The Potential of Transgenic Hybrid Aspen Plants with a Recombinant Lac Gene from the Fungus Trametes hirsuta to Degrade Trichlorophenol
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Genetic Transformation
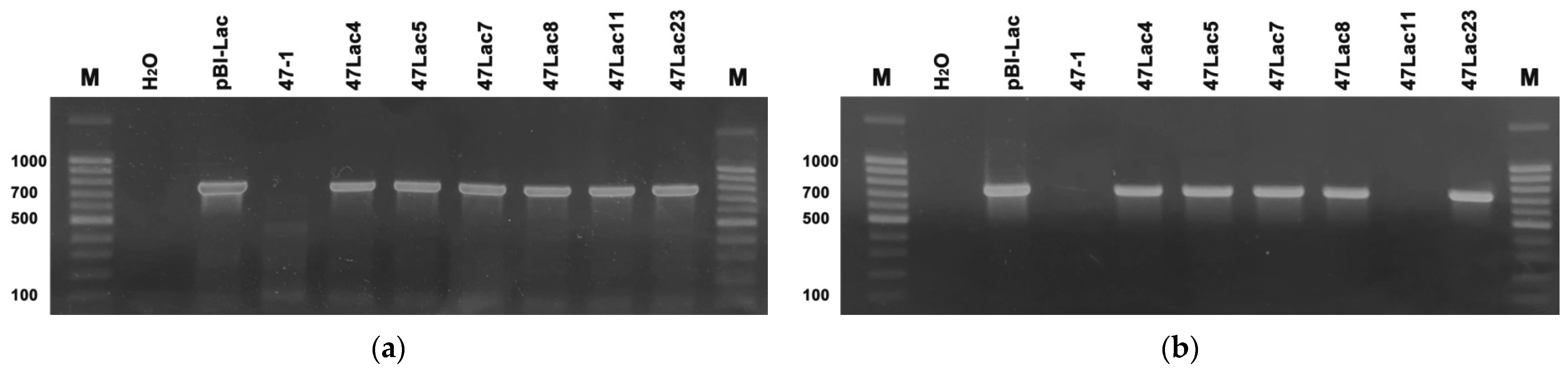
2.2. Confirmation of the Presence of Recombinant Gene
2.3. Gene Expression Analysis (RT-qPCR)
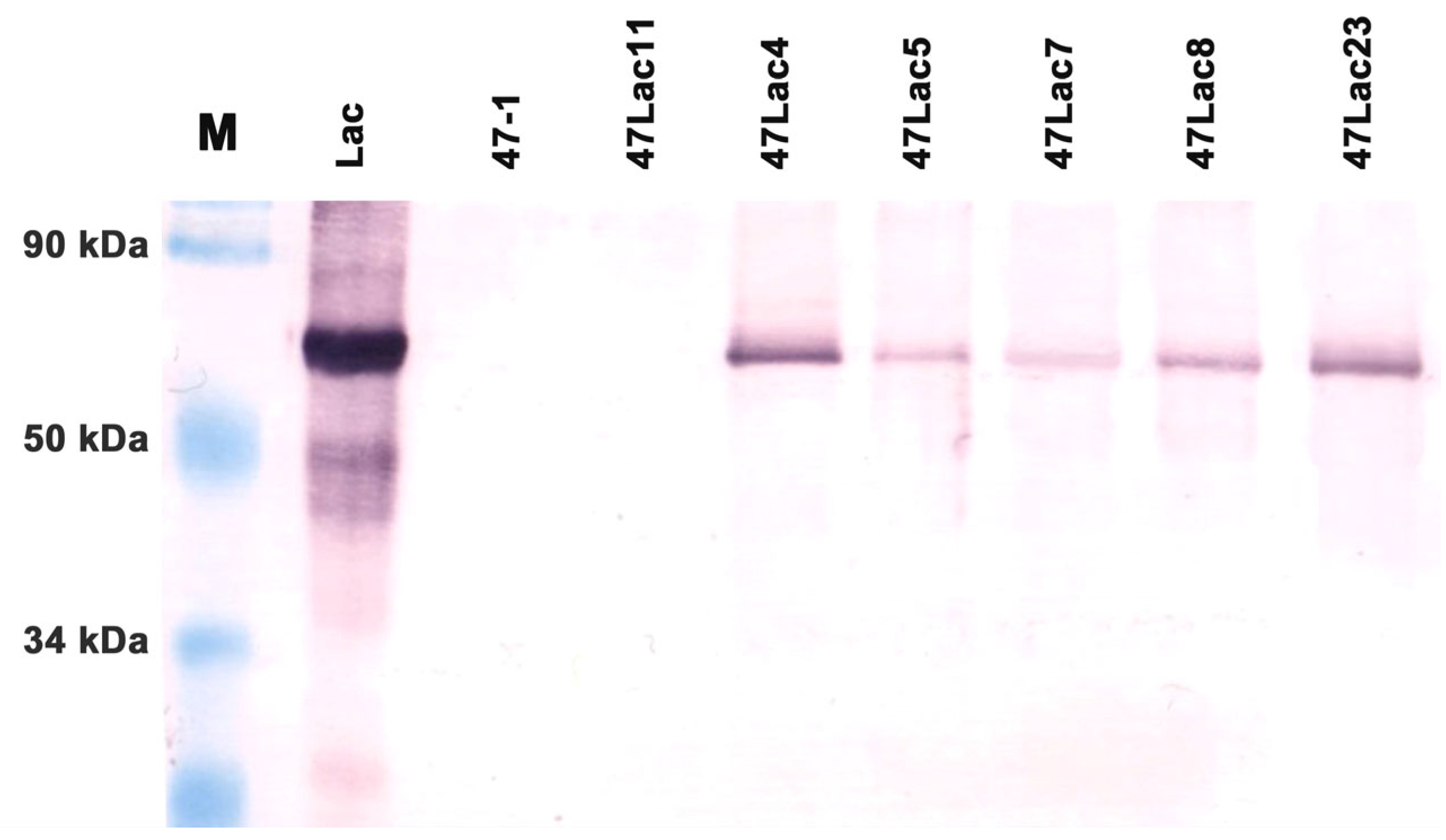
2.4. Quantitative Western Blot Analysis and Determination of Total Protein Concentration in Extracts
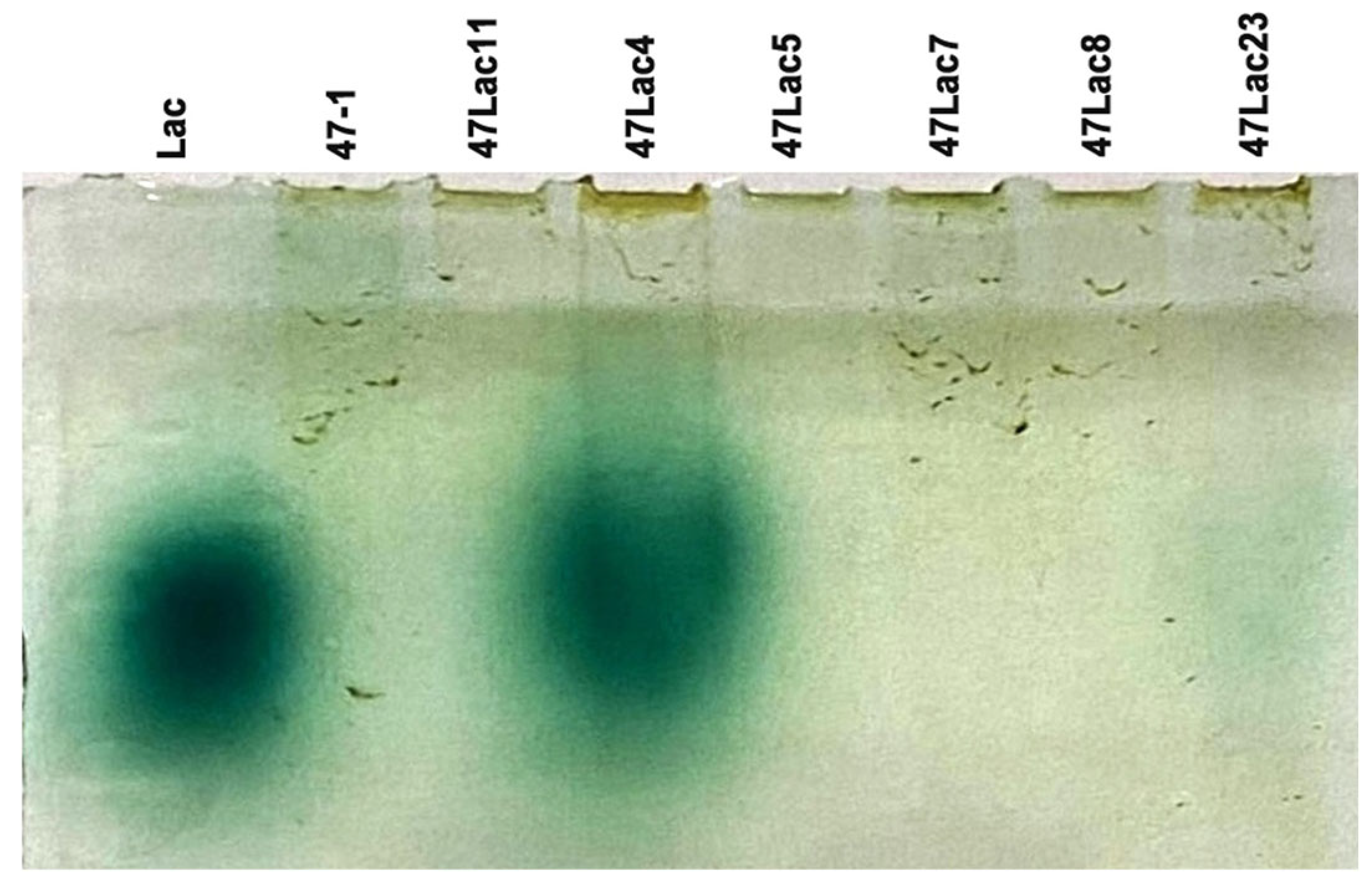
2.5. Native Polyacrylamide Gel Electrophoresis (PAGE) and Zymogram Analysis
2.6. Growth Indicators
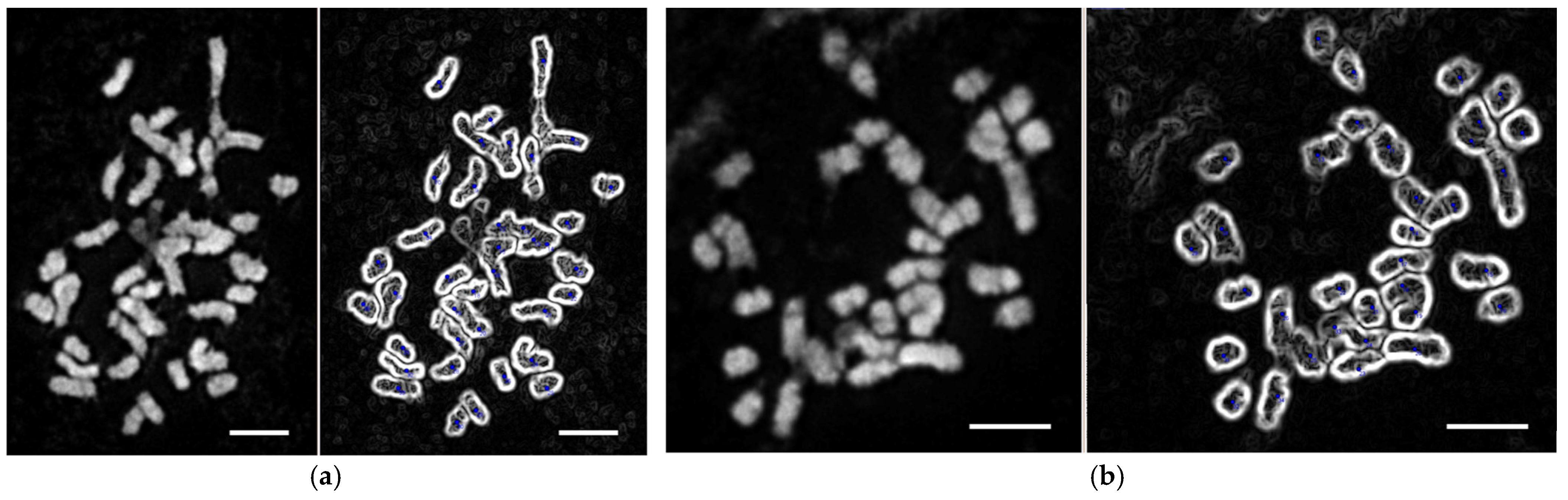
2.7. Determination of Karyotype
2.8. Analysis of Main Wood Components
2.9. Analysis of Phenolic Degradation
2.10. Statistical Data Analysis
3. Results
3.1. Confirmation of Transgenic Status
3.2. Gene Expression of the Transgenic Gene Lac and the Native Genes of Lignin Biosynthesis
3.3. Plant Biometry Data
3.4. Content of Pentosans (Hemicellulose), Cellulose, and Total Lignin
3.5. Phenolic Degradation Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos, R.L.; Moreira, V.R.; Amaral, M.C.S. Phenolic compounds in water: Review of occurrence, risk, and retention by membrane technology. J. Environ. Manag. 2024, 351, 119772. [Google Scholar] [CrossRef] [PubMed]
- Paasivirta, J.; Heinola, K.; Humppi, T.; Karjalainen, A.; Knuutinen, J.; Mäntykoski, K.; Paukku, R.; Piilola, T.; Surma-Aho, K.; Tarhanen, J.; et al. Polychlorinated phenols, guaiacols and catechols in environment. Chemosphere 1985, 14, 469–491. [Google Scholar] [CrossRef]
- Czaplicka, M. Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 2004, 322, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and biotechnological applications of fungal laccase. 3 Biotech 2016, 6, 15. [Google Scholar] [CrossRef]
- Aghapour, A.A.; Moussavi, G.; Yaghmaeian, K. Investigating the performance of a novel cyclic rotating-bed biological reactor compared with a sequencing continuous-inflow reactor for biodegradation of catechol in wastewater. Bioresour. Technol. 2013, 138, 369–372. [Google Scholar] [CrossRef]
- Arora, P.K.; Bae, H. Bacterial degradation of chlorophenols and their derivatives. Microb. Cell Factories 2014, 13, 31. [Google Scholar] [CrossRef]
- Tzou, Y.-M.; Wang, S.-L.; Liu, J.-C.; Huang, Y.-Y.; Chen, J.-H. Removal of 2, 4, 6-trichlorophenol from a solution by humic acids repeatedly extracted from a peat soil. J. Hazard. Mater. 2008, 152, 812–819. [Google Scholar] [CrossRef]
