An RB1CC1 Missense Variant in Nova Scotia Duck Tolling Retrievers with Degenerative Encephalopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Cases and Controls
2.2. Molecular Genetic Analyses
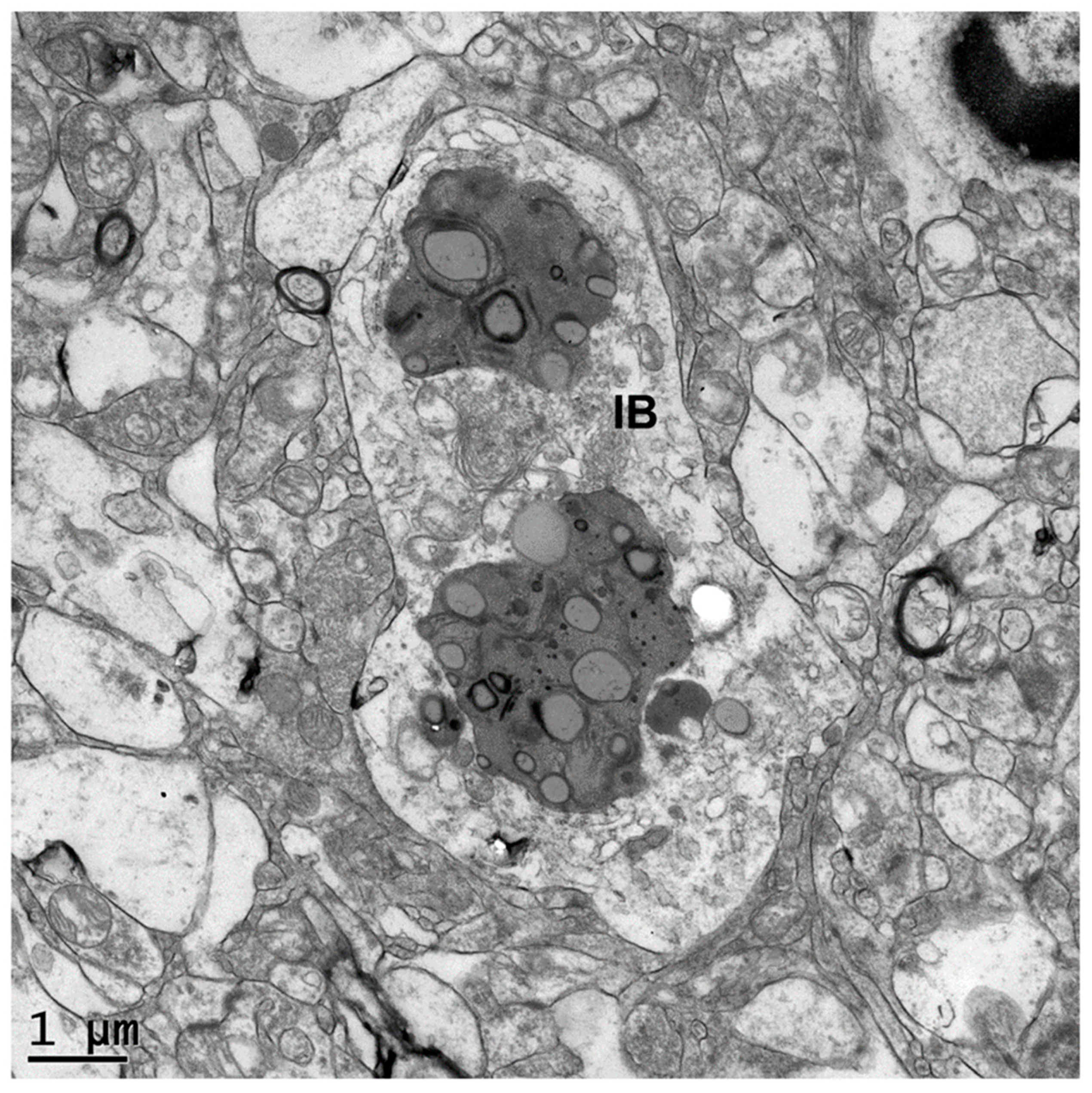
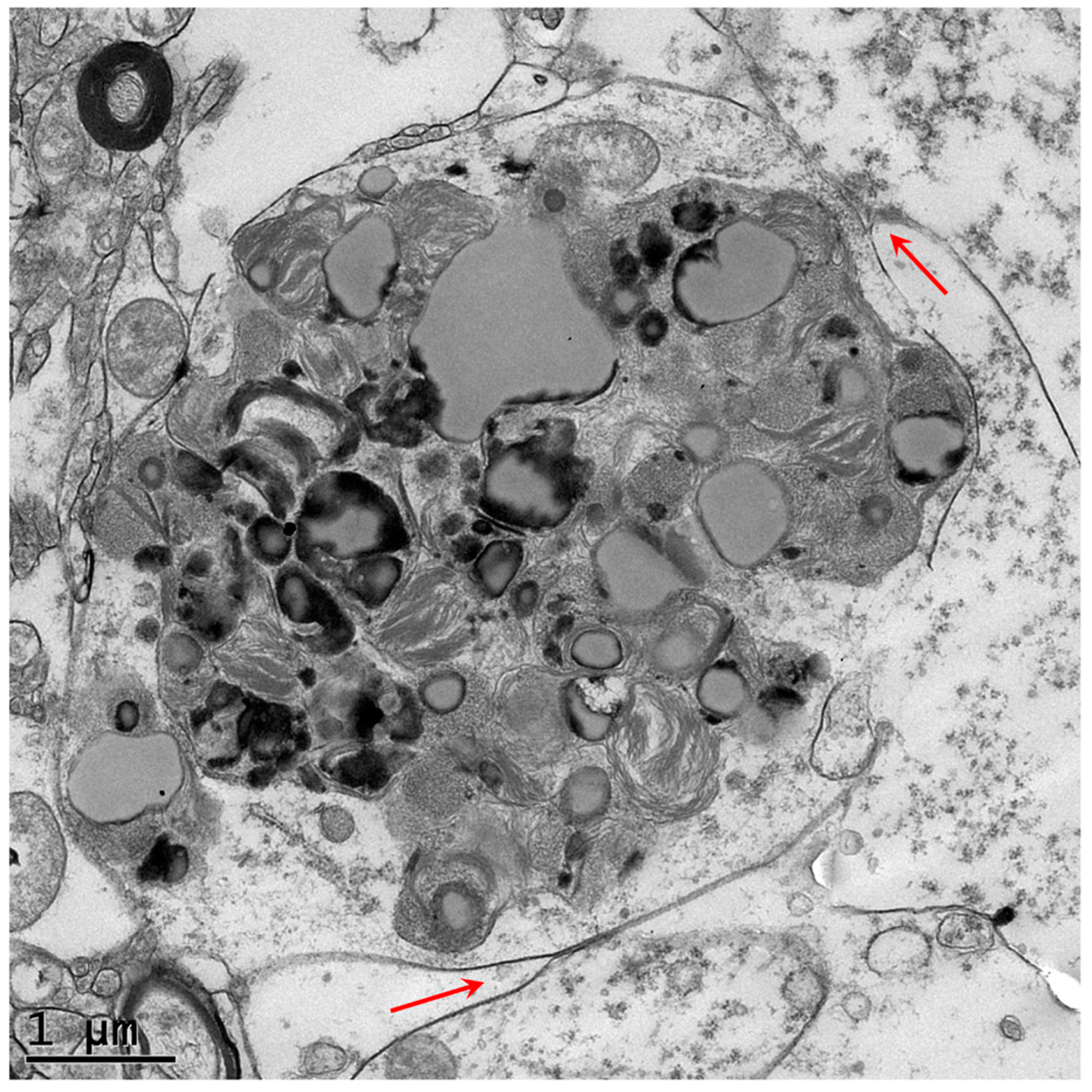
2.3. Electron Microscopy
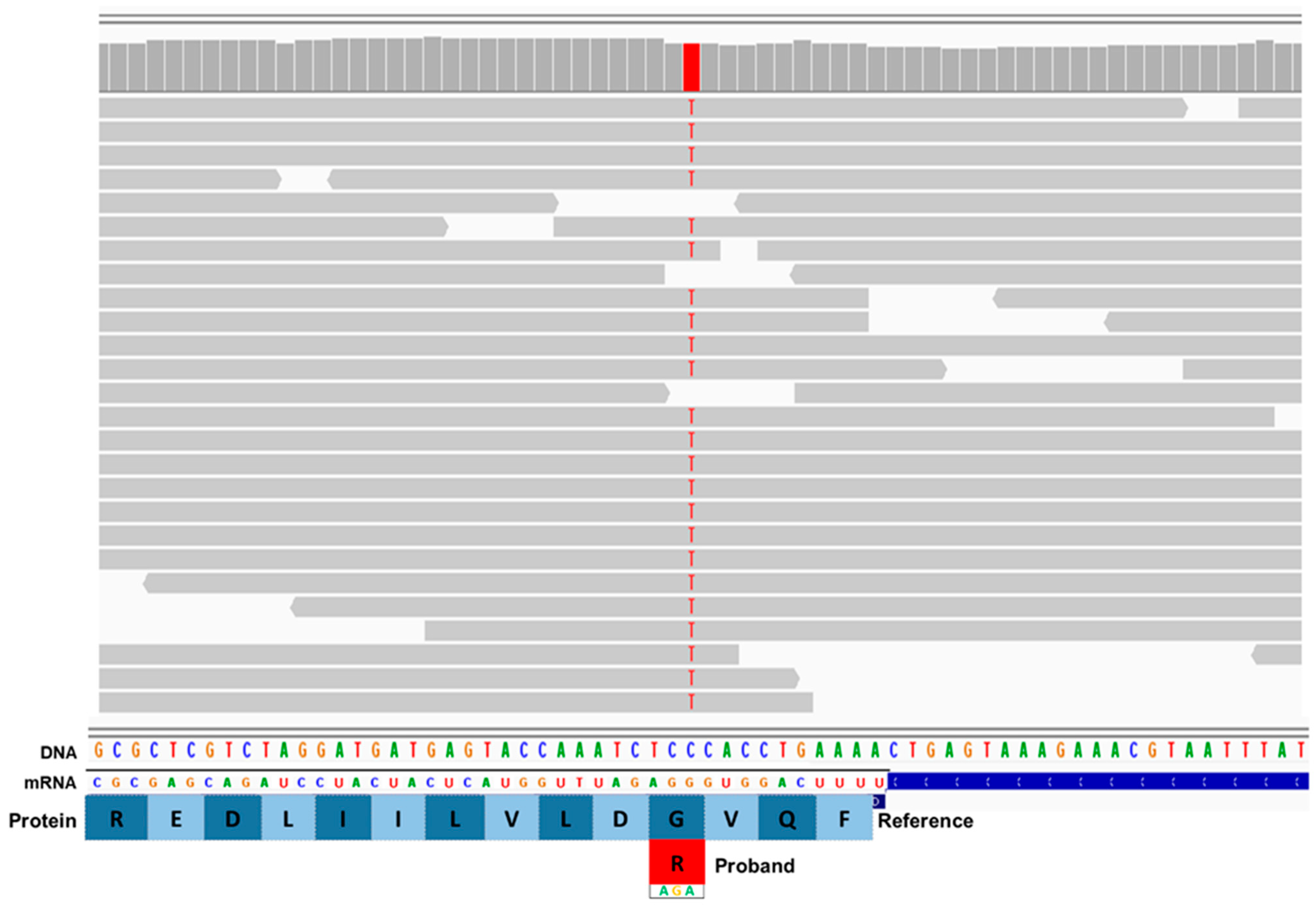
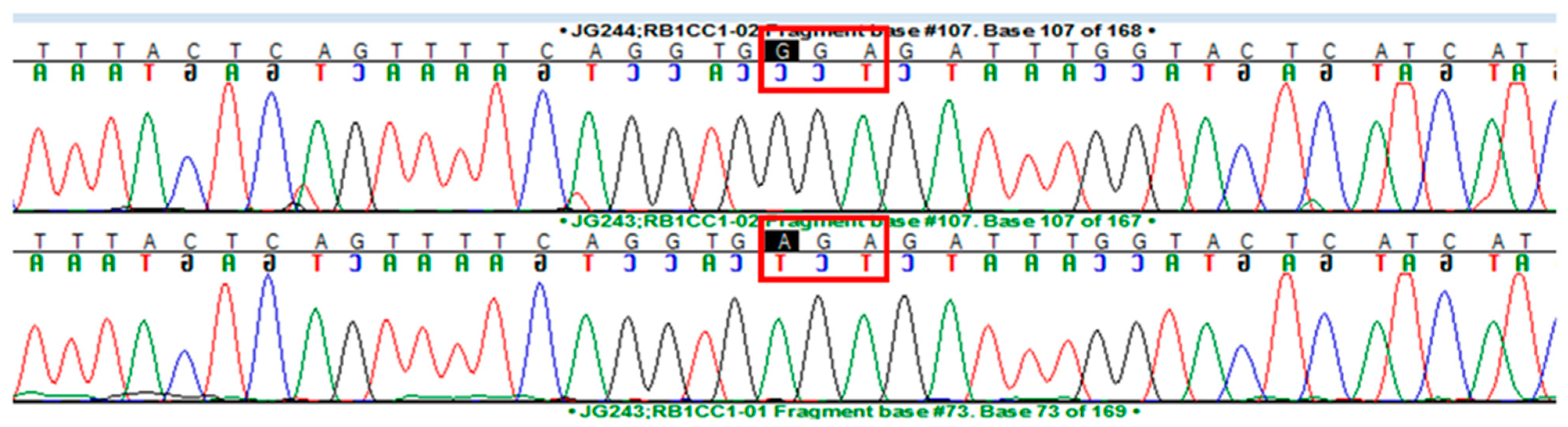
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, E.N.; Dawson, L.J.; Rose, J.H.; Van Meervenne, S.; Frykman, O.; Rohdin, C.; Leijon, A.; Soerensen, K.E.; Jarnegren, J.; Johnson, G.C.; et al. Degenerative Encephalopathy in Nova Scotia Duck Tolling Retrievers Presenting with a Rapid Eye Movement Sleep Behavior Disorder. J. Vet. Intern. Med. 2016, 30, 1681–1689. [Google Scholar] [CrossRef]
- Katz, M.L.; Khan, S.; Awano, T.; Shahid, S.A.; Siakotos, A.N.; Johnson, G.S. A Mutation in the CLN8 Gene in English Setter Dogs with Neuronal Ceroid-Lipofuscinosis. Biochem. Biophys. Res. Commun. 2005, 327, 541–547. [Google Scholar] [CrossRef]
- Zeng, R.; Coates, J.R.; Johnson, G.C.; Hansen, L.; Awano, T.; Kolicheski, A.; Ivansson, E.; Perloski, M.; Lindblad-Toh, K.; O’Brien, D.P.; et al. Breed Distribution of SOD1 Alleles Previously Associated with Canine Degenerative Myelopathy. J. Vet. Intern. Med. 2014, 28, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain W1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Gahl, W.A. Lysosomal Storage Diseases. Transl. Sci. Rare Dis. 2017, 2, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Bullock, G.; Johnson, G.S.; Mhlanga-Mutangadura, T.; Petesch, S.C.; Thompson, S.; Goebbels, S.; Katz, M.L. Lysosomal Storage Disease Associated with a CNP Sequence Variant in Dalmatian Dogs. Gene 2022, 830, 146513. [Google Scholar] [CrossRef] [PubMed]
- Furuta, A.; Kikuchi, H.; Fujita, H.; Yamada, D.; Fujiwara, Y.; Kabuta, T.; Nishino, I.; Wada, K.; Uchiyama, Y. Property of Lysosomal Storage Disease Associated with Midbrain Pathology in the Central Nervous System of Lamp-2-Deficient Mice. Am. J. Pathol. 2015, 185, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Wesselborg, S.; Stork, B. ATG13: Just a Companion, or an Executor of the Autophagic Program? Autophagy 2014, 10, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Noda, N.N.; Mizushima, N. Atg101: Not Just an Accessory Subunit in the Autophagy-Initiation Complex. Cell Struct. Funct. 2016, 41, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.-I.; Kanki, T. How Autophagy Eats Large Mitochondria: Autophagosome Formation Coupled with Mitochondrial Fragmentation. Autophagy 2017, 13, 980–981. [Google Scholar] [CrossRef]
