Effects of Levetiracetam on Episodic Ataxia Type 2 and Spinocerebellar Ataxia Type 6 with Episodic Ataxic Symptoms: A Case Series
Abstract
1. Introduction
2. Case Reports
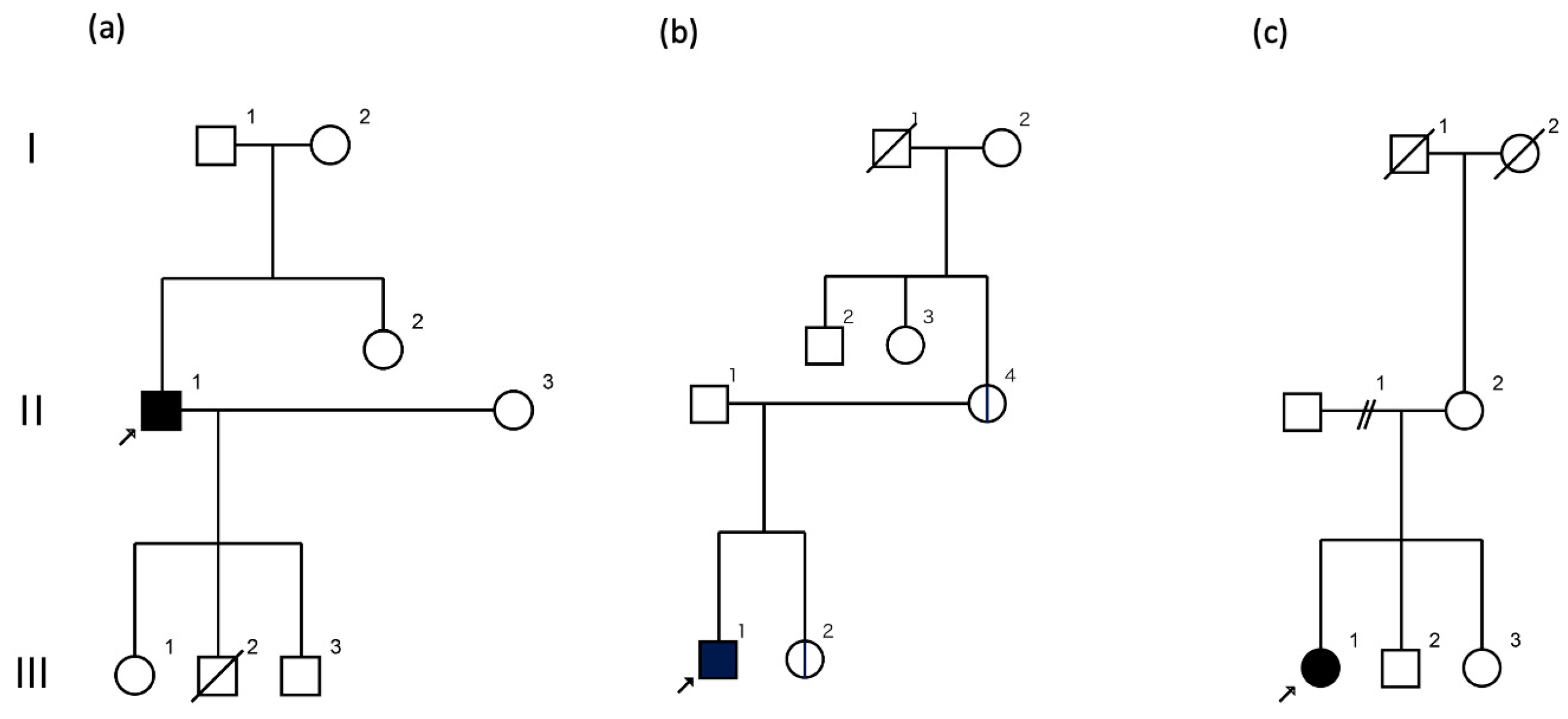
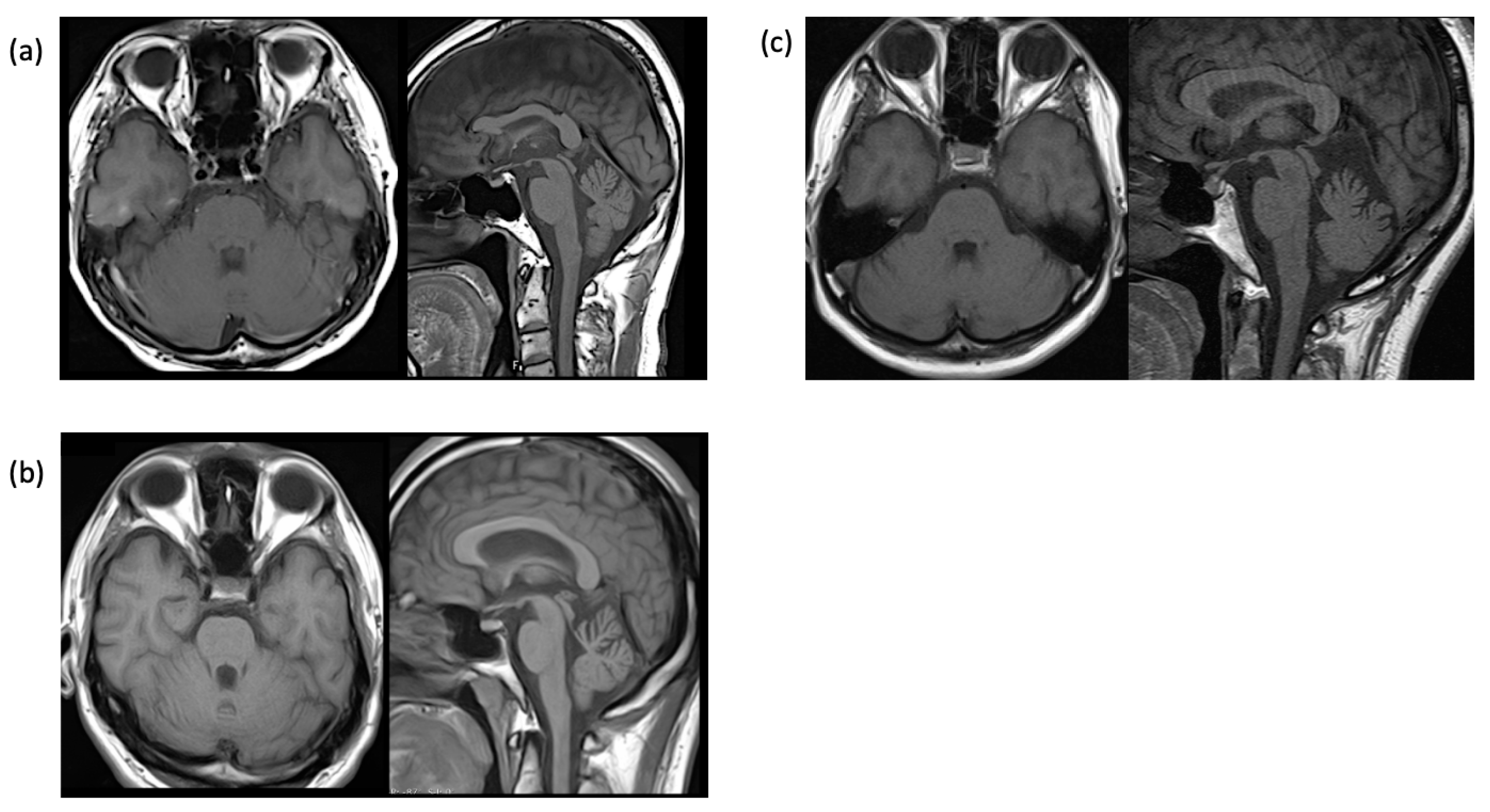
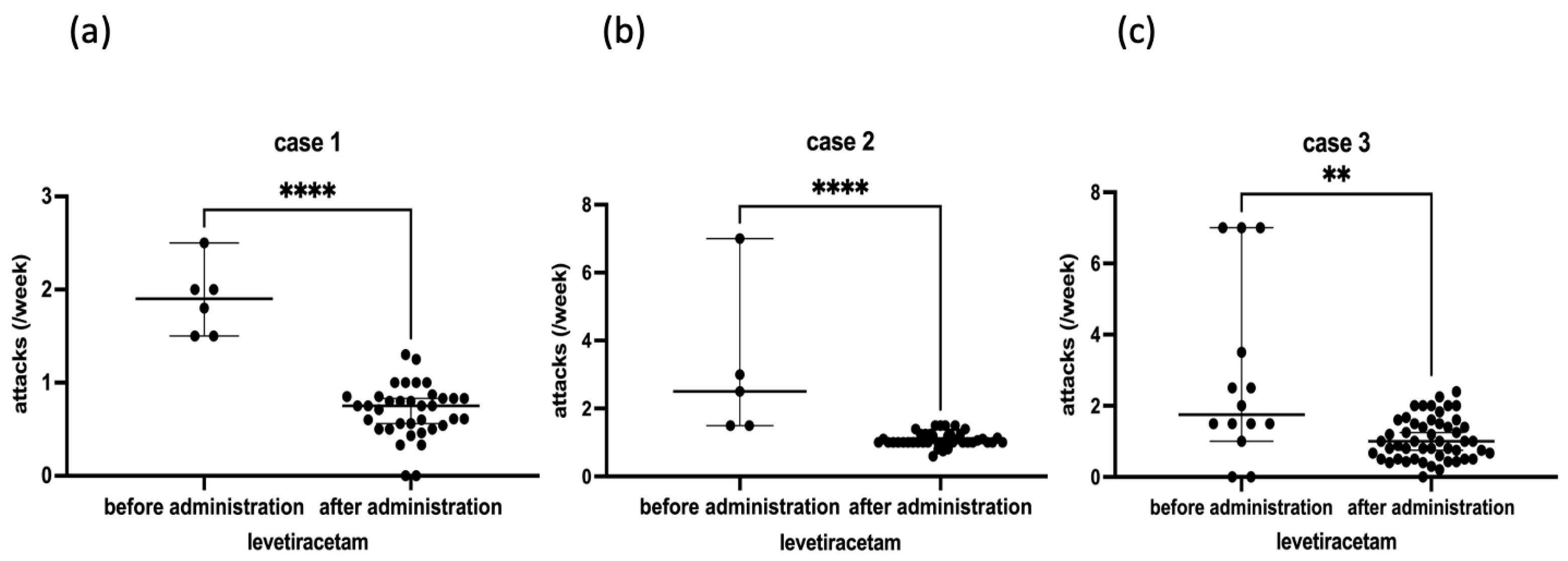
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olszewska, D.A.; Shetty, A.; Rajalingam, R.; Rodriguez-Antiguedad, J.; Hamed, M.; Huang, J.; Breza, M.; Rasheed, A.; Bahr, N.; Madoev, H.; et al. Genotype-phenotype relations for episodic ataxia genes: MDSGene systematic review. Eur. J. Neurol. 2023, 30, 3377–3393. [Google Scholar] [CrossRef] [PubMed]
- Ophoff, R.A.; Terwindt, G.M.; Vergouwe, M.N.; van Eijk, R.; Oefner, P.J.; Hoffman, S.M.; Lamerdin, J.E.; Mohrenweiser, H.W.; Bulman, D.E.; Ferrari, M.; et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996, 87, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Zhuchenko, O.; Bailey, J.; Bonnen, P.; Ashizawa, T.; Stockton, D.W.; Amos, C.; Dobyns, W.B.; Subramony, S.H.; Zoghbi, H.Y.; Lee, C.C. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat. Genet. 1997, 15, 62–69. [Google Scholar] [CrossRef]