- Bollag, J.M.; Chu, H.L.; Rao, M.A.; Gianfreda, L. Enzymatic oxidative transformation of chlorophenol mixtures. J. Environ. Qual. 2003, 32, 63–69. [Google Scholar] [CrossRef]
- Chandra, R.; Chowdhary, P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ. Sci. Process. Impacts 2015, 17, 326–342. [Google Scholar] [CrossRef]
- Viswanath, B.; Rajesh, B.; Janardhan, A.; Kumar, A.P.; Narasimha, G. Fungal laccases and their applications in bioremediation. Enzym. Res. 2014, 2014, 163242. [Google Scholar] [CrossRef]
- Hirai, H.; Kashima, Y.; Hayashi, K.; Sugiura, T.; Yamagishi, K.; Kawagishi, H.; Nishida, T. Efficient expression of laccase gene from white-rot fungus Schizophyllum communein a transgenic tobacco plant. FEMS Microbiol. Lett. 2008, 286, 130–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, B.; Yan, Y.; Xu, J.; Fu, X.; Han, H.; Gao, J.; Li, Z.; Wang, L.; Tian, Y.; Peng, R. Heterologous expression and characterization of a laccase from Laccaria bicolor in Pichia pastoris. Biotechnol. Biotechnol. Equip. 2016, 30, 63–68. [Google Scholar] [CrossRef]
- Licinio, A.; Laur, J.; Pitre, F.E.; Labrecque, M. Willow and herbaceous species’ phytoremediation potential in Zn-contaminated farm field soil in Eastern Québec, Canada: A greenhouse feasibility study. Plants 2023, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, R.K. Genetic Improvement of Poplar. In Genetic Engineering of Crop Plants for Food and Health Security; Tiwari, S., Koul, B., Eds.; Springer: Singapore, 2024; Volume 1, pp. 199–211. [Google Scholar] [CrossRef]
- Sharan, S.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Transgenic poplar for resistance against pest and pathogen attack in forests: An overview. Front. For. Glob. Change 2024, 7, 1490562. [Google Scholar] [CrossRef]
- Ye, X.; Busov, V.; Zhao, N.; Meilan, R.; McDonnell, L.M.; Coleman, H.D.; Mansfield, S.D.; Chen, F.; Li, Y.; Cheng, Z.M. Transgenic Populus trees for forest products, bioenergy, and functional genomics. Crit. Rev. Plant Sci. 2011, 30, 415–434. [Google Scholar] [CrossRef]
- Bruegmann, T.; Polak, O.; Deecke, K.; Nietsch, J.; Fladung, M. Poplar Transformation. In Transgenic Plants: Methods and Protocols; Kumar, S., Barone, P., Smith, M., Eds.; Humana Press: New York, NY, USA, 2018; Volume 12, pp. 165–177. [Google Scholar] [CrossRef]
