Genome-Wide Identification, Gene Duplication, and Expression Pattern of NPC2 Gene Family in Parnassius glacialis
Abstract
1. Introduction
2. Materials and Methods
2.1. The Identification of the NPC2 Gene Family
2.2. Analysis of Physicochemical Properties, Secondary Structure, and Subcellular Localization
2.3. Analysis of Multiple Sequence Alignment and Phylogenetic
2.4. An Analysis of the Conserved Motifs, Gene Structures, and Characteristic Domains
2.5. An Analysis of the Chromosome Distribution, Collinearity, and Gene Duplication
2.6. An Analysis of the Cis-Regulatory Elements
2.7. Genome Annotation and Kyoto Encyclopedia of Genes and Genome (KEGG) Pathway Enrichment
2.8. RNA Extraction and Quantitative RT-PCR
3. Results
3.1. Genome-Wide Identification and Physicochemical Properties of NPC2 Genes
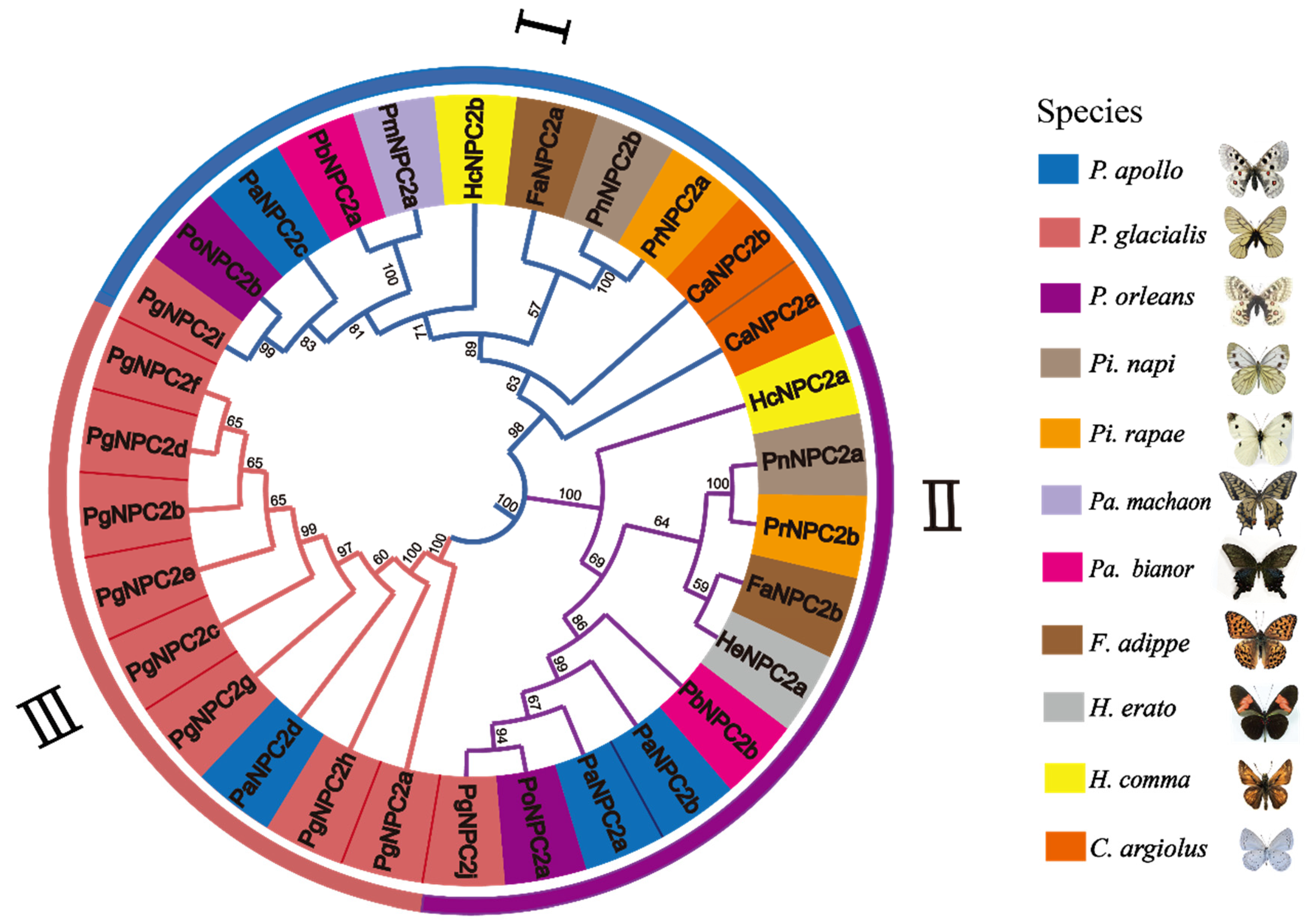
3.2. A Phylogenetic Analysis of the NPC2 Gene Family Members in Representative Butterfly Genomes
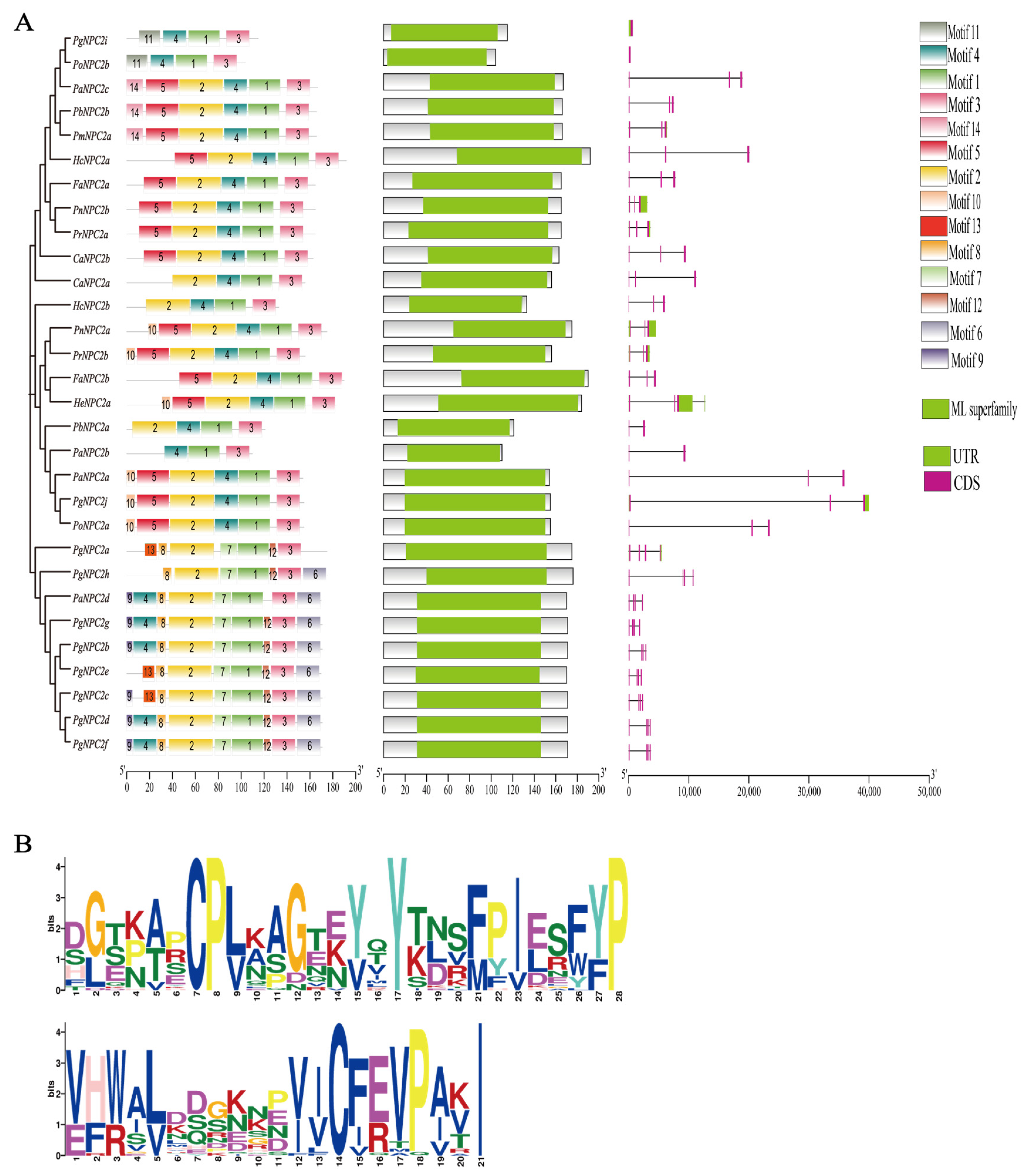