- Jodice, C.; Mantuano, E.; Veneziano, L.; Trettel, F.; Sabbadini, G.; Calandriello, L.; Francia, A.; Spadaro, M.; Pierelli, F.; Salvi, F.; et al. Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum. Mol. Genet. 1997, 6, 1973–1978. [Google Scholar] [CrossRef]
- Griggs, R.C.; Moxley, R.T., 3rd; Lafrance, R.A.; McQuillen, J. Hereditary paroxysmal ataxia: Response to acetazolamide. Neurology 1978, 28, 1259–1264. [Google Scholar] [CrossRef]
- Strupp, M.; Kalla, R.; Dichgans, M.; Freilinger, T.; Glasauer, S.; Brandt, T. Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology 2004, 62, 1623–1625. [Google Scholar] [CrossRef] [PubMed]
- Muth, C.; Teufel, J.; Schols, L.; Synofzik, M.; Franke, C.; Timmann, D.; Mansmann, U.; Strupp, M. Fampridine and Acetazolamide in EA2 and Related Familial EA: A Prospective Randomized Placebo-Controlled Trial. Neurol. Clin. Pract. 2021, 11, e438–e446. [Google Scholar] [CrossRef]
- Lee, H.; Jang, D.H.; Jang, J.H.; Kim, T. Effectiveness of levetiracetam in an acetazolamide-unresponsive patient with episodic ataxia type 2 by a novel CACNA1A nonsense mutation. Eur. J. Neurol. 2017, 24, e43–e44. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, M.; Takiyama, Y.; Ueno, N.; Nishizawa, M. A sporadic case of episodic ataxia with nystagmus (EA-2). Rinsho Shinkeigaku 1998, 38, 446–449. [Google Scholar]
