Assessment of Stress and Immune Gene Expression in Australasian Snapper (Chrysophrys auratus) Exposed to Chronic Temperature Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design of Chronic Temperature Stress Trial
2.2. Gene Target Sequences
2.3. RNA Extraction
2.4. Gene Expression Analysis—NanoString nCounter Analysis System
2.5. Statistical Analyses
3. Results
3.1. Fish Growth and Behaviour in the Chronic Temperature Stress Experiment
3.2. Gene Expression in the Chronic Temperature Stress Experiment
3.3. Correlations of Liver and Head Kidney Gene Expression with Fin Gene Expression
3.4. Comparison of Gene Expression Between Chronic and Acute Exposure to Temperature Differences
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.S.; Nestor, V.; Golbuu, Y.; Palumbi, S.R. Coral bleaching resistance variation is linked to differential mortality and skeletal growth during recovery. Evol. Appl. 2023, 16, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Palomar, G.; Wos, G.; Stoks, R.; Sniegula, S. Latitude-specific urbanization effects on life history traits in the damselfly Ischnura elegans. Evol. Appl. 2023, 16, 1503–1515. [Google Scholar] [CrossRef]
- Szwejser, E.; Verburg-van Kemenade, B.M.L.; Maciuszek, M.; Chadzinska, M. Estrogen-dependent seasonal adaptations in the immune response of fish. Horm. Behav. 2017, 88, 15–24. [Google Scholar] [CrossRef]
- Pinzón, J.H.; Kamel, B.; Burge, C.A.; Harvell, C.D.; Medina, M.; Weil, E.; Mydlarz, L.D. Whole transcriptome analysis reveals changes in expression of immune-related genes during and after bleaching in a reef-building coral. R. Soc. Open Sci. 2015, 2, 140214. [Google Scholar] [CrossRef]
- Libro, S.; Kaluziak, S.T.; Vollmer, S.V. RNA-seq profiles of immune related genes in the Staghorn Coral Acropora cervicornis infected with white band disease. PLoS ONE 2013, 8, e81821. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.J. Modulation of the immune system of fish by their environment. Fish Shellfish Immun. 2008, 25, 373–383. [Google Scholar] [CrossRef]