- Łukaszkiewicz, J.; Długoński, A.; Fortuna-Antoszkiewicz, B.; Fialová, J. The potential of poplars (Populus L.) for the sustainable environment of cities. Land 2024, 13, 593. [Google Scholar] [CrossRef]
- Rogers, P.C.; Pinno, B.D.; Šebesta, J.; Albrectsen, B.R.; Li, G.; Ivanova, N.; Kusbach, A.; Kuuluvainen, T.; Landhausser, S.M.; Liu, H.; et al. A global view of aspen: Conservation science for widespread keystone systems. Glob. Ecol. Conserv. 2020, 21, e00828. [Google Scholar] [CrossRef]
- Robinson, K.M.; Möller, L.; Bhalerao, R.P.; Hertzberg, M.; Nilsson, O.; Jansson, S. Variation in non-target traits in genetically modified hybrid aspens does not exceed natural variation. New Biotechnol. 2021, 64, 27–36. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Cheng, X.; Sun, J.; Marita, J.M.; Ralph, J.; Chiang, V.L. Combinatorial modification of multiple lignin traits in trees through multigene cotransformation. Proc. Natl. Acad. Sci. USA 2003, 100, 4939–4944. [Google Scholar] [CrossRef]
- Lebedev, V.G.; Schestibratov, K.A.; Shadrina, T.E.; Bulatova, I.V.; Abramochkin, D.G.; Miroshnikov, A.I. Cotransformation of aspen and birch with three T-DNA regions from two different replicons in one Agrobacterium tumefaciens strain. Russ. J. Genet. 2010, 46, 1282–1289. [Google Scholar] [CrossRef]
- Hjältén, J.; Axelsson, E.P.; Whitham, T.G.; LeRoy, C.J.; Julkunen-Tiitto, R.; Wennström, A.; Pilate, G. Increased resistance of Bt aspens to Phratora vitellinae (Coleoptera) leads to increased plant growth under experimental conditions. PLoS ONE 2012, 7, e30640. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.G.; Faskhiev, V.N.; Kovalenko, N.P.; Schestibratov, K.A.; Miroshnikov, A.I. Testing transgenic aspen plants with bar gene for herbicide resistance under semi-natural conditions. Acta Nat. 2016, 8, 92–101. [Google Scholar] [CrossRef]
- Yu, D.; Wildhagen, H.; Tylewicz, S.; Miskolczi, P.C.; Bhalerao, R.P.; Polle, A. Abscisic acid signalling mediates biomass trade-off and allocation in poplar. New Phytol. 2019, 223, 1192–1203. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Y.; Chaffin, T.A.; Lee, J.H.; Poindexter, M.R.; Ahkami, A.H.; Blumwald, E.; Stewart, C.N., Jr. Performance of abiotic stress-inducible synthetic promoters in genetically engineered hybrid poplar (Populus tremula × Populus alba). Front. Plant Sci. 2022, 13, 1011939. [Google Scholar] [CrossRef]