- Corona Velazquez, A.F.; Jackson, W.T. So Many Roads: The Multifaceted Regulation of Autophagy Induction. Mol. Cell Biol. 2018, 38, e00303-18. [Google Scholar] [CrossRef] [PubMed]
- Yamano, K.; Youle, R.J. Two Different Axes CALCOCO2-RB1CC1 and OPTN-ATG9A Initiate PRKN-Mediated Mitophagy. Autophagy 2020, 16, 2105–2107. [Google Scholar] [CrossRef]
- Yamamoto, H.; Zhang, S.; Mizushima, N. Autophagy Genes in Biology and Disease. Nat. Rev. Genet. 2023, 24, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sandford, E.; Gatica, D.; Qiu, Y.; Liu, X.; Zheng, Y.; Schulman, B.A.; Xu, J.; Semple, I.; Ro, S.-H.; et al. Mutation in ATG5 Reduces Autophagy and Leads to Ataxia with Developmental Delay. eLife 2016, 5, e12245. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.J.; Guissart, C.; Olahova, M.; Sasorith, S.; Piron-Prunier, F.; Suomi, F.; Zhang, D.; Martinez-Lopez, N.; Leboucq, N.; Bahr, A.; et al. Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N. Engl. J. Med. 2021, 384, 2406–2417. [Google Scholar] [CrossRef] [PubMed]
- Almannai, M.; Marafi, D.; Abdel-Salam, G.M.H.; Zaki, M.S.; Duan, R.; Calame, D.; Herman, I.; Levesque, F.; Elbendary, H.M.; Hegazy, I.; et al. El-Hattab-Alkuraya Syndrome Caused by Biallelic WDR45B Pathogenic Variants: Further Delineation of the Phenotype and Genotype. Clin. Genet. 2022, 101, 530–540. [Google Scholar] [CrossRef]
- Seidahmed, M.Z.; Hamad, M.H.; AlBakheet, A.; Elmalik, S.A.; AlDrees, A.; Al-Sufayan, J.; Alorainy, I.; Ghozzi, I.M.; Colak, D.; Salih, M.A.; et al. Ancient Founder Mutation in RUBCN: A Second Unrelated Family Confirms Salih Ataxia (SCAR15). BMC Neurol. 2020, 20, 207. [Google Scholar] [CrossRef]
- Guo, A.; Lun, P.; Chen, J.; Li, Q.; Chang, K.; Li, T.; Pan, D.; Zhang, J.; Zhou, J.; Wang, K.; et al. Association Analysis of Risk Genes Identified by SCHEMA with Schizophrenia in the Chinese Han Population. Psychiatr. Genet. 2022, 32, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Lappas, A.S.; Ioannou, M.; Christodoulou, N.G. Histopathological Evidence of Cellular Alterations in the Dentate Gyrus Is Associated with Aberrant RB1CC1-ATG16L1 Expression in the Hippocampus among Older Adults with Chronic Schizophrenia: A Pilot Post-Mortem Study. Schizophr. Res. 2025, 275, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.