; Schrage, A.J.; et al. Association of OPRD1 polymorphisms with heroin dependence in a large case-control series. Addict. Biol. 2014, 19, 111–121. [Google Scholar] [PubMed]
- Lindell, A.E.; Zimmermann-Kogadeeva, M.; Patil, K.R. Multimodal interactions of drugs, natural compounds and pollutants with the gut microbiota. Nat. Rev. Microbiol. 2022, 20, 431–443. [Google Scholar] [PubMed]
- Krystal, J.H.; Staley, J.; Mason, G.; Petrakis, I.L.; Kaufman, J.; Harris, R.A.; Gelernter, J.; Lappalainen, J. γ-aminobutyric acid type A receptors and alcoholism: Intoxication, dependence, vulnerability, and treatment. Arch. Gen. Psychiatry 2006, 63, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Naassila, M.; Pierrefiche, O. GluN2B subunit of the NMDA receptor: The keystone of the effects of alcohol during neurodevelopment. Neurochem. Res. 2019, 44, 78–88. [Google Scholar]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Starkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar]
- Peterson, V.L.; Jury, N.J.; Cabrera-Rubio, R.; Draper, L.A.; Crispie, F.; Cotter, P.D.; Dinan, T.G.; Holmes, A.; Cryan, J.F. Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 2017, 323, 172–176. [Google Scholar]
- Bjørkhaug, S.T.; Aanes, H.; Neupane, S.P.; Bramness, J.G.; Malvik, S.; Henriksen, C.; Skar, V.; Medhus, A.W.; Valeur, J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes 2019, 10, 663–675. [Google Scholar]
- Litwinowicz, K.; Choroszy, M.; Waszczuk, E. Changes in the composition of the human intestinal microbiome in alcohol use disorder: A systematic review. Am. J. Drug Alcohol Abus. 2020, 46, 4–12. [Google Scholar]
- Addolorato, G.; Ponziani, F.R.; Dionisi, T.; Mosoni, C.; Vassallo, G.A.; Sestito, L.; Petito, V.; Picca, A.; Marzetti, E.; Tarli, C.; et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. 2020, 40, 878–888. [Google Scholar]
- Du, Y.; Li, L.; Gong, C.; Li, T.; Xia, Y. The diversity of the intestinal microbiota in patients with alcohol use disorder and its relationship to alcohol consumption and cognition. Front. Psychiatry 2022, 13, 1054685. [Google Scholar]
- Baltazar-Díaz, T.A.; González-Hernández, L.A.; Aldana-Ledesma, J.M.; Peña-Rodríguez, M.; Vega-Magaña, A.N.; Zepeda-Morales, A.S.M.; López-Roa, R.I.; Del Toro-Arreola, S.; Martínez-López, E.; Salazar-Montes, A.M.; et al. Escherichia/Shigella, SCFAs, and metabolic pathways—The triad that orchestrates intestinal dysbiosis in patients with decompensated alcoholic cirrhosis from Western Mexico. Microorganisms 2022, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.H.; Mizuno, Y.; Volkow, N.D.; Howes, O.D. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.; Tognotti, E. Brief history of the medical and non-medical use of amphetamine-like psychostimulants. Exp. Neurol. 2021, 342, 113754. [Google Scholar] [CrossRef] [PubMed]
- Asser, A.; Taba, P. Psychostimulants and movement disorders. Front. Neurol. 2015, 6, 75. [Google Scholar] [CrossRef]
- Ning, T.; Gong, X.; Xie, L.; Ma, B. Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front. Microbiol. 2017, 8, 1620. [Google Scholar] [CrossRef]
- Cook, R.R.; Fulcher, J.A.; Tobin, N.H.; Li, F.; Lee, D.J.; Woodward, C.; Javanbakht, M.; Brookmeyer, R.; Shoptaw, S.; Bolan, R.; et al. Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Sci. Rep. 2019, 9, 14840. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Liu, X.; Liu, G.; Zeng, K.; Wang, G. Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci. Rep. 2021, 11, 18178. [Google Scholar] [CrossRef]
- He, L.; Yang, B.Z.; Ma, Y.J.; Wen, L.; Liu, F.; Zhang, X.J.; Liu, T.Q. Differences in clinical features and gut microbiota between individuals with methamphetamine casual use and methamphetamine use disorder. Front. Cell. Infect. Microbiol. 2023, 13, 1103919. [Google Scholar] [CrossRef]
- Volpe, G.E.; Ward, H.; Mwamburi, M.; Dinh, D.; Bhalchandra, S.; Wanke, C.; Kane, A.V. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J. Stud. Alcohol Drugs 2014, 75, 347–357. [Google Scholar] [CrossRef]