- Tsarev, A.P. Growth and breeding of aspen in Russia. Silvae Genet. 2013, 62, 153–160. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, P.; Parmar, N.; Shandil, R.K.; Aggarwal, G.; Gaur, A.; Srivastava, D.K. Achievements and prospects of genetic engineering in poplar: A review. New For. 2021, 52, 889–920. [Google Scholar] [CrossRef]
- Jefferson, R.A. Assaying chimeric genes in plants: The GUS fusion system. Plant Mol. Biol. Rep. 1987, 5, 387–405. [Google Scholar] [CrossRef]
- Rebrikov, D.N.; Stepanova, E.V.; Koroleva, O.V.; Budarina, Z.I.; Zakharova, M.V.; Yurkova, T.V.; Solonin, A.S.; Belova, O.V.; Leont’evsky, A.A. Laccase of the lignolytic fungus Trametes hirsuta: Purification and characterization of the enzyme, and cloning and primary structure of the gene. Appl. Biochem. Microbiol. 2006, 42, 564–572. [Google Scholar] [CrossRef]
- Abyanova, A.R.; Chulkin, A.M.; Vavilova, E.A.; Fedorova, T.V.; Loginov, D.S.; Koroleva, O.V.; Benevolensky, S.V. Heterologous production of the Trametes hirsuta laccase in the fungus Penicillium canescens. Appl. Biochem. Microbiol. 2010, 46, 342–347. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propag. Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kovalenko, N.P.; Subbotina, N.M.; Shestibratov, K.A. Modified CTAB protocol for the isolation of high-quality RNA from silver and downy birch leaves. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 3105–3110. [Google Scholar]
- Kovalitskaya, Y.A.; Kovalenko, N.P.; Shestibratov, K.A. Populus tremula plants with reduced expression of the 4-coumarate-CoA ligase gene demonstrate defects of the rhizogenesis. Int. J. Eng. Technol. 2018, 7, 1139–1144. [Google Scholar] [CrossRef]
- Wang, P.; Dudareva, N.; Morgan, J.A.; Chapple, C. Genetic manipulation of lignocellulosic biomass for bioenergy. Curr. Opin. Chem. Biol. 2015, 29, 32–39. [Google Scholar] [CrossRef]
- Castro-Rodríguez, V.; García-Gutiérrez, A.; Canales, J.; Cañas, R.A.; Kirby, E.G.; Avila, C.; Cánovas, F.M. Poplar trees for phytoremediation of high levels of nitrate and applications in bioenergy. Plant Biotechnol. J. 2016, 14, 299–312. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem. J. 1988, 254, 877–884. [Google Scholar] [CrossRef]
- Shani, Z.; Dekel, M.; Tsabary, G.; Goren, R.; Shoseyov, O. Growth enhancement of transgenic poplar plants by overexpression of Arabidopsis thaliana endo-1,4-β-glucanase (cel1). Mol. Breed. 2004, 14, 321–330. [Google Scholar] [CrossRef]