-L.; Thompson, D.A.; et al. Deletion of Autophagy Inducer RB1CC1 Results in Degeneration of the Retinal Pigment Epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Yeo, S.; Karsli-Uzunbas, G.; White, E.; Mizushima, N.; Virgin, H.W.; Guan, J.-L. Elevated P62/SQSTM1 Determines the Fate of Autophagy-Deficient Neural Stem Cells by Increasing Superoxide. J. Cell Biol. 2016, 212, 545–560. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Yeo, S.; Liang, C.-C.; Okamoto, T.; Sun, S.; Wen, J.; Guan, J.-L. Distinct Roles of Autophagy-Dependent and -Independent Functions of FIP200 Revealed by Generation and Analysis of a Mutant Knock-in Mouse Model. Genes Dev. 2016, 30, 856–869. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Yu, R.-H.; Zhou, S.; Tang, P.-P.; Zhang, R.; Wu, Y.-X.; Xu, R.; Wei, J.-M.; Wang, Y.-Y.; Zhang, J.-L.; et al. TAX1BP1 and FIP200 Orchestrate Non-Canonical Autophagy of P62 Aggregates for Mouse Neural Stem Cell Maintenance. Zool. Res. 2024, 45, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yeo, S.; Haas, M.A.; Guan, J.-L. Autophagy Gene FIP200 in Neural Progenitors Non-Cell Autonomously Controls Differentiation by Regulating Microglia. J. Cell Biol. 2017, 216, 2581–2596. [Google Scholar] [CrossRef]
- Liang, C.-C.; Wang, C.; Peng, X.; Gan, B.; Guan, J.-L. Neural-Specific Deletion of FIP200 Leads to Cerebellar Degeneration Caused by Increased Neuronal Death and Axon Degeneration. J. Biol. Chem. 2010, 285, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Deneubourg, C.; Ramm, M.; Smith, L.J.; Baron, O.; Singh, K.; Byrne, S.C.; Duchen, M.R.; Gautel, M.; Eskelinen, E.-L.; Fanto, M.; et al. The Spectrum of Neurodevelopmental, Neuromuscular and Neurodegenerative Disorders Due to Defective Autophagy. Autophagy 2022, 18, 496–517. [Google Scholar] [CrossRef]
- Hahn, K.; Rohdin, C.; Jagannathan, V.; Wohlsein, P.; Baumgartner, W.; Seehusen, F.; Spitzbarth, I.; Grandon, R.; Drogemuller, C.; Jaderlund, K.H. TECPR2 Associated Neuroaxonal Dystrophy in Spanish Water Dogs. PLoS ONE 2015, 10, e0141824. [Google Scholar] [CrossRef] [PubMed]
- Tamim-Yecheskel, B.-C.; Fraiberg, M.; Kokabi, K.; Freud, S.; Shatz, O.; Marvaldi, L.; Subic, N.; Brenner, O.; Tsoory, M.; Eilam-Altstadter, R.; et al. A Tecpr2 Knockout Mouse Exhibits Age-Dependent Neuroaxonal Dystrophy Associated with Autophagosome Accumulation. Autophagy 2021, 17, 3082–3095. [Google Scholar] [CrossRef]