- Denier, C.; Ducros, A.; Vahedi, K.; Joutel, A.; Thierry, P.; Ritz, A.; Castelnovo, G.; Deonna, T.; Gerard, P.; Devoize, J.L.; et al. High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology 1999, 52, 1816–1821. [Google Scholar] [CrossRef]
- Shimazaki, H.; Nakao, K.; Ishikawa, K.; Takiyama, Y.; Nakano, I. A case of spinocerebellar ataxia type 6 with its initial symptom of episodic ataxia-like phenotype. No To Shinkei 2006, 58, 63–67. [Google Scholar] [PubMed]
- Na, S.; Kim, T. Efficacy of levetiracetam in patients with episodic ataxia type 2 caused by CACNA1A mutation: Three case reports. Neurol. Sci. 2021, 42, 3897–3899. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Kalla, R.; Claassen, J.; Adrion, C.; Mansmann, U.; Klopstock, T.; Freilinger, T.; Neugebauer, H.; Spiegel, R.; Dichgans, M.; et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology 2011, 77, 269–275. [Google Scholar] [CrossRef]
- Guida, S.; Trettel, F.; Pagnutti, S.; Mantuano, E.; Tottene, A.; Veneziano, L.; Fellin, T.; Spadaro, M.; Stauderman, K.; Williams, M.; et al. Complete loss of P/Q calcium channel activity caused by a CACNA1A missense mutation carried by patients with episodic ataxia type 2. Am. J. Hum. Genet. 