- Bylesjö, M.; Segura, V.; Soolanayakanahally, R.Y.; Rae, A.M.; Trygg, J.; Gustafsson, P.; Jansson, S.; Street, N.R. LAMINA: A tool for rapid quantification of leaf size and shape parameters. BMC Plant Biol. 2008, 8, 82. [Google Scholar] [CrossRef]
- Muravenko, O.V.; Amosova, A.V.; Samatadze, T.E.; Popov, K.V.; Poletaev, A.I.; Zelenin, A.V. 9-Aminoacridine: An efficient reagent to improve human and plant chromosome banding patterns and to standardize chromosome image analysis. Cytom. Part A J. Int. Soc. Anal. Cytol. 2003, 51, 52–57. [Google Scholar] [CrossRef]
- Amosova, A.V.; Bolsheva, N.L.; Zoshchuk, S.A.; Twardovska, M.O.; Yurkevich, O.Y.; Andreev, I.O.; Samatadze, T.E.; Badaeva, E.D.; Kunakh, V.A.; Muravenko, O.V. Comparative molecular cytogenetic characterization of seven Deschampsia (Poaceae) species. PLoS ONE 2017, 12, e0175760. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.E.; Martin, T.M.; Pauly, M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part I: Lignin. J. Vis. Exp. 2010, 37, e1745. [Google Scholar] [CrossRef]
- Kurschner, K.; Hanak, A. Determination of cellulose. Z. Utersuch Lebensm. 1930, 59, 448–485. [Google Scholar]
- Obolenskaya, A.V.; Elnitskaya, Z.P.; Leonovich, A.A. Laboratory Work on the Chemistry of Wood; Ekologiya: Moscow, Russia, 1991; p. 320, (In Russian with English Abstract). [Google Scholar]
- Wilson, W.K.; Mandel, J. Determination of pentosans. TAPPI J. 1960, 43, 998. [Google Scholar]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, C.N.M.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef]
- Carabajal, M.; Perullini, M.; Jobbágy, M.; Ullrich, R.; Hofrichter, M.; Levin, L. Removal of phenol by immobilization of Trametes versicolor in silica-alginate-fungus biocomposites and loofa sponge. CLEAN Soil Air Water 2015, 44, 180–188. [Google Scholar] [CrossRef]
- Gaitan, I.J.; Medina, S.C.; González, J.C.; Rodríguez, A.; Espejo, Á.J.; Osma, J.F.; Sarria, V.; Alméciga-Díaz, C.J.; Sánchez, O.F. Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresour. Technol. 2011, 102, 3632–3635. [Google Scholar] [CrossRef]
- Fukuda, T.; Uchida, H.; Takashima, Y.; Uwajima, T.; Kawabata, T.; Suzuki, M. Degradation of bisphenol A by purified laccase from Trametes villosa. Biochem. Biophys. Res. Commun. 2001, 284, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Grassi, E.; Scodeller, P.; Filiel, N.; Carballo, R.; Levin, L. Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int. Biodeterior. Biodegrad. 2011, 65, 635–643. [Google Scholar] [CrossRef]