- Symonds, J.E.; Walker, S.P.; Ven, I.; Marchant, A.; Irvine, G.W.; Pether, S. Developing broodstock resources for farmed marine fish. Proc. N. Z. Soc. Anim. Prod. 2012, 72, 222–226. [Google Scholar]
- Symonds, J.E.; Clarke, S.M.; King, N.; Walker, S.P.; Blanchard, B.; Sutherland, D.; Roberts, R.; Preece, M.A.; Tate, M.; Buxton, P.; et al. Developing Successful Breeding Programs for New Zealand Aquaculture: A Perspective on Progress and Future Genomic Opportunities. Front. Genet. 2019, 10, 27. [Google Scholar] [CrossRef]
- Symonds, J.; King, N.; Camara, M.; Ragg, N.; Hilton, Z.; Walker, S.; Roberts, R.; Malpot, E.; Preece, M.; Amer, P.; et al. New Zealand aquaculture selective breeding: From theory to industry application for three flagship species. In Proceedings of the World Congress on Genetics Applied to Livestock Production (WCGALP), Auckland, New Zealand, 11–16 February 2018; p. 1035. [Google Scholar]
- Bowering, L.R.; McArley, T.J.; Devaux, J.B.; Hickey, A.J.; Herbert, N.A. Metabolic resilience of the Australasian snapper (Chrysophrys auratus) to marine heatwaves and hypoxia. Front. Physiol. 2023, 14, 1215442. [Google Scholar] [CrossRef]
- Samuels, G.; Hegarty, L.; Fantham, W.; Ashton, D.; Blommaert, J.; Wylie, M.J.; Moran, D.; Wellenreuther, M. Generational breeding gains in a new species for aquaculture, the Australasian snapper (Chrysophrys auratus). Aquaculture 2024, 586, 740482. [Google Scholar]
- Moran, D.; Schleyken, J.; Flammensbeck, C.; Fantham, W.; Ashton, D.; Wellenreuther, M. Enhanced survival and growth in the selectively bred Chrysophrys auratus (Australasian snapper, tāmure). Aquaculture 2023, 563, 738970. [Google Scholar] [CrossRef]
- Murata, O.; Harada, T.; Miyashita, S.; Izumi, K.-i.; Maeda, S.; Kato, K.; Kumai, H. Selective breeding for growth in red sea bream. Fish. Sci. 1996, 62, 845–849. [Google Scholar]