- Gerace, E.; Baldi, S.; Salimova, M.; Di Gloria, L.; Curini, L.; Cimino, V.; Nannini, G.; Russo, E.; Pallecchi, M.; Ramazzotti, M.; et al. Oral and fecal microbiota perturbance in cocaine users: Can rTMS-induced cocaine abstinence support eubiosis restoration? iScience 2023, 26, 106627. [Google Scholar] [CrossRef]
- Stein, C. New concepts in opioid analgesia. Expert Opin. Investig. Drugs 2018, 27, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [PubMed]
- Trang, T.; Al-Hasani, R.; Salvemini, D.; Salter, M.W.; Gutstein, H.; Cahill, C.M. Pain and poppies: The good, the bad, and the ugly of opioid analgesics. J. Neurosci. 2015, 35, 13879–13888. [Google Scholar]
- Valentino, R.J.; Volkow, N.D. Untangling the complexity of opioid receptor function. Neuropsychopharmacology 2018, 43, 2514–2520. [Google Scholar] [CrossRef]
- Rosenberg, P.H.; Renkonen, O.V. Antimicrobial activity of bupivacaine and morphine. Anesthesiology 1985, 62, 178–179. [Google Scholar]
- Grimmond, T.R.; Brownridge, P. Antimicrobial activity of bupivacaine and pethidine. Anaesth. Intensive Care 1986, 14, 418–420. [Google Scholar]
- Kesici, S.; Demırci, M.; Kesici, U. Efeitos antimicrobianos do fentanil e da bupivacaína: Estudo in vitro [Antimicrobial effects of fentanyl and bupivacaine]. Braz. J. Anesthesiol. 2020, 70, 357–363. [Google Scholar]
- Sheagren, J.N.; Barsoum, I.S.; Lin, M.Y. Methadone: Antimicrobial activity and interaction with antibiotics. Antimicrob. Agents Chemother. 1977, 12, 748–750. [Google Scholar]
- Tamanai-Shacoori, Z.; Shacoori, V.; Jolivet-Gougeon, A.; Vo Van, J.M.; Repere, M.; Donnio, P.Y.; Bonnaure-Mallet, M. The antibacterial activity of tramadol against bacteria associated with infectious complications after local or regional anesthesia. Anesth. Analg. 2007, 105, 524–527. [Google Scholar]
- Cruz-Lebrón, A.; Johnson, R.; Mazahery, C.; Troyer, Z.; Joussef-Piña, S.; Quiñones-Mateu, M.E.; Strauch, C.M.; Hazen, S.L.; Levine, A.D. Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes 2021, 13, 1946368. [Google Scholar] [CrossRef]
- Herlihy, B.; Roy, S. Gut-microbiome implications in opioid use disorder and related behaviors. Adv. Drug Alcohol Res. 2022, 2, 10311. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [PubMed]
- Farzam, H.; Farahani, A.; Tafkik, A.; Gorgin Karaji, A.; Mohajeri, P.; Rezaei, M.; Jalalvandi, F. Antibacterial effect of tramadol against Staphylococcus aureus and Pseudomonas aeruginosa: An in vivo study. New Microbes New Infect. 2018, 24, 42–46. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Z.; Wang, H.; Shen, Z.; Guo, Y.; Gao, Y.; Chen, X.; Wu, Q.; Li, X.; Wang, K. Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci. Rep. 2017, 7, 3628. [Google Scholar] [CrossRef]
- Acharya, C.; Betrapally, N.S.; Gillevet, P.M.; Sterling, R.K.; Akbarali, H.; White, M.B.; Ganapathy, D.; Fagan, A.; Sikaroodi, M.; Bajaj, J.S. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 319–331. [Google Scholar]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic potential of cannabis, cannabidiol, and cannabinoid-based pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Ali, E.; Almagboul, A.; Khogali, S.; Gergeir, U. Antimicrobial activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar]
- Panee, J.; Gerschenson, M.; Chang, L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J. Neuroimmune Pharmacol. 2018, 13, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Kouraki, A.; Gohir, S.; Turnbull, J.; Kelly, A.; Chapman, V.; Barrett, D.A.; Bulsiewicz, W.J.; Valdes, A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes 2021, 13, 1997559. [Google Scholar] [PubMed]
- Benowitz, N.L. Pharmacology of nicotine: Addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar]
- Rice, M.E.; Cragg, S.J. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004, 7, 583–584. [Google Scholar] [CrossRef]