- Navada, K.K.; Kulal, A. Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int. Biodeterior. Biodegrad. 2019, 138, 63–69. [Google Scholar] [CrossRef]
- Rodionov, A.V. Polyploidy and interspecific hybridization in the evolution of flowering plants. Vavilovskii Zhurnal Genet. Sel. 2013, 17, 2. [Google Scholar]
- Furukawa, T.; Sawaguchi, C.; Watanabe, A.; Takahashi, M.; Nigorikawa, M.; Furukawa, K.; Iimura, Y.; Kajita, S.; Oguchi, T.; Ito, Y.; et al. Application of fungal laccase fused with cellulose-binding domain to develop low-lignin rice plants. J. Biosci. Bioeng. 2013, 116, 616–619. [Google Scholar] [CrossRef]
- Van Eerde, A.; Várnai, A.; Wang, Y.; Paruch, L.; Jameson, J.-K.; Qiao, F.; Geir Eiken, H.; Su, H.; Eijsink, V.G.H.; Liu, J. Successful production and ligninolytic activity of a bacterial laccase, Lac51, made in Nicotiana benthamiana via transient expression. Front. Plant Sci. 2022, 13, 912293. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cezard, L.; Le Bris, P.; Borrega, N.; Herve, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 Results in Tissue-Specific Alterations to Lignification of Arabidopsis thaliana Stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef]
- Vidyagina, E.O.; Subbotina, N.M.; Belyi, V.A.; Lebedev, V.G.; Krutovsky, K.V.; Shestibratov, K.A. Various effects of the expression of the xyloglucanase gene from Penicillium canescens in transgenic aspen under semi-natural conditions. BMC Plant Biol. 2020, 20, 251. [Google Scholar] [CrossRef]
- Mukherjee, D.; Ferreira, N.G.C.; Saha, N.C. Effects of 2,4,6-Trichlorophenol on Clarias batrachus: A biomarkers approach. Environ. Sci. Pollut. Res. 2022, 29, 47011–47024. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Podkoscielny, P.; Dabrowski, A.; Marijuk, O.V. Heterogeneity of active carbons in adsorption of phenol aqueous solutions. Appl. Surf. Sci. 2003, 205, 297–303. [Google Scholar] [CrossRef]
- Benbachir, H.; Gaffour, H.; Mokhtari, M. Photodegradation of 2,4,6-trichlorophenol using natural hematite modified with chloride of zirconium oxide. React. Kinet. Mech. Catal. 2017, 122, 635–653. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Chen, B.; Yang, F.; Li, B.; Li, H.; Jiang, Z.; Song, H. Show more effective biodegradation of chlorophenols, sulfonamides, and their mixtures by bacterial laccase immobilized on chitin. Ecotoxicol. Environ. Saf. 2023, 256, 114856. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-D.; Li, Q.-J.; Luo, B.; Chen, X.-Y. Ex planta phytoremediation of trichlorophenol and phenolic allelochemicals via an engineered secretory laccase. Nat. Biotechnol. 2004, 22, 893–897. [Google Scholar] [CrossRef]