- Ashton, D.T.; Hilario, E.; Jaksons, P.; Ritchie, P.A.; Wellenreuther, M. Genetic diversity and heritability of economically important traits in captive Australasian snapper (Chrysophrys auratus). Aquaculture 2019, 505, 190–198. [Google Scholar] [CrossRef]
- Baesjou, J.P.; Wellenreuther, M. Genetic signatures of domestication selection in the Australasian snapper (Chrysophrys auratus). Genes 2021, 12, 1737. [Google Scholar]
- Ashton, D.T.; Ritchie, P.A.; Wellenreuther, M. High-Density Linkage Map and QTLs for Growth in Snapper (Chrysophrys auratus). G3-Genes Genom Genet. 2019, 9, 1027–1035. [Google Scholar] [CrossRef]
- Catanach, A.; Crowhurst, R.; Deng, C.; David, C.; Bernatchez, L.; Wellenreuther, M. The genomic pool of standing structural variation outnumbers single nucleotide polymorphism by threefold in the marine teleost Chrysophrys auratus. Mol. Ecol. 2019, 28, 1210–1223. [Google Scholar] [CrossRef]
- Wellenreuther, M.; Le Luyer, J.; Cook, D.; Ritchie, P.A.; Bernatchez, L. Domestication and Temperature Modulate Gene Expression Signatures and Growth in the Australasian Snapper Chrysophrys auratus. G3-Genes Genom Genet. 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Blommaert, J.; Sandoval-Castillo, J.; Beheregaray, L.; Wellenreuther, M. Peering into the gaps: Long-read sequencing illuminates structural variants and genomic evolution in the Australasian snapper. Genomics 2024, 116, 110929. [Google Scholar] [CrossRef]
- Aguirre-Liguori, J.A.; Ramírez-Barahona, S.; Gaut, B.S. The evolutionary genomics of species’ responses to climate change. Nat. Ecol. Evol. 2021, 5, 1350–1360. [Google Scholar]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.R. Evolution, climate change, and extreme events. Science 2017, 357, 451–452. [Google Scholar] [PubMed]
- Urban, M.C.; Bocedi, G.; Hendry, A.P.; Mihoub, J.-B.; Pe’er, G.; Singer, A.; Bridle, J.; Crozier, L.; De Meester, L.; Godsoe, W.; et al. Improving the forecast for biodiversity under climate change. Science 2016, 353, aad8466. [Google Scholar]