2001, 68, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Dorgans, K.; Salvi, J.; Bertaso, F.; Bernard, L.; Lory, P.; Doussau, F.; Mezghrani, A. Characterization of the dominant inheritance mechanism of Episodic Ataxia type 2. Neurobiol. Dis. 2017, 106, 110–123. [Google Scholar] [CrossRef]
- Kordasiewicz, H.B.; Thompson, R.M.; Clark, H.B.; Gomez, C.M. C-termini of P/Q-type Ca2+ channel alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum. Mol. Genet. 2006, 15, 1587–1599. [Google Scholar] [CrossRef]
- Contreras-Garcia, I.J.; Cardenas-Rodriguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gomez-Manzo, S.; Hernandez-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macias, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef]
- Mark, M.D.; Schwitalla, J.C.; Groemmke, M.; Herlitze, S. Keeping Our Calcium in Balance to Maintain Our Balance. Biochem. Biophys. Res. Commun. 2017, 483, 1040–1050. [Google Scholar] [CrossRef]
- Ishikawa, T.; Tomatsu, S.; Tsunoda, Y.; Lee, J.; Hoffman, D.S.; Kakei, S. Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PLoS ONE 2014, 9, e108774. [Google Scholar] [CrossRef]
- Angehagen, M.; Margineanu, D.G.; Ben-Menachem, E.; Ronnback, L.; Hansson, E.; Klitgaard, H. Levetiracetam reduces caffeine-induced Ca2+ transients and epileptiform potentials in hippocampal neurons. Neuroreport 2003, 14, 471–475. [Google Scholar] [CrossRef]