- Pavia, C.S.; Pierre, A.; Nowakowski, J. Antimicrobial activity of nicotine against a spectrum of bacterial and fungal pathogens. J. Med. Microbiol. 2000, 49, 675–676. [Google Scholar]
- Zaidi, M.I.; Wattoo, F.H.; Wattoo, M.H.S.; Tirmizi, S.A.; Salman, S. Antibacterial activities of nicotine and its zinc complex. Afr. J. Microbiol. Res. 2012, 6, 5134–5137. [Google Scholar]
- Biedermann, L.; Zeitz, J.; Mwinyi, J.; Sutter-Minder, E.; Rehman, A.; Ott, S.J.; Steurer-Stey, C.; Frei, A.; Frei, P.; Scharl, M.; et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 2013, 8, e59260. [Google Scholar]
- Vogtmann, E.; Flores, R.; Yu, G.; Freedman, N.D.; Shi, J.; Gail, M.H.; Dye, B.A.; Wang, G.Q.; Klepac-Ceraj, V.; Paster, B.J.; et al. Association between tobacco use and the upper gastrointestinal microbiome among Chinese men. Cancer Causes Control 2015, 26, 581–588. [Google Scholar]
- Opstelten, J.L.; Plassais, J.; van Mil, S.W.; Achouri, E.; Pichaud, M.; Siersema, P.D.; Oldenburg, B.; Cervino, A.C. Gut microbial diversity is reduced in smokers with Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 2070–2077. [Google Scholar]
- Lee, S.H.; Yun, Y.; Kim, S.J.; Lee, E.J.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.L.; Kim, H.N.; Lee, J.H. Association between cigarette smoking status and composition of gut microbiota: Population-based cross-sectional study. J. Clin. Med. 2018, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Savin, Z.; Kivity, S.; Yonath, H.; Yehuda, S. Smoking and the intestinal microbiome. Arch. Microbiol. 2018, 200, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, E.R.; Shah, A.; Koloski, N.; Walker, M.M.; Talley, N.J.; Morrison, M.; Holtmann, G.J. Influence of cigarette smoking on the human duodenal mucosa-associated microbiota. Microbiome 2018, 6, 150. [Google Scholar] [CrossRef]
- Stewart, C.J.; Auchtung, T.A.; Ajami, N.J.; Velasquez, K.; Smith, D.P.; De La Garza, R., II; Salas, R.; Petrosino, J.F. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: A pilot study. PeerJ 2018, 6, e4693. [Google Scholar] [CrossRef]
- Nolan-Kenney, R.; Wu, F.; Hu, J.; Yang, L.; Kelly, D.; Li, H.; Jasmine, F.; Kibriya, M.G.; Parvez, F.; Shaheen, I.; et al. The association between smoking and gut microbiome in Bangladesh. Nicotine Tob. Res. 2020, 22, 1339–1346. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, Y.; Chen, L.; Qi, Y.; He, J.; Hu, M.; Zhang, Y.; Fan, L.; Yang, T.; Wang, L.; et al. The effects of cigarettes and alcohol on intestinal microbiota in healthy men. J. Microbiol. 2020, 58, 926–937. [Google Scholar] [CrossRef]
- Antinozzi, M.; Giffi, M.; Sini, N.; Gallè, F.; Valeriani, F.; De Vito, C.; Liguori, G.; Romano Spica, V.; Cattaruzza, M.S. Cigarette smoking and human gut microbiota in healthy adults: A systematic review. Biomedicines 2022, 10, 510. [Google Scholar] [CrossRef]
- Mirnics, Z.; Kövi, Z.; Tanyi, Z.; Grezsa, F. Adolescent drug use, relational variables and personality factors. Psychiatr. Danub. 2021, 33, 656–665. [Google Scholar]
- Hamdan-Mansour, A.; Mahmoud, K.; Shibi, A.; Arabiat, D. Impulsivity and sensation-seeking personality traits as predictors of substance use among university students. J. Psychosoc. Nurs. Ment. Health Serv. 2018, 56, 57–63. [Google Scholar]
- Mahoney, J.; Thompson-Lake, D.; Cooper, K.; Verrico, C.; Newton, T.; De La Garza, R., II. A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. J. Psychopharmacol. 2015, 29, 50–56. [Google Scholar]
- Ismael, F.; Baltieri, D. Role of personality traits in cocaine craving throughout an outpatient psychosocial treatment program. Braz. J. Psychiatry 2014, 36, 24–31. [Google Scholar] [PubMed]
- Wingo, T.; Nesil, T.; Choi, J.; Li, M. Novelty seeking and drug addiction in humans and animals: From behavior to molecules. J. Neuroimmune Pharmacol. 2016, 11, 456–470. [Google Scholar] [PubMed]