- Fraiberg, M.; Tamim-Yecheskel, B.-C.; Kokabi, K.; Subic, N.; Heimer, G.; Eck, F.; Nalbach, K.; Behrends, C.; Ben-Zeev, B.; Shatz, O.; et al. Lysosomal Targeting of Autophagosomes by the TECPR Domain of TECPR2. Autophagy 2021, 17, 3096–3108. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Tzekov, R.; Li, H.; McDowell, J.H.; Gao, G.; Smith, W.C.; Tang, S.; Kaushal, S. Inhibition or Stimulation of Autophagy Affects Early Formation of Lipofuscin-Like Autofluorescence in the Retinal Pigment Epithelium Cell. Int. J. Mol. Sci. 2017, 18, 728. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Guo, E.; Yang, J.; Yang, Y.; Liu, S.; Jiang, X.; Hu, Q.; Dirsch, O.; Dahmen, U.; Zhang, C.; et al. Young Plasma Reverses Age-Dependent Alterations in Hepatic Function through the Restoration of Autophagy. Aging Cell 2018, 17, e12708. [Google Scholar] [CrossRef] [PubMed]
- Nian, F.-S.; Li, L.-L.; Cheng, C.-Y.; Wu, P.-C.; Lin, Y.-T.; Tang, C.-Y.; Ren, B.-S.; Tai, C.-Y.; Fann, M.-J.; Kao, L.-S.; et al. Rab18 Collaborates with Rab7 to Modulate Lysosomal and Autophagy Activities in the Nervous System: An Overlapping Mechanism for Warburg Micro Syndrome and Charcot-Marie-Tooth Neuropathy Type 2B. Mol. Neurobiol. 2019, 56, 6095–6105. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, S.A.; Shah, H.V.; Kanfer, G.; Pickrell, A.M.; Holtzclaw, L.A.; Ward, M.E.; Youle, R.J. Loss of TAX1BP1-Directed Autophagy Results in Protein Aggregate Accumulation in the Brain. Mol. Cell 2020, 80, 779–795.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-W.; Wang, H.-J.; Tan, Y.-Z.; Wang, Y.-L.; Yu, S.-N.; Li, Z.-H. Reducing Lipofuscin Accumulation and Cardiomyocytic Senescence of Aging Heart by Enhancing Autophagy. Exp. Cell Res. 2021, 403, 112585. [Google Scholar] [CrossRef] [PubMed]
- Borner, J.H.; Rawashdeh, O.; Rami, A. Exacerbated Age-Related Hippocampal Alterations of Microglia Morphology, β-Amyloid and Lipofuscin Deposition and Presenilin Overexpression in Per1−/−-Mice. Antioxidants 2021, 10, 1330. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Koskela, A.; Blasiak, J.; Kaarniranta, K. Autophagy in Drusen Biogenesis Secondary to Age-Related Macular Degeneration. Acta Ophthalmol. 2024, 102, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Gao, Y.-X.; Yuan, Q.-Z.; Zhang, M. NLRP3 and Autophagy in Retinal Ganglion Cell Inflammation in Age-Related Macular Degeneration: Potential Therapeutic Implications. Int. J. Ophthalmol. 2024, 17, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Sosulski, M.L.; Gongora, R.; Danchuk, S.; Dong, C.; Luo, F.; Sanchez, C.G. Deregulation of Selective Autophagy during Aging and Pulmonary Fibrosis: The Role of TGFbeta1. Aging Cell 2015, 14, 774–783. [Google Scholar] [CrossRef]