- Nardello, R.; Plicato, G.; Mangano, G.D.; Gennaro, E.; Mangano, S.; Brighina, F.; Raieli, V.; Fontana, A. Two distinct phenotypes, hemiplegic migraine and episodic Ataxia type 2, caused by a novel common CACNA1A variant. BMC Neurol. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Verriello, L.; Pauletto, G.; Nilo, A.; Lonigro, I.; Betto, E.; Valente, M.; Curcio, F.; Gigli, G.L. Epilepsy and episodic ataxia type 2: Family study and review of the literature. J. Neurol. 2021, 268, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Onset | 10 y | 13 y | 24 y |
| Age at examination, gender | 66 y, M | 39 y, M | 46 y, F |
| Body weight (kg) | 57 | 70 | 52 |
| Race | Asian | Asian | Asian |
| Interictal nystagmus | + | + | − |
| Ictal duration | 30 min–12 h | 10–30 min | 2–3 h |
| Ictal symptoms (IS): dysarthria | + | + | + |
| IS: Limb ataxia | + | + | − |
| IS: Truncal ataxia | + | + | + |
| Migraine | − | + | − |
| EEG | intermittent delta activity | spike and wave, intermittent theta activity | normal |
| Brain MRI: cerebellar atrophy | +− | + | + |
| Brain SPECT: cerebellar blood flow | → | ↓ | → |