- Dugyala, S.; Ptacek, T.; Simon, J.; Li, Y.; Frohlich, F. Putative modulation of the gut microbiome by probiotics enhances preference for novelty in a preliminary double-blind placebo-controlled study in ferrets. Anim. Microbiome 2020, 2, 14. [Google Scholar]
- Hofford, R.; Mervosh, N.; Euston, T.; Meckel, K.; Orr, A.; Kiraly, D. Alterations in microbiome composition and metabolic byproducts drive behavioral and transcriptional responses to morphine. Neuropsychopharmacology 2021, 46, 2062–2072. [Google Scholar]
- Kiraly, D.; Walker, D.; Calipari, E.; Labonte, B.; Issler, O.; Pena, C.; Ribeiro, E.A.; Russo, S.J.; Nestler, E.J. Alterations of the host microbiome affect behavioral responses to cocaine. Sci. Rep. 2016, 6, 35455. [Google Scholar]
- Lee, K.; Vuong, H.; Nusbaum, D.; Hsiao, E.; Evans, C.; Taylor, A. The gut microbiota mediates reward and sensory responses associated with regimen selective morphine dependence. Neuropsychopharmacology 2018, 43, 2606–2614. [Google Scholar]
- Leeuwendaal, N.; Cryan, J.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Cheng, J.; Wolever, T. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur. J. Clin. Nutr. 2017, 71, 953–958. [Google Scholar]
- Zallar, L.; Farokhnia, M.; Tunstall, B.; Vendruscolo, L.; Leggio, L. The role of the ghrelin system in drug addiction. Int. Rev. Neurobiol. 2017, 136, 89–119. [Google Scholar]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar]
- García-Cabrerizo, R.; Carbia, C.; Riordan, K.; Schellekens, H.; Cryan, J. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021, 157, 1495–1524. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Engel, J.; Jerlhag, E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS ONE 2013, 8, e69010. [Google Scholar] [CrossRef] [PubMed]
- Harasta, A.; Power, J.; von Jonquieres, G.; Karl, T.; Drucker, D.; Housley, G.; Schneider, M.; Klugmann, M. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology 2015, 40, 1969–1978. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Martín-Núñez, G.; Roca-Rodríguez, M.; Cardona, F.; Coin-Aragüez, L.; Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Muñoz-Garach, A.; Fernández-García, J.C.; Moreno-Indias, I.; et al. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. J. Clin. Med. 2019, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jin, T. The incretin hormone GLP-1 and mechanisms underlying its secretion. J. Diabetes 2016, 8, 753–765. [Google Scholar]
- Kolli, U.; Roy, S. The role of the gut microbiome and microbial metabolism in mediating opioid-induced changes in the epigenome. Front. Microbiol. 2023, 14, 1233194. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Epigenetic mechanisms in aging: Extrinsic factors and gut microbiome. Genes 2024, 15, 1599. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Dhillon, R.S.; Denu, J.M.; Carey, H.V. Metabolic programming of the epigenome: Host and gut microbial metabolite interactions with host chromatin. Transl. Res. 2017, 189, 30–50. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Wu, S.E.; Hashimoto-Hill, S.; Woo, V.; Eshleman, E.M.; Whitt, J.; Engleman, L.; Karns, R.; Denson, L.A.; Haslam, D.B.; Alenghat, T. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 2020, 586, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [PubMed]
- Lund, P.J.; Gates, L.A.; Leboeuf, M.; Smith, S.A.; Chau, L.; Lopes, M.; Friedman, E.S.; Saiman, Y.; Kim, M.S.; Shoffler, C.A.; et al. Stable isotope tracing in vivo reveals a metabolic bridge linking the microbiota to host histone acetylation. Cell Rep. 2022, 41, 111809. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.H.; Köferle, A.; Beck, S. From profiles to function in epigenomics. Nat. Rev. Genet. 2017, 18, 51–66. [Google Scholar]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar]