- Bao, W.; Peng, R.; Zhang, Z.; Tian, Y.; Zhao, W.; Xue, Y.; Gao, J.; Yao, Q. Expression, characterization and 2,4,6-trichlorophenol degradation of laccase from Monilinia fructigena. Mol. Biol. Rep. 2011, 39, 3871–3877. [Google Scholar] [CrossRef]
- The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
| Gene | NCBI GenBank Accession Number | Primer Nucleotide Sequence (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Act | MN196665.1 | TATGCCCTCCCACATGCCAT | CATCTGCTGGAAGGTGCTGA |
| UBQ | PQ155116.1 | GTTGATTTTTGCTGGGAAGC | GATCTTGGCCTTCACGTTGT |
| LacTh | KP027478 | ATTCTCAGTGGTGCCCACAGACGG | ATGGACGCGTTCGACGGAAG |
| CAD6 | KF145199.1 | GGTAGGAAGCAAAGTTGAAAAGTTC | TAGCCTCCGTACGTGGTGGTTCCAT |
| CCoAOMT1 | KX227458.1 | GTACCTCACATACTTCCTCATTGGT | TGGAAGTTTTGATTTCATCTTTG |
| CCR1 | MW928493.1 | AGTTTTCCATACTGCTTCTGTC | ACAACATCAGGGCTCCTATTGGGGT |
| Pxp 3-4 | X97351.1 | CAGCTTACTCCAACATTTTATGACC | GTATTGTCCAACAAAAGTGAACCA |
| 4CL | MK256749.1 | CTTTGTTAATAGCCCATCCAG | TGATTTCACAGCAAATGCAAC |
| MYB152 | PQ178447.1 | TCGTATCTGAACTGGACCAAAATAG | AGGGACTAAGATTTCATGGGGTTC |
| Line | Lac | CAD6 | CCoAOMT1 | CCR1 | Pxp3-4 | 4CL | MYB152 |
|---|---|---|---|---|---|---|---|
| 47-1 (control) | - | 1 | 1 | 1 | 1 | 1 | 1 |
| 47Lac4 | 1 | 0.44 | 0.89 | 1.26 | 2.53 | 1.35 | 1.65 |
| 47Lac5 | 0.031 | 0.52 | 0.75 | 0.66 | 1.54 | 0.74 | 1.39 |
| 47Lac7 | 0.0013 | 0.48 | 0.75 | 0.8 | 1.26 | 0.68 | 2.26 |
| 47Lac8 | 0.159 | 0.37 | 0.85 | 0.51 | 1.49 | 0.8 | 1.15 |
| 47Lac11 (control) | - | 0.59 | 0.78 | 0.7 | 0.95 | 0.84 | 0.96 |
| 47Lac23 | 0.346 | 0.26 | 0.3 | 0.38 | 0.75 | 0.47 | 0.94 |
| Line | Lac Relative Expression Level ± Stand. Error |
|---|---|
| 47-1 (control) | - |
| 47Lac5 | 3.48 ± 0.13 |
| 47Lac7 | 1.00 ± 0.03 |
| 47Lac8 | 32.11 ± 2.34 |
| 47Lac23 | 45.89 ± 4.55 |
| Line | Height, cm | Stem Diameter, mm | Volume, m3 | Number of Branches | Leaf Area, mm2 |
|---|---|---|---|---|---|
| 47-1 (control) | 288.6 ± 8.61 | 19.53 ± 0.57 | 0.110 | 34.3 ± 1.73 | 5716.0 ± 182 |
| 47Lac5 | 285.3 ± 10.43 | 18.68 ± 0.78 | 0.100 | 29.1 ± 0.98 * | 6028.9 ± 170 |
| 47Lac7 | 268.7 ± 9.35 | 18.88 ± 0.79 | 0.096 | 36.8 ± 1.77 | 6585.2 ± 153 |