- Winicki, N.M.; Nanavati, A.P.; Morrell, C.H.; Moen, J.M.; Axsom, J.E.; Krawczyk, M.; Petrashevskaya, N.N.; Beyman, M.G.; Ramirez, C.; Alfaras, I.; et al. A Small Erythropoietin Derived Non-Hematopoietic Peptide Reduces Cardiac Inflammation, Attenuates Age Associated Declines in Heart Function and Prolongs Healthspan. Front. Cardiovasc. Med. 2022, 9, 1096887. [Google Scholar] [CrossRef]
- Terman, A.; Kurz, T.; Gustafsson, B.; Brunk, U.T. The Involvement of Lysosomes in Myocardial Aging and Disease. Curr. Cardiol. Rev. 2008, 4, 107–115. [Google Scholar] [CrossRef]
- Turco, E.; Witt, M.; Abert, C.; Bock-Bierbaum, T.; Su, M.-Y.; Trapannone, R.; Sztacho, M.; Danieli, A.; Shi, X.; Zaffagnini, G.; et al. FIP200 Claw Domain Binding to P62 Promotes Autophagosome Formation at Ubiquitin Condensates. Mol. Cell 2019, 74, 330–346.e11. [Google Scholar] [CrossRef] [PubMed]
- Turco, E.; Witt, M.; Abert, C.; Bock-Bierbaum, T.; Su, M.-Y.; Trapannone, R.; Sztacho, M.; Danieli, A.; Shi, X.; Zaffagnini, G.; et al. How RB1CC1/FIP200 Claws Its Way to Autophagic Engulfment of SQSTM1/P62-Ubiquitin Condensates. Autophagy 2019, 15, 1475–1477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Gong, X.; Wang, Y.; Zhang, Y.; Tang, Y.; Zhou, X.; Liu, H.; Huang, Y.; Zhang, J.; et al. Mechanistic Insights into the Interactions of TAX1BP1 with RB1CC1 and Mammalian ATG8 Family Proteins. Proc. Natl. Acad. Sci. USA 2024, 121, e2315550121. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Zhang, M.; Zhou, Z.; Wu, P.; Peng, C.; Wang, Y.; Gong, X.; Li, Y.; Wang, Y.; Xu, X.; et al. Structural and Biochemical Advances on the Recruitment of the Autophagy-Initiating ULK and TBK1 Complexes by Autophagy Receptor NDP52. Sci. Adv. 2021, 7, eabi6582. [Google Scholar] [CrossRef]
- Assar, E.A.; Tumbarello, D.A. Loss of the Essential Autophagy Regulators FIP200 or Atg5 Leads to Distinct Effects on Focal Adhesion Composition and Organization. Front. Cell Dev. Biol. 2020, 8, 733. [Google Scholar] [CrossRef]
- Yeo, S.K.; Guan, J.-L. Regulation of Immune Checkpoint Blockade Efficacy in Breast Cancer by FIP200: A Canonical-Autophagy-Independent Function. Cell Stress 2020, 4, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Yi, F.; Yeo, S.; Haas, M.; Tang, X.; Guan, J.-L. Non-Canonical Function of FIP200 Is Required for Neural Stem Cell Maintenance and Differentiation by Limiting TBK1 Activation and P62 Aggregate Formation. Sci. Rep. 2021, 11, 23907. [Google Scholar] [CrossRef]