- Engevik, M.A.; Morra, C.N.; Röth, D.; Engevik, K.; Spinler, J.K.; Devaraj, S.; Crawford, S.E.; Estes, M.K.; Kalkum, M.; Versalovic, J. Microbial metabolic capacity for intestinal folate production and modulation of host folate receptors. Front. Microbiol. 2019, 10, 2305. [Google Scholar]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B vitamins and their roles in gut health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef]
- McAuley, E.; McNulty, H.; Hughes, C.; Strain, J.J.; Ward, M. Riboflavin status, MTHFR genotype and blood pressure: Current evidence and implications for personalised nutrition. Proc. Nutr. Soc. 2016, 75, 405–414. [Google Scholar]
- Patel, A.B.; He, Y.; Radhakrishnan, I. Histone acetylation and deacetylation—Mechanistic insights from structural biology. Gene 2023, 890, 147798. [Google Scholar]
- Siudeja, K.; Srinivasan, B.; Xu, L.; Rana, A.; de Jong, J.; Nollen, E.A.; Jackowski, S.; Sanford, L.; Hayflick, S.; Sibon, O.C. Impaired coenzyme A metabolism affects histone and tubulin acetylation in Drosophila and human cell models of pantothenate kinase associated neurodegeneration. EMBO Mol. Med. 2011, 3, 755–766. [Google Scholar]
- Pacey, S.; Workman, P.; Sarker, D. Pharmacokinetics and pharmacodynamics in drug development. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 2845–2848. [Google Scholar]
- Doogue, M.P.; Polasek, T.M. The ABCD of clinical pharmacokinetics. Ther. Adv. Drug Saf. 2013, 4, 5–7. [Google Scholar] [PubMed]
- Cussotto, S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Psychotropics and the microbiome: A chamber of secrets…. Psychopharmacology 2019, 236, 1411–1432. [Google Scholar] [PubMed]
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2021, 11, 1789–1812. [Google Scholar]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile acids and their derivatives as potential modifiers of drug release and pharmacokinetic profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef]
- Kinzi, J.; Grube, M.; Meyer Zu Schwabedissen, H.E. OATP2B1—The underrated member of the organic anion transporting polypeptide family of drug transporters? Biochem. Pharmacol. 2021, 188, 114534. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Pohl, K.; Moodley, P.; Dhanda, A. The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1498–1506. [Google Scholar] [CrossRef]
- Patel, M.; Taskar, K.S.; Zamek-Gliszczynski, M.J. Importance of hepatic transporters in clinical disposition of drugs and their metabolites. J. Clin. Pharmacol. 2016, 56, S23–S39. [Google Scholar]
- Lakshmanan, M. Drug metabolism. In Introduction to Basics of Pharmacology and Toxicology; Raj, G., Raveendran, R., Eds.; Springer: Singapore, 2019; pp. 99–116. [Google Scholar]
- de Campos, E.G.; de Almeida, O.G.G.; De Martinis, E.C.P. The role of microorganisms in the biotransformation of psychoactive substances and its forensic relevance: A critical interdisciplinary review. Forensic Sci. Res. 2023, 8, 173–184. [Google Scholar]
- Otles, S.; Özyurt, V.H. Biotransformation in the production of secondary metabolites. Stud. Nat. Prod. Chem. 2021, 68, 435–457. [Google Scholar]
- Yousuf, M.; Jamil, W.; Mammadova, K. Microbial bioconversion: A regio-specific method for novel drug design and toxicolog-ical study of metabolites. Curr. Pharm. Biotechnol. 2019, 20, 1156–1162. [Google Scholar] [PubMed]
- Bresler, M.M.; Rosser, S.J.; Basran, A.; Bruce, N.C. Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Appl. Environ. Microbiol. 2000, 66, 904–908. [Google Scholar]
- Britt, A.J.; Bruce, N.C.; Lowe, C.R. Identification of a cocaine esterase in a strain of Pseudomonas maltophilia. J. Bacteriol. 1992, 174, 2087–2094. [Google Scholar]
- Lister, D.L.; Sproulé, R.F.; Britt, A.J.; Lowe, C.R.; Bruce, N.C. Degradation of cocaine by a mixed culture of Pseudomonas fluorescens MBER and Comamonas acidovorans MBLF. Appl. Environ. Microbiol. 1996, 62, 94–99. [Google Scholar]
- Mustafa, S.A. The development of bacterial carboxylesterase biological recognition elements for cocaine detection. Mol. Biotechnol. 2018, 60, 601–607. [Google Scholar]