- Bentley-Hewitt, K.L.; Flammensbeck, C.K.; Crowhurst, R.N.; Hedderley, D.I.; Wellenreuther, M. Development of a Novel Stress and Immune Gene Panel for the Australasian Snapper (Chrysophrys auratus). Genes 2024, 15, 1390. [Google Scholar] [CrossRef] [PubMed]
- Troell, M.; Naylor, R.L.; Metian, M.; Beveridge, M.; Tyedmers, P.H.; Folke, C.; Arrow, K.J.; Barrett, S.; Crépin, A.-S.; Ehrlich, P.R.; et al. Does aquaculture add resilience to the global food system? Proc. Natl. Acad. Sci. USA 2014, 111, 13257–13263. [Google Scholar]
- Sgro, C.M.; Lowe, A.J.; Hoffmann, A.A. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 2011, 4, 326–337. [Google Scholar] [CrossRef]
- Seebacher, F.; White, C.R.; Franklin, C.E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 2015, 5, 61–66. [Google Scholar]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar]
- Brinchmann, M.F. Immune relevant molecules identified in the skin mucus of fish using -omics technologies. Mol. Biosyst. 2016, 12, 2056–2063. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Bentley-Hewitt, K.L. Influence of Dietary Avocado on Gut Health in Rats. Plant Foods Hum. Nutr. 2017, 72, 321–323. [Google Scholar] [CrossRef]
- Payne, R.W. GenStat. Wiley Interdiscip. Rev. Comput. Stat. 2009, 1, 255–258. [Google Scholar]
- Ndandala, C.B.; Dai, M.; Mustapha, U.F.; Li, X.; Liu, J.; Huang, H.; Li, G.; Chen, H. Current research and future perspectives of GH and IGFs family genes in somatic growth and reproduction of teleost fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- Kokou, F.; Adamidou, S.; Karacostas, L.; Sarropoulou, E. Sample size matters in dietary gene expression studies—A case study in the gilthead sea bream (sparus aurata l.). Aquac. Rep. 2016, 3, 82–87. [Google Scholar] [CrossRef][Green Version]
- Espinosa, C.; Cuesta, A.; Esteban, M.Á. Effects of dietary polyvinylchloride microparticles on general health, immune status and expression of several genes related to stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 68, 251–259. [Google Scholar] [CrossRef]