- Xue, X.; Ma, L.; Zhang, X.; Xu, X.; Guo, S.; Wang, Y.; Qiu, S.; Cui, J.; Guo, W.; Yu, Y.; et al. Tumour Cells Are Sensitised to Ferroptosis via RB1CC1-Mediated Transcriptional Reprogramming. Clin. Transl. Med. 2022, 12, e747. [Google Scholar] [CrossRef]
- Yang, Y.; Klionsky, D.J. A Novel Role of ATG9A and RB1CC1/FIP200 in Mediating Cell-Death Checkpoints to Repress TNF Cytotoxicity. Autophagy 2023, 19, 1617–1618. [Google Scholar] [CrossRef]
- Chen, P.; Duan, Y.; Lu, X.; Chen, L.; Zhang, W.; Wang, H.; Hu, R.; Liu, S. RB1CC1 Functions as a Tumor-Suppressing Gene in Renal Cell Carcinoma via Suppression of PYK2 Activity and Disruption of TAZ-Mediated PDL1 Transcription Activation. Cancer Immunol. Immunother. 2021, 70, 3261–3275. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, K.; Hao, W.; Wu, Y.; Patil, G.; Hua, F.; Sun, Y.; Huang, C.; Ritchey, J.; Jones, C.; et al. FIP200 Restricts RNA Virus Infection by Facilitating RIG-I Activation. Commun. Biol. 2021, 4, 921. [Google Scholar] [CrossRef]
- Okamoto, T.; Yeo, S.K.; Hao, M.; Copley, M.R.; Haas, M.A.; Chen, S.; Guan, J.-L. FIP200 Suppresses Immune Checkpoint Therapy Responses in Breast Cancers by Limiting AZI2/TBK1/IRF Signaling Independent of Its Canonical Autophagy Function. Cancer Res. 2020, 80, 3580–3592. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Wang, C.; Guan, J.-L. Role of FIP200 in Inflammatory Processes beyond Its Canonical Autophagy Function. Biochem. Soc. Trans. 2020, 48, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.M.; Dowdle, W.E.; DeJesus, R.; Wang, Z.; Bergman, P.; Kobylarz, M.; Lindeman, A.; Xavier, R.J.; McAllister, G.; Nyfeler, B.; et al. Autophagy-Independent Lysosomal Targeting Regulated by ULK1/2-FIP200 and ATG9. Cell Rep. 2017, 20, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Abbi, S.; Ueda, H.; Zheng, C.; Cooper, L.A.; Zhao, J.; Christopher, R.; Guan, J.-L. Regulation of Focal Adhesion Kinase by a Novel Protein Inhibitor FIP200. Mol. Biol. Cell 2002, 13, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Melkoumian, Z.K.; Peng, X.; Gan, B.; Wu, X.; Guan, J.-L. Mechanism of Cell Cycle Regulation by FIP200 in Human Breast Cancer Cells. Cancer Res. 2005, 65, 6676–6684. [Google Scholar] [CrossRef]
- Choi, J.D.; Ryu, M.; Ae Park, M.; Jeong, G.; Lee, J.-S. FIP200 Inhibits β-Catenin-Mediated Transcription by Promoting APC-Independent β-Catenin Ubiquitination. Oncogene 2013, 32, 2421–2432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, C.; Liang, C.-C.; Bian, Z.C.; Zhu, Y.; Guan, J.-L. FIP200 Is Required for Maintenance and Differentiation of Postnatal Neural Stem Cells. Nat. Neurosci. 2013, 16, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Chano, T.; Inoue, H.; Isono, T.; Koiwai, O.; Okabe, H. Rb1cc1 Is Critical for Myoblast Differentiation through Rb1 Regulation. Virchows Arch. 2005, 447, 643–648. [Google Scholar] [CrossRef]