- Brim, R.L.; Nance, M.R.; Youngstrom, D.W.; Narasimhan, D.; Zhan, C.G.; Tesmer, J.J.; Sunahara, R.K.; Woods, J.H. A thermally stable form of bacterial cocaine esterase: A potential therapeutic agent for treatment of cocaine abuse. Mol. Pharmacol. 2010, 77, 593–600. [Google Scholar]
- Chen, X.; Deng, J.; Cui, W.; Hou, S.; Zhang, J.; Zheng, X.; Ding, X.; Wei, H.; Zhou, Z.; Kim, K.; et al. Development of Fc-fused cocaine hydrolase for cocaine addiction treatment: Catalytic and pharmacokinetic properties. AAPS J. 2018, 20, 53. [Google Scholar] [CrossRef]
- Howell, L.; Nye, J.; Stehouwer, J.; Voll, R.; Narasimhan, D.; Nichols, J.; Sunahara, R.; Goodman, M.; Carroll, F.; Woods, J.; et al. A thermostable bacterial cocaine esterase rapidly eliminates cocaine from brain in nonhuman primates. Transl. Psychiatry 2014, 4, e407. [Google Scholar] [CrossRef]
- Narasimhan, D.; Woods, J.H.; Sunahara, R.K. Bacterial cocaine esterase: A protein-based therapy for cocaine overdose and addiction. Future Med. Chem. 2012, 4, 137–150. [Google Scholar]
- Zhang, T.; Zheng, X.; Zhou, Z.; Chen, X.; Jin, Z.; Deng, J.; Zhan, C.G.; Zheng, F. Clinical potential of an enzyme-based novel therapy for cocaine overdose. Sci. Rep. 2017, 7, 15303. [Google Scholar] [CrossRef] [PubMed]
- Coutts, R.T.; Foster, B.C. Metabolism of amphetamines by Mycobacterium smegmatis. Can. J. Microbiol. 1980, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.C.; Wilson, D.L.; Marwood, T.; Ethier, J.C.; Zamecnik, J. Microbial transformation of 3,4-methylenedioxy-N-methylamphetamine and 3,4-methylenedioxyamphetamine. Can. J. Microbiol. 1996, 42, 851–854. [Google Scholar]
- Kumar, K.; Dhoke, G.V.; Sharma, A.K.; Jaiswal, S.K.; Sharma, V.K. Mechanistic elucidation of amphetamine metabolism by tyramine oxidase from human gut microbiota using molecular dynamics simulations. J. Cell. Biochem. 2019, 120, 11206–11215. [Google Scholar]
- Bruce, N.C.; Wilmot, C.J.; Jordan, K.N.; Stephens, L.D.; Lowe, C.R. Microbial degradation of the morphine alkaloids. Purification and characterization of morphine dehydrogenase from Pseudomonas putida M10. Biochem. J. 1991, 274, 875–880. [Google Scholar] [CrossRef]
- Walker, E.H.; French, C.E.; Rathbone, D.A.; Bruce, N.C. Mechanistic studies of morphine dehydrogenase and stabilization against covalent inactivation. Biochem. J. 2000, 345, 687–692. [Google Scholar] [CrossRef]
- Boonstra, B.; Rathbone, D.A.; Bruce, N.C. Engineering novel biocatalytic routes for production of semisynthetic opiate drugs. Biomol. Eng. 2001, 18, 41–47. [Google Scholar]
- Hailes, A.M.; Bruce, N.C. Biological synthesis of the analgesic hydromorphone, an intermediate in the metabolism of morphine, by Pseudomonas putida M10. Appl. Environ. Microbiol. 1993, 59, 2166–2170. [Google Scholar]
- Long, M.T.; Hailes, A.M.; Kirby, G.W.; Bruce, N.C. Transformations of morphine alkaloids by Pseudomonas putida M10. Appl. Environ. Microbiol. 1995, 61, 3645–3649. [Google Scholar]
- Shraddha, N.; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef] [PubMed]
- Lister, D.L.; Kanungo, G.; Rathbone, D.A.; Bruce, N.C. Transformations of codeine to important semisynthetic opiate derivatives by Pseudomonas putida M10. FEMS Microbiol. Lett. 1999, 181, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.A.; Reddy, G.S.; Vatvars, A. Identification of transformation products arising from bacterial oxidation of codeine by Streptomyces griseus. Appl. Environ. Microbiol. 1985, 50, 831–836. [Google Scholar] [CrossRef]
- Kyslíková, E.; Babiak, P.; Stepánek, V.; Zahradnik, J.; Palyzova, A.; Maresova, H.; Valesova, R.; Hajicek, J.; Kyslik, P. Biotransformation of codeine to 14-OH-codeine derivatives by Rhizobium radiobacter R89-1. J. Mol. Catal. B Enzym. 2013, 87, 1–5. [Google Scholar] [CrossRef]
- Niknam, S.; Faramarzi, M.A.; Abdi, K.; Yazdi, M.T.; Amini, M.; Rastegar, H. Bioconversion of codeine to semi-synthetic opiate derivatives by the cyanobacterium Nostoc muscorum. World J. Microbiol. Biotechnol. 2010, 26, 119–123. [Google Scholar] [CrossRef]