| 47Lac8 | 294.0 ± 9.71 | 21.01 ± 10.01 | 0.130 | 30.9 ± 1.3 | 6661.8 ± 171 * |
| 47Lac11 (control) | 279.0 ± 12.6 | 20.33 ± 0.95 | 0.115 | 37.0 ± 1.44 | 5718.4 ± 165 |
| 47Lac23 | 277.8 ± 11.53 | 19.23 ± 0.75 | 0.103 | 38.6 ± 1.2 * | 3653.5 ± 155 * |
| Line | Pentosans (Hemicellulose) | Cellulose | Lignin |
|---|---|---|---|
| 47-1 (control) | 19.0 ± 0.99 | 41.8 ± 1.56 | 30.3 ± 1.50 |
| 47Lac5 | 16.9 ± 0.78 | 42.1 ± 1.67 | 29.9 ± 1.59 |
| 47Lac7 | 17.8 ± 0.63 | 42.3 ± 1.88 | 28.9 ± 1.21 |
| 47Lac8 | 18.0 ± 0.73 | 42.8 ± 1.70 | 29.2 ± 1.33 |
| 47Lac11 (control) | 18.6 ± 0.87 | 41.3 ± 1.95 | 29.4 ± 1.40 |
| 47Lac23 | 17.8 ± 0.85 | 43.2 ± 1.73 | 30.1 ± 1.49 |
| Line | Residual 2,4,6-TCP, µM |
|---|---|
| 47-1 (control) | 1.793 ± 0.874 |
| 47Lac4 | 0.363 ± 0.030 |
| 47Lac5 | 0.414 ± 0.035 |
| 47Lac8 | 0.199 ± 0.037 |
| 47Lac23 | 0.783 ± 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidyagina, E.O.; Subbotina, N.M.; Belova, E.N.; Kovalitskaya, Y.A.; Evdokimov, V.A.; Belyi, V.A.; Kochetov, A.P.; Surin, A.K.; Krutovsky, K.V.; Shestibratov, K.A. The Potential of Transgenic Hybrid Aspen Plants with a Recombinant Lac Gene from the Fungus Trametes hirsuta to Degrade Trichlorophenol. Genes 2025, 16, 298. https://doi.org/10.3390/genes16030298
Vidyagina EO, Subbotina NM, Belova EN, Kovalitskaya YA, Evdokimov VA, Belyi VA, Kochetov AP, Surin AK, Krutovsky KV, Shestibratov KA. The Potential of Transgenic Hybrid Aspen Plants with a Recombinant Lac Gene from the Fungus Trametes hirsuta to Degrade Trichlorophenol. Genes. 2025; 16(3):298. https://doi.org/10.3390/genes16030298
Chicago/Turabian StyleVidyagina, Elena O., Natalia M. Subbotina, Eugenia N. Belova, Yulia A. Kovalitskaya, Vyacheslav A. Evdokimov, Vladimir A. Belyi, Alexey P. Kochetov, Alexey K. Surin, Konstantin V. Krutovsky, and Konstantin A. Shestibratov. 2025. "The Potential of Transgenic Hybrid Aspen Plants with a Recombinant Lac Gene from the Fungus Trametes hirsuta to Degrade Trichlorophenol" Genes 16, no. 3: 298. https://doi.org/10.3390/genes16030298
APA StyleVidyagina, E. O., Subbotina, N. M., Belova, E. N., Kovalitskaya, Y. A., Evdokimov, V. A., Belyi, V. A., Kochetov, A. P., Surin, A. K., Krutovsky, K. V., & Shestibratov, K. A. (2025). The Potential of Transgenic Hybrid Aspen Plants with a Recombinant Lac Gene from the Fungus Trametes hirsuta to Degrade Trichlorophenol. Genes, 16(3), 298. https://doi.org/10.3390/genes16030298