- Chano, T.; Saeki, Y.; Serra, M.; Matsumoto, K.; Okabe, H. Preferential Expression of RB1-Inducible Coiled-Coil 1 in Terminal Differentiated Musculoskeletal Cells. Am. J. Pathol. 2002, 161, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gan, B.; Wu, X.; Guan, J.-L. Inactivation of FIP200 Leads to Inflammatory Skin Disorder, but Not Tumorigenesis, in Conditional Knock-out Mouse Models. J. Biol. Chem. 2009, 284, 6004–6013. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Guan, J.-L. FIP200, an Essential Component of Mammalian Autophagy Is Indispensible for Fetal Hematopoiesis. Autophagy 2011, 7, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wei, S.; Gan, B.; Peng, X.; Zou, W.; Guan, J.-L. Suppression of Autophagy by FIP200 Deletion Inhibits Mammary Tumorigenesis. Genes Dev. 2011, 25, 1510–1527. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Guan, J.-L. Suppression of Autophagy by FIP200 Deletion Impairs DNA Damage Repair and Increases Cell Death upon Treatments with Anticancer Agents. Mol. Cancer Res. 2011, 9, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Molusky, M.M.; Song, J.; Hu, C.-R.; Fang, F.; Rui, C.; Mathew, A.V.; Pennathur, S.; Liu, F.; Cheng, J.-X.; et al. Autophagy Deficiency by Hepatic FIP200 Deletion Uncouples Steatosis from Liver Injury in NAFLD. Mol. Endocrinol. 2013, 27, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fang, F.; Yuan, H.; Yang, D.; Chen, Y.; Williams, L.; Goldstein, S.A.; Krebsbach, P.H.; Guan, J.-L. Suppression of Autophagy by FIP200 Deletion Leads to Osteopenia in Mice through the Inhibition of Osteoblast Terminal Differentiation. J. Bone Miner. Res. 2013, 28, 2414–2430. [Google Scholar] [CrossRef]
- Li, Y.; Gan, C.; Zhang, S.; Zhou, X.; Li, X.; Wei, Y.; Yang, J.; Wu, M. FIP200 Is Involved in Murine Pseudomonas Infection by Regulating HMGB1 Intracellular Translocation. Cell Physiol. Biochem. 2014, 33, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Iyengar, R.; Li-Harms, X.; Joo, J.H.; Wright, C.; Lavado, A.; Horner, L.; Yang, M.; Guan, J.-L.; Frase, S.; et al. The Autophagy-Inducing Kinases, ULK1 and ULK2, Regulate Axon Guidance in the Developing Mouse Forebrain via a Noncanonical Pathway. Autophagy 2018, 14, 796–811. [Google Scholar] [CrossRef]