- Cameron, G.W.; Jordan, K.N.; Holt, P.J.; Baker, P.B.; Lowe, C.R.; Bruce, N.C. Identification of a heroin esterase in Rhodococcus sp. strain H1. Appl. Environ. Microbiol. 1994, 60, 3881–3883. [Google Scholar] [CrossRef]
- Stabler, P.J.; Bruce, N.C. Oxidation of morphine to 2,2′-bimorphine by Cylindrocarpon didymum. Appl. Environ. Microbiol. 1998, 64, 4106–4108. [Google Scholar] [CrossRef]
- Chaudhary, V.; Leisch, H.; Moudra, A.; Allen, B.; De Luca, V.; Cox, D.P.; Hudlicky, T. Biotransformations of morphine alkaloids by fungi: N-demethylations, oxidations, and reductions. Collect. Czechoslov. Chem. Commun. 2009, 74, 1179–1193. [Google Scholar] [CrossRef]
- Gibson, M.; Soper, C.J.; Parfitt, R.T.; Sowell, G.J. Studies on the mechanism of microbial N-demethylation of codeine by cell-free extracts of Cunninghamella bainieri. Enzyme Microb. Technol. 1984, 6, 471–475. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Shaari, K.; Verpoorte, R. Biotransformation of tetrahydrocannabinol. Phytochem. Rev. 2016, 15, 921–934. [Google Scholar] [CrossRef]
- Rashidi, H.; Akhtar, M.T.; van der Kooy, F.; Verpoorte, R.; Duetz, W.A. Hydroxylation and further oxidation of delta 9-tetrahydrocannabinol by alkane-degrading bacteria. Appl. Environ. Microbiol. 2009, 75, 7135–7141. [Google Scholar] [PubMed]
- Binder, M.; Popp, A. Microbial transformation of cannabinoids. Part 3. Major metabolites of (3R, 4R)-∆1-tetrahydrocannabinol. Helv. Chim. Acta 1980, 63, 2515–2518. [Google Scholar]
- Robertson, L.W.; Koh, S.W.; Huff, S.R.; Malhotra, R.K.; Ghosh, A. Microbiological oxidation of the pentyl side chain of cannabinoids. Experientia 1978, 34, 1020–1022. [Google Scholar] [PubMed]
- Robertson, L.W.; Lyle, M.A.; Billets, S. Biotransformation of cannabinoids by Syncephalastrum racemosum. Biol. Mass Spectrom. 1975, 2, 266–271. [Google Scholar] [CrossRef]
- Merali, Z.; Ross, S.; Paré, G. The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metabol. Drug Interact. 2014, 29, 143–151. [Google Scholar] [CrossRef]
- Willey, D.L.; Caswell, D.A.; Lowe, C.R.; Bruce, N.C. Nucleotide sequence and over-expression of morphine dehydrogenase, a plasmid-encoded gene from Pseudomonas putida M10. Biochem. J. 1993, 290, 539–544. [Google Scholar] [CrossRef]
- French, C.E.; Bruce, N.C. Bacterial morphinone reductase is related to old yellow enzyme. Biochem. J. 1995, 312, 671–678. [Google Scholar]
- Abalain, J.H.; Di Stefano, S.; Amet, Y.; Quemener, E.; Abalain-Colloc, M.L.; Floch, H.H. Cloning, DNA sequencing and expression of (3-17)β hydroxysteroid dehydrogenase from Pseudomonas testosteroni. J. Steroid Biochem. Mol. Biol. 1993, 44, 133–139. [Google Scholar]
- Yaver, D.S.; Overjero, M.D.; Xu, F.; Nelson, B.A.; Brown, K.M.; Halkier, T.; Bernauer, S.; Brown, S.H.; Kauppinen, S. Molecular characterization of laccase genes from the basidiomycete Coprinus cinereus and heterologous expression of the laccase lcc1. Appl. Environ. Microbiol. 1999, 65, 4943–4948. [Google Scholar]
- Basheer, S.; Rashid, N.; Akram, M.S.; Akhtar, M. A highly stable laccase from Bacillus subtilis strain R5: Gene cloning and characterization. Biosci. Biotechnol. Biochem. 2018, 83, 436–445. [Google Scholar]
- Chmelová, D.; Ondrejovič, M.; Miertuš, S. Laccases as effective tools in the removal of pharmaceutical products from aquatic systems. Life 2024, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.C.; Waterman, M.R.; Zhao, B. Streptomyces cytochromes P450: Applications in drug metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, D.A.; Holt, P.J.; Lowe, C.R.; Bruce, N.C. Molecular analysis of the Rhodococcus sp. strain H1 her gene and characterization of its product, a heroin esterase, expressed in Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 2062–2066. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. World Drug Report 2022; United Nations: New York, NY, USA, 2022.