- Jeffries, K.M.; Teffer, A.; Michaleski, S.; Bernier, N.J.; Heath, D.D.; Miller, K.M. The use of non-lethal sampling for transcriptomics to assess the physiological status of wild fishes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110629. [Google Scholar]
- Zhang, Z.P.; Wells, M.C.; Boswell, M.G.; Beldorth, I.; Kirk, L.M.; Wang, Y.L.; Wang, S.L.; Savage, M.; Walter, R.B.; Booth, R.E. Identification of robust hypoxia biomarker candidates from fin of medaka (Oryzias latipes). Comp. Biochem. Phys. C 2012, 155, 11–17. [Google Scholar] [CrossRef]
- Madeira, C.; Madeira, D.; Diniz, M.S.; Cabral, H.N.; Vinagre, C. Comparing biomarker responses during thermal acclimation: A lethal vs non-lethal approach in a tropical reef clownfish. Comp. Biochem. Phys. A 2017, 204, 104–112. [Google Scholar] [CrossRef]
- Flanagan, S.; Ryan, A.; Gisolfi, C.; Moseley, P. Tissue-specific HSP70 response in animals undergoing heat stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1995, 268, R28–R32. [Google Scholar]
- Takle, H.; Baeverfjord, G.; Lunde, M.; Kolstad, K.; Andersen, O. The effect of heat and cold exposure on HSP70 expression and development of deformities during embryogenesis of Atlantic salmon (Salmo salar). Aquaculture 2005, 249, 515–524. [Google Scholar]
- CJ, O.; GE, H. Thermal history-dependent expression of the hsp70 gene in purple sea urchins: Biogeographic patterns and the effect of temperature acclimation. J. Exp. Mar. Biol. Ecol. 2005, 327, 134. [Google Scholar]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–34. [Google Scholar]
- Guo, H.; Dixon, B. Understanding acute stress-mediated immunity in teleost fish. Fish Shellfish Immunol. Rep. 2021, 2, 100010. [Google Scholar] [PubMed]
- Teletchea, F.; Fontaine, P. Levels of domestication in fish: Implications for the sustainable future of aquaculture. Fish Fish. 2014, 15, 181–195. [Google Scholar]