- Oestreich, A.K.; Chadchan, S.B.; Medvedeva, A.; Lydon, J.P.; Jungheim, E.S.; Moley, K.H.; Kommagani, R. The Autophagy Protein, FIP200 (RB1CC1) Mediates Progesterone Responses Governing Uterine Receptivity and Decidualization. Biol. Reprod. 2020, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Vogel, P.; Li-Harms, X.; Wang, B.; Kundu, M. ATG14 and RB1CC1 Play Essential Roles in Maintaining Muscle Homeostasis. Autophagy 2021, 17, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; White, E. Autophagy in PDGFRA+ Mesenchymal Cells Is Required for Intestinal Homeostasis and Mammalian Survival. Autophagy 2023, 19, 726–728. [Google Scholar] [CrossRef]
- Yang, F.; Kalantari, S.; Ruan, B.; Sun, S.; Bian, Z.; Guan, J.-L. Autophagy Inhibition Prevents Lymphatic Malformation Progression to Lymphangiosarcoma by Decreasing Osteopontin and Stat3 Signaling. Nat. Commun. 2023, 14, 978. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Hu, J.-S.; Zhang, G.-Q.; Chen, H.-Z.; Yuan, Y.; Li, Y.-L.; Lv, X.-J.; Tian, F.-Y.; Pan, S.-H.; et al. RB1CC1-Enhanced Autophagy Facilitates PSCs Activation and Pancreatic Fibrogenesis in Chronic Pancreatitis. Cell Death Dis. 2018, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bodnar, D.; Chen, C.-Y.; Sanchez-Andrade, G.; Sanderson, M.; Shi, J.; Meilleur, K.G.; Hurles, M.E.; Gerety, S.S.; Tsai, E.A.; et al. Rare Genetic Variants Impact Muscle Strength. Nat. Commun. 2023, 14, 3449. [Google Scholar] [CrossRef] [PubMed]
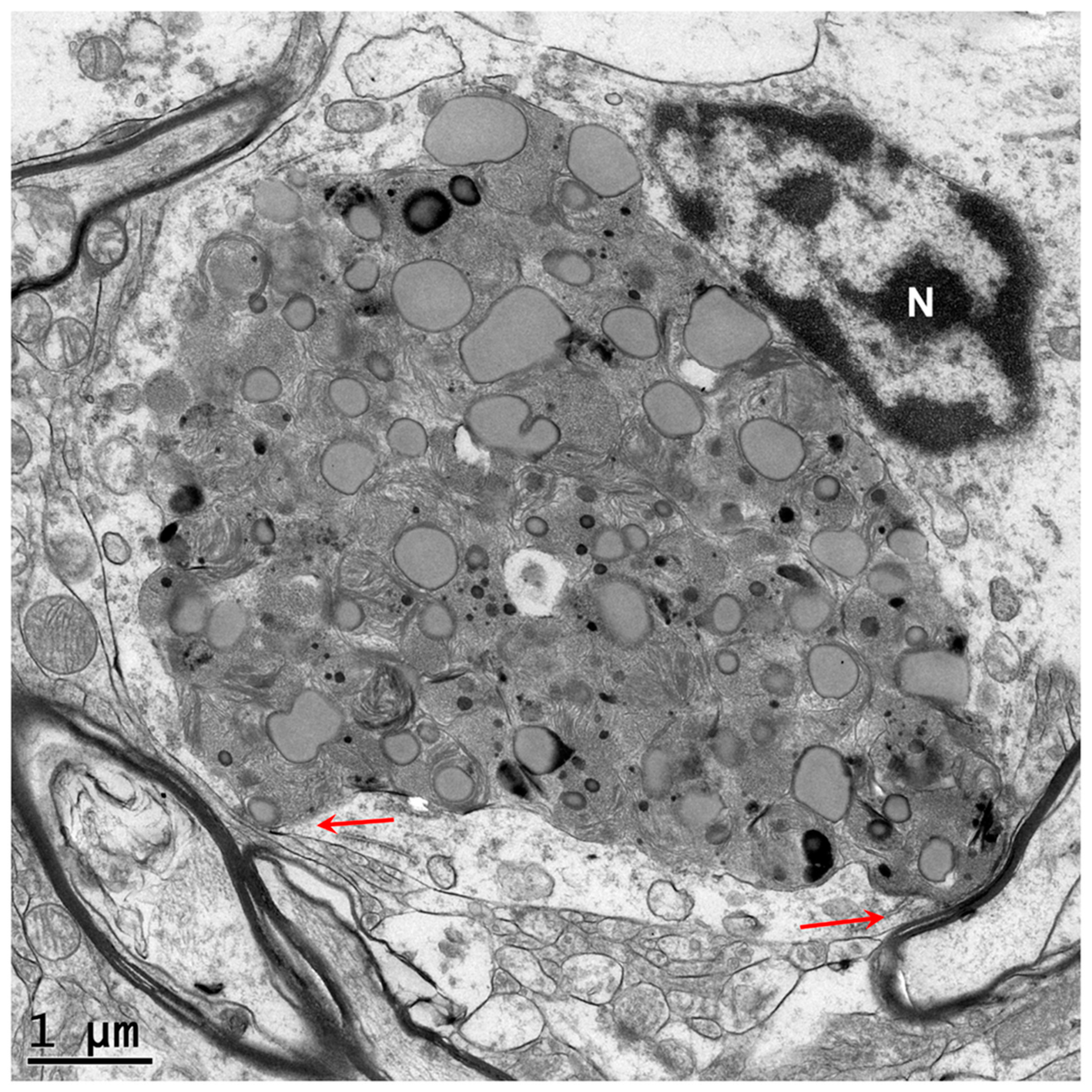
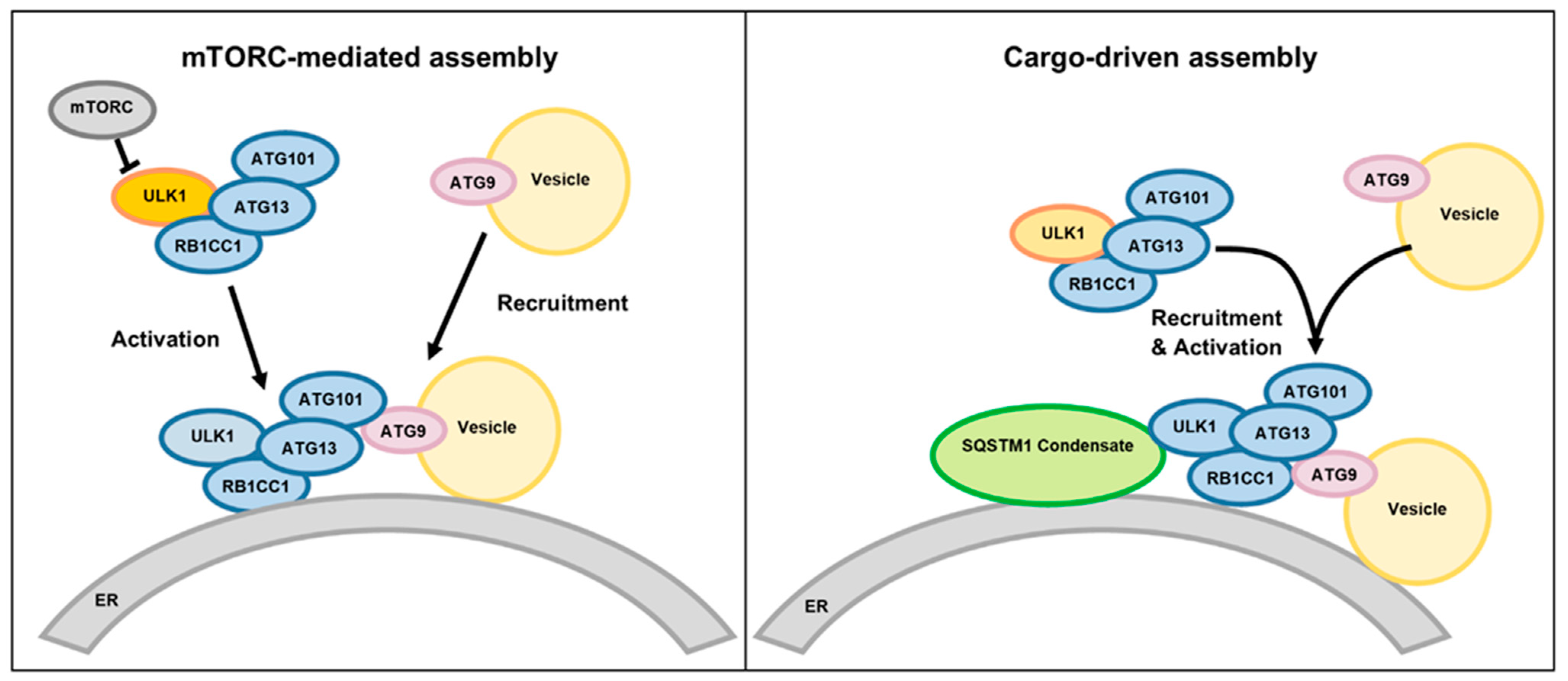
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Bullock, G.; O’Brien, D.P.; Johnson, G.S.; Katz, M.L. An RB1CC1 Missense Variant in Nova Scotia Duck Tolling Retrievers with Degenerative Encephalopathy. Genes 2025, 16, 269. https://doi.org/10.3390/genes16030269
Guo J, Bullock G, O’Brien DP, Johnson GS, Katz ML. An RB1CC1 Missense Variant in Nova Scotia Duck Tolling Retrievers with Degenerative Encephalopathy. Genes. 2025; 16(3):269. https://doi.org/10.3390/genes16030269
Chicago/Turabian StyleGuo, Juyuan, Garrett Bullock, Dennis P. O’Brien, Gary S. Johnson, and Martin L. Katz. 2025. "An RB1CC1 Missense Variant in Nova Scotia Duck Tolling Retrievers with Degenerative Encephalopathy" Genes 16, no. 3: 269. https://doi.org/10.3390/genes16030269
APA StyleGuo, J., Bullock, G., O’Brien, D. P., Johnson, G. S., & Katz, M. L. (2025). An RB1CC1 Missense Variant in Nova Scotia Duck Tolling Retrievers with Degenerative Encephalopathy. Genes, 16(3), 269. https://doi.org/10.3390/genes16030269