- Nielsen, D.A.; Nielsen, E.M.; Dasari, T.; Spellicy, C.J. Pharmacogenetics of addiction therapy. Methods Mol. Biol. 2014, 1175, 589–624. [Google Scholar]
- Zastrozhin, M.S.; Skryabin, V.Y.; Miroshkin, S.S.; Bryun, E.A.; Sychev, D.A. Pharmacogenetics of alcohol addiction: Current perspectives. Appl. Clin. Genet. 2019, 12, 131–140. [Google Scholar]
- Lohoff, F.W. Pharmacotherapies and personalized medicine for alcohol use disorder: A review. Pharmacogenomics 2020, 21, 1117–1138. [Google Scholar] [CrossRef]
- Bailey, K.R.; Cheng, C. Conference Scene: The great debate: Genome-wide association studies in pharmacogenetics research, good or bad? Pharmacogenomics 2010, 11, 305–308. [Google Scholar] [CrossRef]
- Liu, X.; Tong, X.; Zhu, J.; Tian, L.; Jie, Z.; Zou, Y.; Lin, X.; Liang, H.; Li, W.; Ju, Y.; et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021, 7, 117. [Google Scholar]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar]
- Cantor, R.M.; Lange, K.; Sinsheimer, J.S. Prioritizing GWAS results: A review of statistical methods and recommendations for their application. Am. J. Human Genet. 2010, 86, 6–22. [Google Scholar]
- Crist, R.C.; Reiner, B.C.; Berrettini, W.H. A review of opioid addiction genetics. Curr. Opin. Psychol. 2019, 27, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Q.; Wigdor, E.M.; Malawsky, D.S.; Campbell, P.; Samocha, K.E.; Chundru, V.K.; Danecek, P.; Lindsay, S.; Marchant, T.; Koko, M.; et al. Examining the role of common variants in rare neurodevelopmental conditions. Nature 2024, 636, 404–411. [Google Scholar] [CrossRef]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Rizkallah, M.R.; Saad, R.; Aziz, R.K. The Human Microbiome Project, personalized medicine and the birth of pharmacomicrobiomics. Curr. Pharmacogenom. Person. Med. 2010, 8, 182–193. [Google Scholar] [CrossRef]
- Aziz, R.K.; Rizkallah, M.R.; Saad, R.; ElRakaiby, M.T. Translating Pharmacomicrobiomics: Three Actionable Challenges/Prospects in 2020. Omics 2020, 24, 60–61. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-generation sequencing technology: Current trends and advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Jimonet, P.; Druart, C.; Blanquet-Diot, S.; Boucinha, L.; Kourula, S.; Le Vacon, F.; Maubant, S.; Rabot, S.; Van de Wiele, T.; Schuren, F.; et al. Gut microbiome integration in drug discovery and development of small molecules. Drug Metab. Dispos. 2024, 52, 274–287. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Psychobiotics: A new perspective on the treatment of stress, anxiety, and depression. Anxiety Stress 2024, 30, 79–93. [Google Scholar]
- Pavlovic, S.; Kotur, N.; Stankovic, B.; Zukic, B.; Gasic, V.; Dokmanovic, L. Pharmacogenomic and pharmacotranscriptomic profiling of childhood acute lymphoblastic leukemia: Paving the way to personalized treatment. Genes 2019, 10, 191. [Google Scholar] [CrossRef]
- Evans, P.; Nagai, T.; Konkashbaev, A.; Zhou, D.; Knapik, E.W.; Gamazon, E.R. Transcriptome-wide association studies (TWAS): Methodologies, applications, and challenges. Curr. Protoc. 2024, 4, e981. [Google Scholar] [PubMed]
- Jabbarzadeh Kaboli, P.; Luo, S.; Chen, Y.; Jomhori, M.; Imani, S.; Xiang, S.; Wu, Z.; Li, M.; Shen, J.; Zhao, Y.; et al. Pharmacotranscriptomic profiling of resistant triple-negative breast cancer cells treated with lapatinib and berberine shows upregulation of PI3K/Akt signaling under cytotoxic stress. Gene 2022, 816, 146171. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, L.; Gao, Y.; Yao, F.; Marti, T.M.; Schmid, R.A.; Peng, R.W. Pharmacotranscriptomic analysis reveals novel drugs and gene networks regulating ferroptosis in cancer. Cancers 2020, 12, 3273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).