- Duarte, C.M.; Marba, N.; Holmer, M. Ecology. Rapid domestication of marine species. Science 2007, 316, 382–383. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929. [Google Scholar] [PubMed]
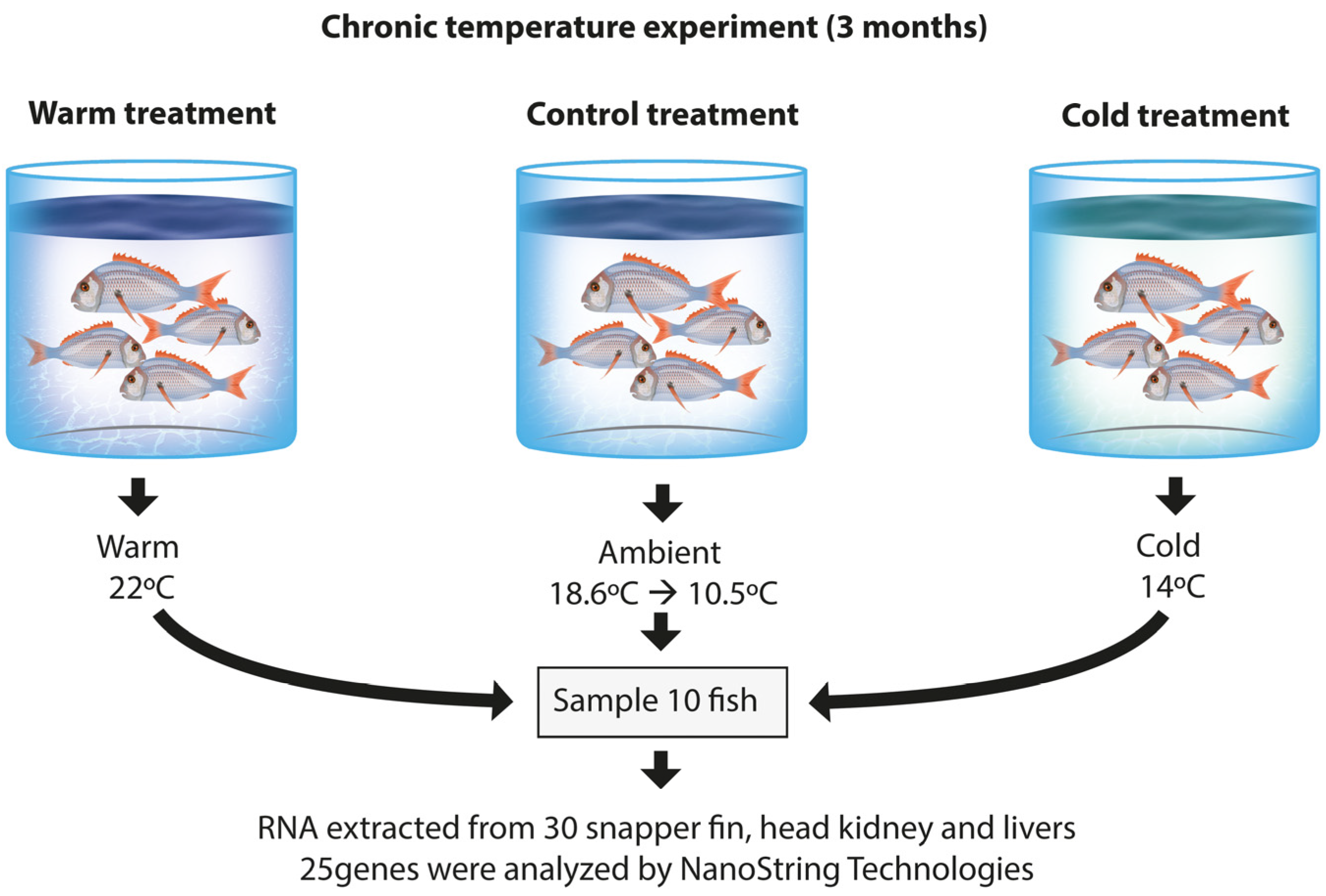
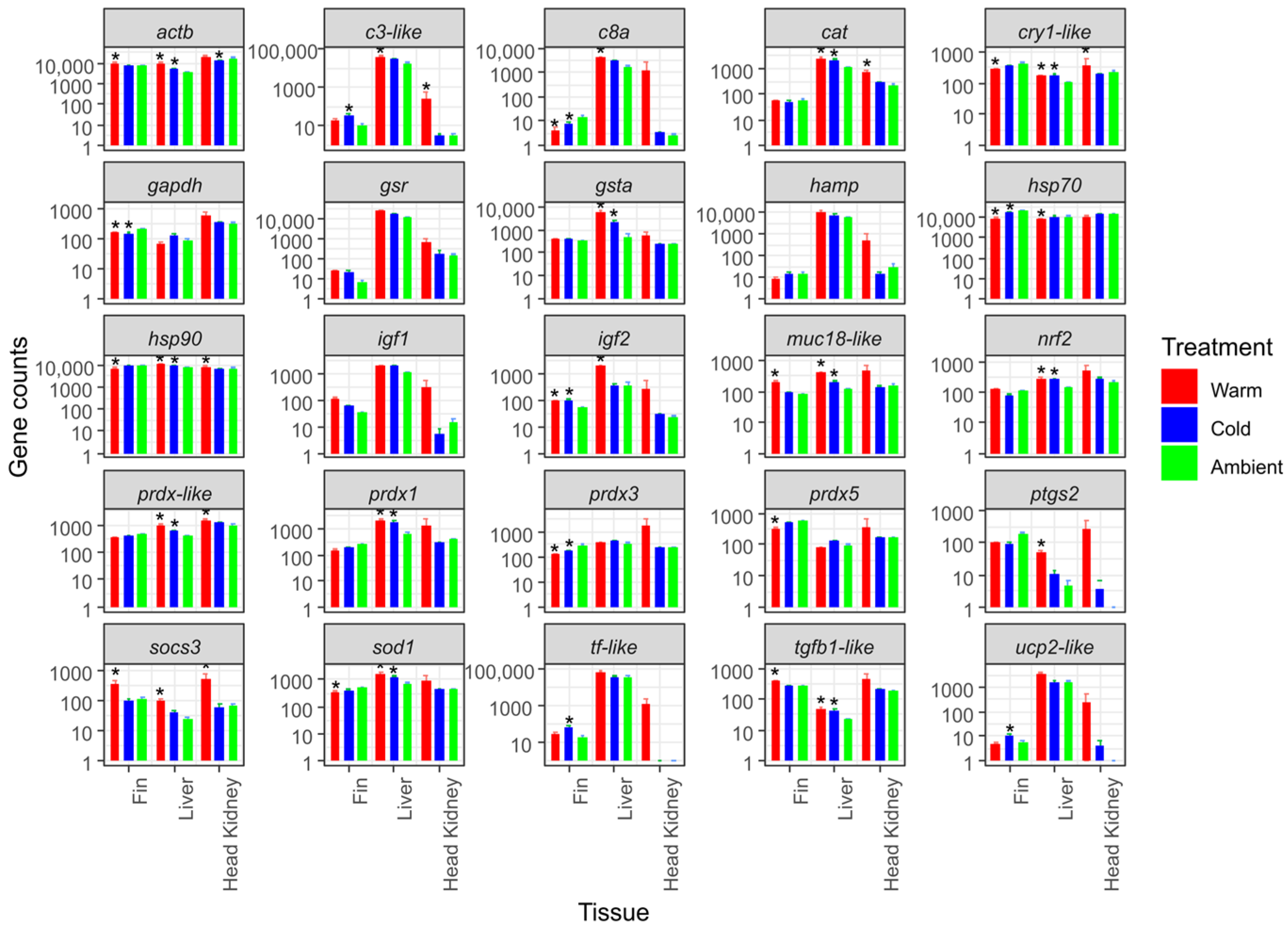
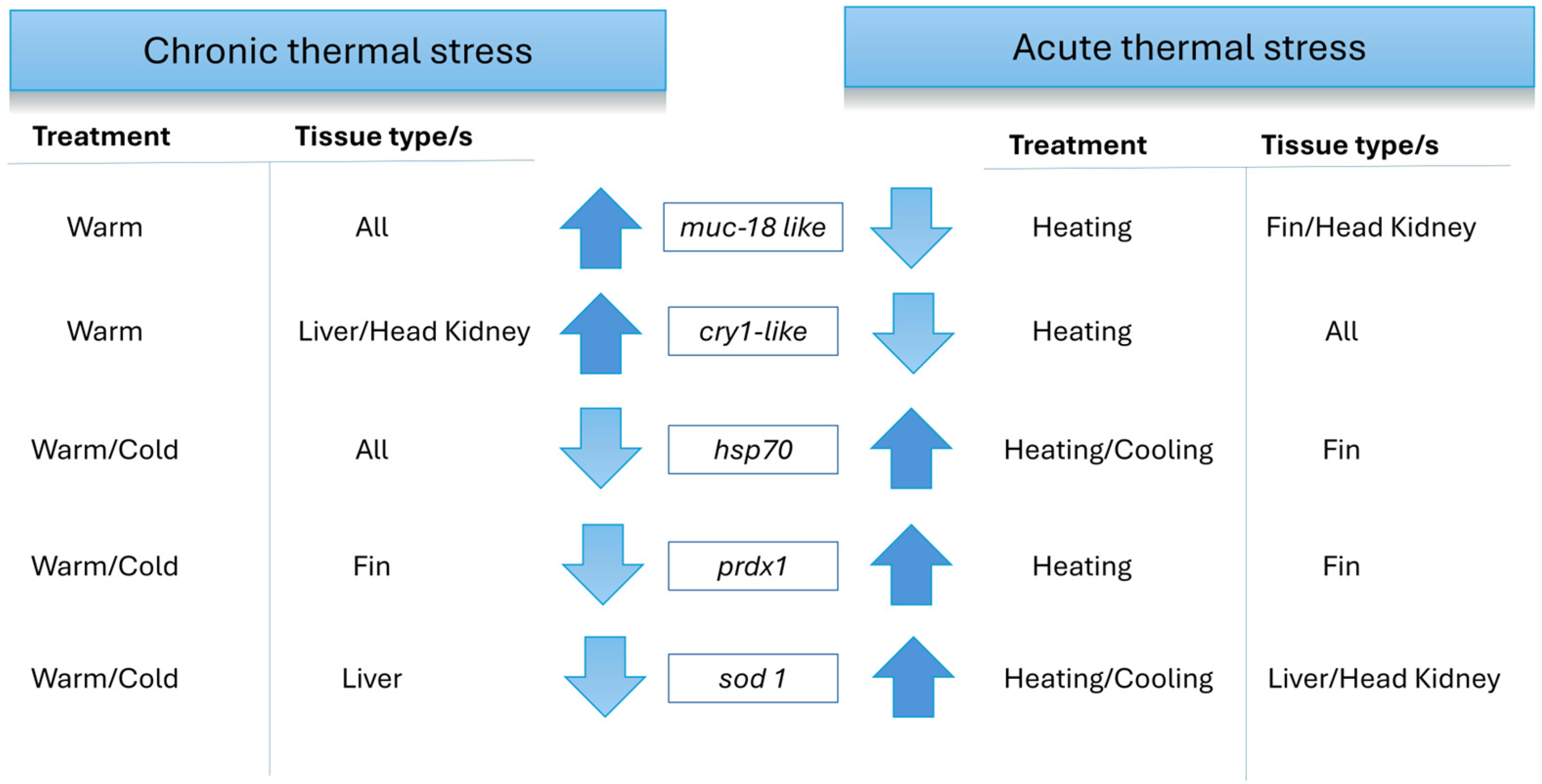
| Treatment Group | Sampling Stage (n = 10) | Fork Length [mm] | Weight [g] |
|---|---|---|---|
| Ambient | Initial | 82.9 ± 2 | 11.3 ± 1 |
| Mid-term | 103.4 ± 2 | 21.7 ± 1 | |
| Final | 104.2 ± 2 | 21.3 ± 1 | |
| Warm treatment | Initial | 80.1 ± 2 | 10.4 ± 1 |
| Mid-term | 130.3 ± 3 | 46.0 ± 4 | |
| Final | 158.1 ± 3 | 88.9 ± 6 | |
| Cold treatment | Initial | 80.1 ± 5 | 11.4 ± 2 |
| Mid-term | 99.2 ± 5 | 21.6 ± 3 | |
| Final | 105.2 ± 5 | 24.8 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bentley-Hewitt, K.; Flammensbeck, C.K.; Hedderley, D.I.; Wellenreuther, M. Assessment of Stress and Immune Gene Expression in Australasian Snapper (Chrysophrys auratus) Exposed to Chronic Temperature Change. Genes 2025, 16, 385. https://doi.org/10.3390/genes16040385
Bentley-Hewitt K, Flammensbeck CK, Hedderley DI, Wellenreuther M. Assessment of Stress and Immune Gene Expression in Australasian Snapper (Chrysophrys auratus) Exposed to Chronic Temperature Change. Genes. 2025; 16(4):385. https://doi.org/10.3390/genes16040385
Chicago/Turabian StyleBentley-Hewitt, Kerry, Christina K. Flammensbeck, Duncan I. Hedderley, and Maren Wellenreuther. 2025. "Assessment of Stress and Immune Gene Expression in Australasian Snapper (Chrysophrys auratus) Exposed to Chronic Temperature Change" Genes 16, no. 4: 385. https://doi.org/10.3390/genes16040385
APA StyleBentley-Hewitt, K., Flammensbeck, C. K., Hedderley, D. I., & Wellenreuther, M. (2025). Assessment of Stress and Immune Gene Expression in Australasian Snapper (Chrysophrys auratus) Exposed to Chronic Temperature Change. Genes, 16(4), 385. https://doi.org/10.3390/genes16040385