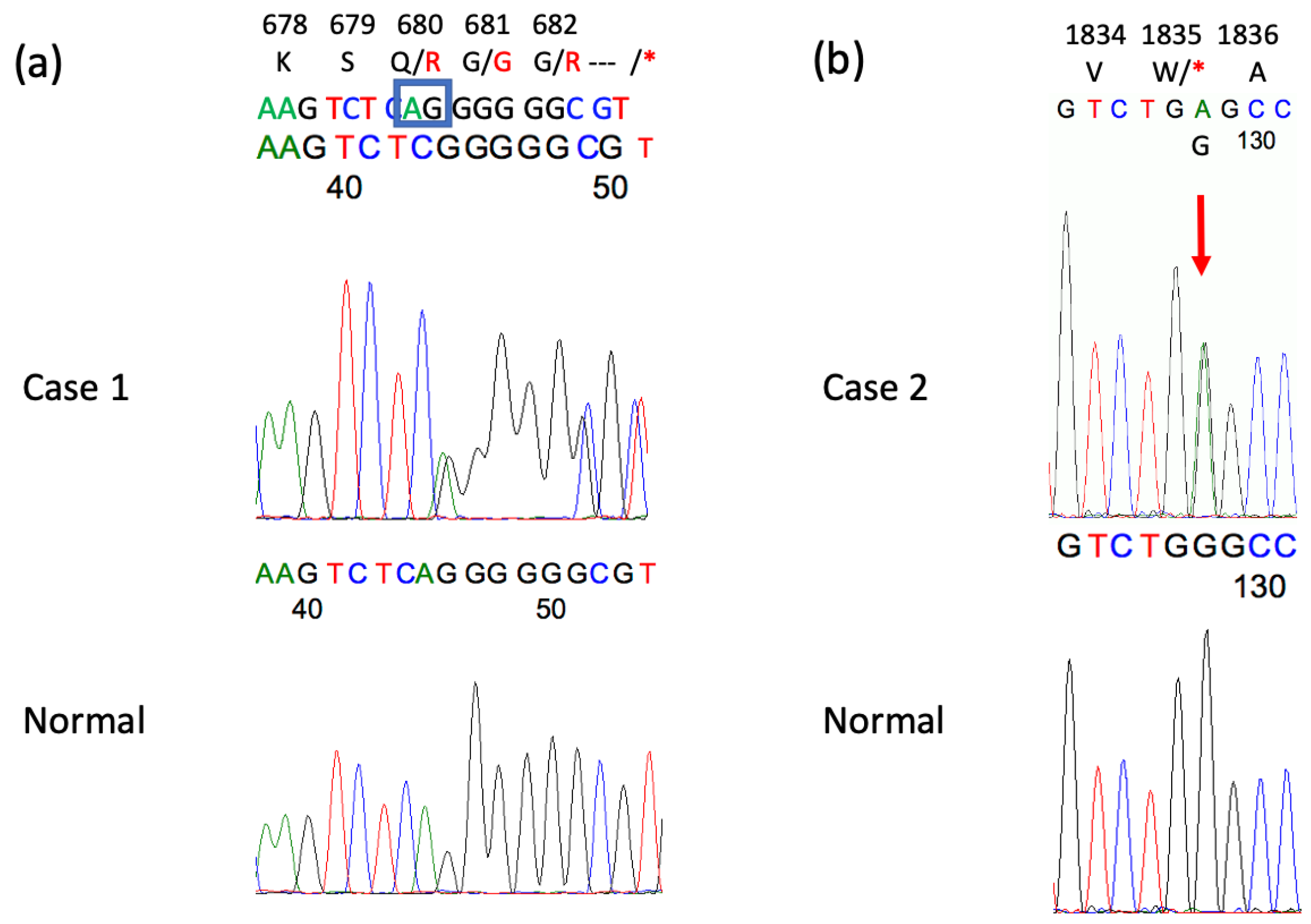
| CACNA1A gene variant/amino acid change | c.2039_2040delAG/p.Q680R fs*16 | c.5505G>A /p.W1835* | − |
| CAG repeat numbers in CACNA1A gene | 7/13 | 12/13 | 13/22 |
| Acetazolamide effect | + | +− | +− |
| Maximum levetiracetam dose (mg/day) | 750 | 1000 | 1000 |
| Levetiracetam effect | ++ | ++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimazaki, H. Effects of Levetiracetam on Episodic Ataxia Type 2 and Spinocerebellar Ataxia Type 6 with Episodic Ataxic Symptoms: A Case Series. Genes 2025, 16, 335. https://doi.org/10.3390/genes16030335
Shimazaki H. Effects of Levetiracetam on Episodic Ataxia Type 2 and Spinocerebellar Ataxia Type 6 with Episodic Ataxic Symptoms: A Case Series. Genes. 2025; 16(3):335. https://doi.org/10.3390/genes16030335
Chicago/Turabian StyleShimazaki, Haruo. 2025. "Effects of Levetiracetam on Episodic Ataxia Type 2 and Spinocerebellar Ataxia Type 6 with Episodic Ataxic Symptoms: A Case Series" Genes 16, no. 3: 335. https://doi.org/10.3390/genes16030335
APA StyleShimazaki, H. (2025). Effects of Levetiracetam on Episodic Ataxia Type 2 and Spinocerebellar Ataxia Type 6 with Episodic Ataxic Symptoms: A Case Series. Genes, 16(3), 335. https://doi.org/10.3390/genes16030335