3.3. Conserved Motifs, Characteristic Domains, and Gene Structures of NPC2 Genes
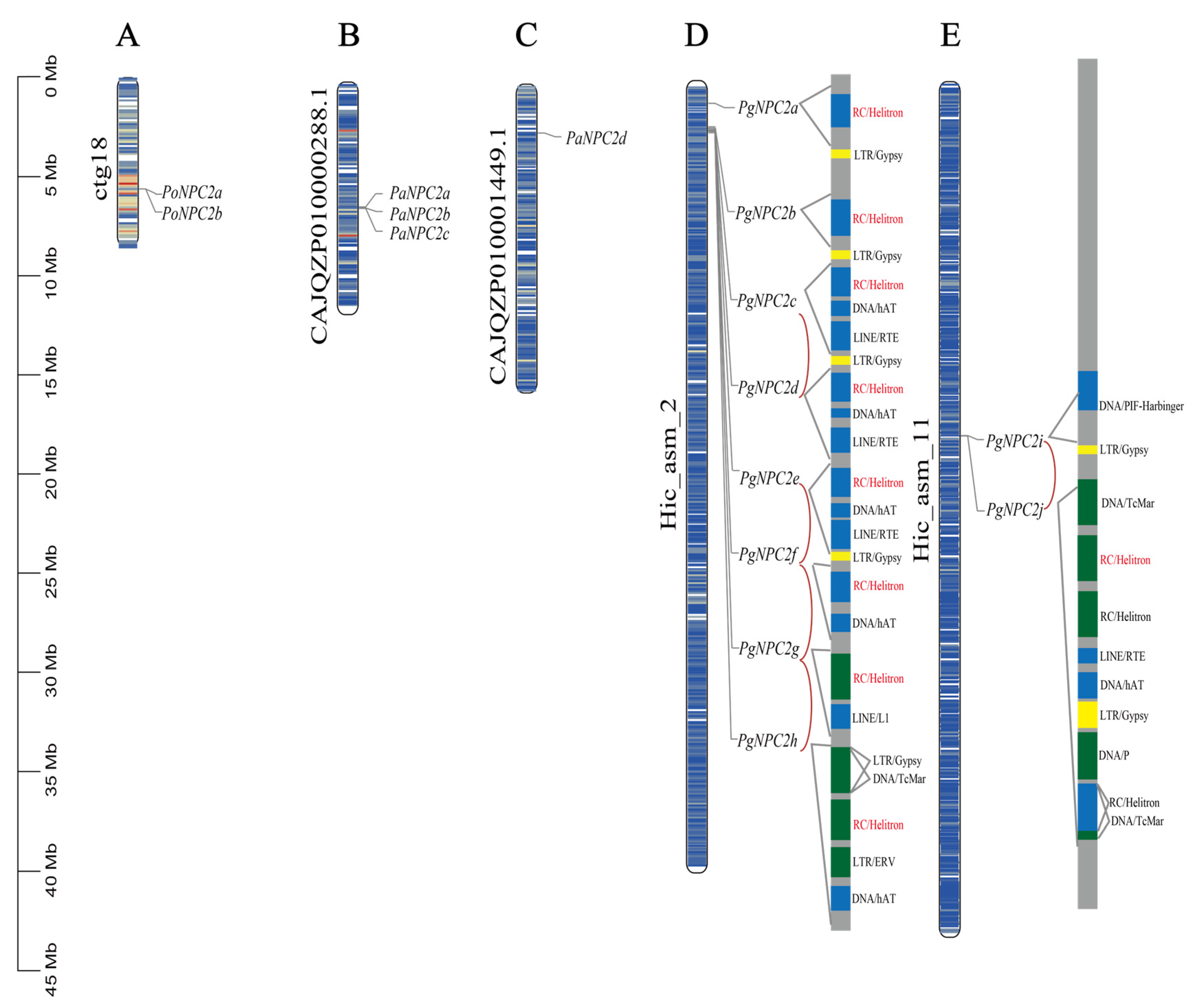
3.4. Chromosomal Location, Gene Duplication, and Collinearity Analysis
3.5. Analysis of Cis-Regulatory Elements of NPC2 Genes
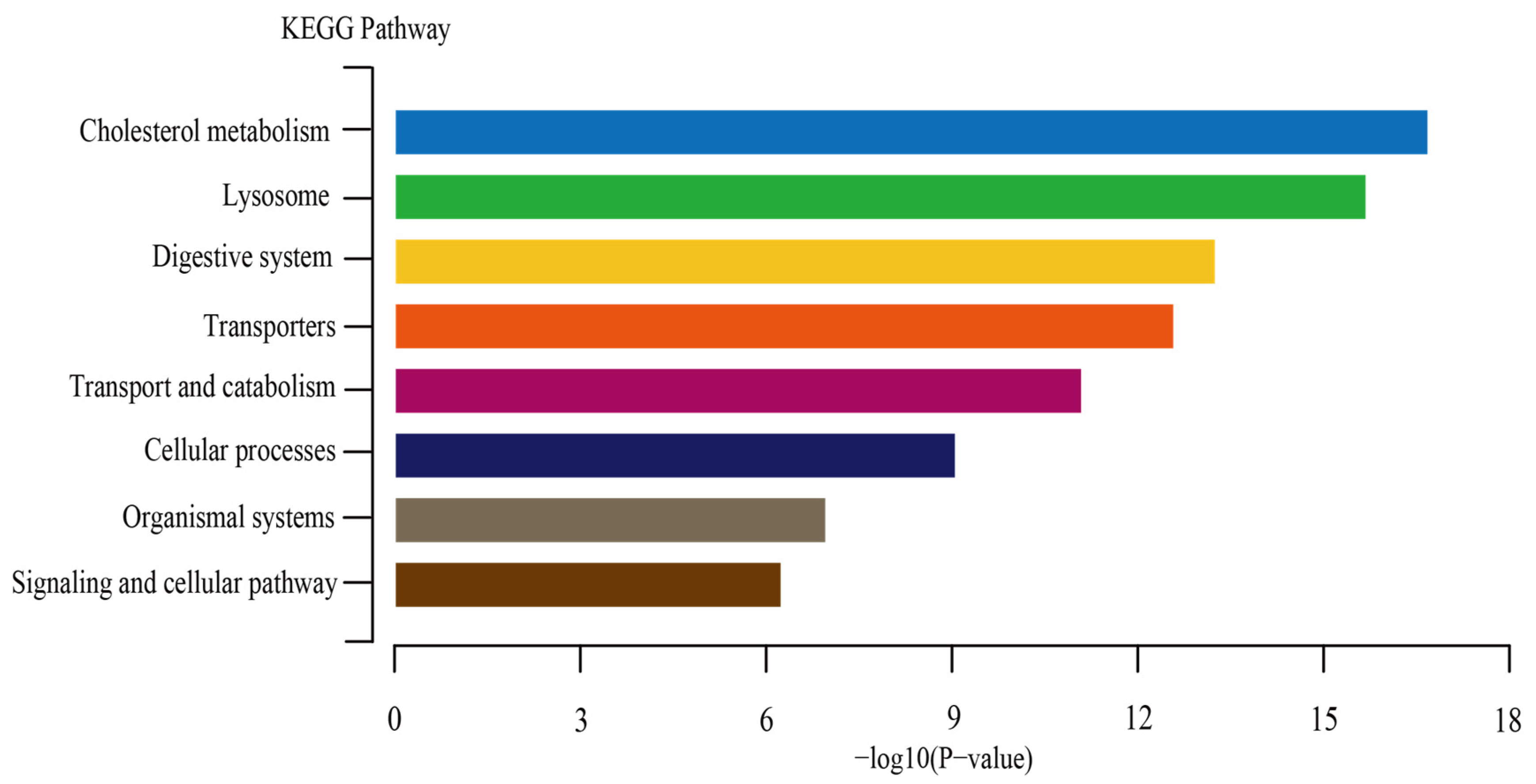
3.6. The KEGG Enrichment Analysis for PgNPC2 Genes of P. glacialis
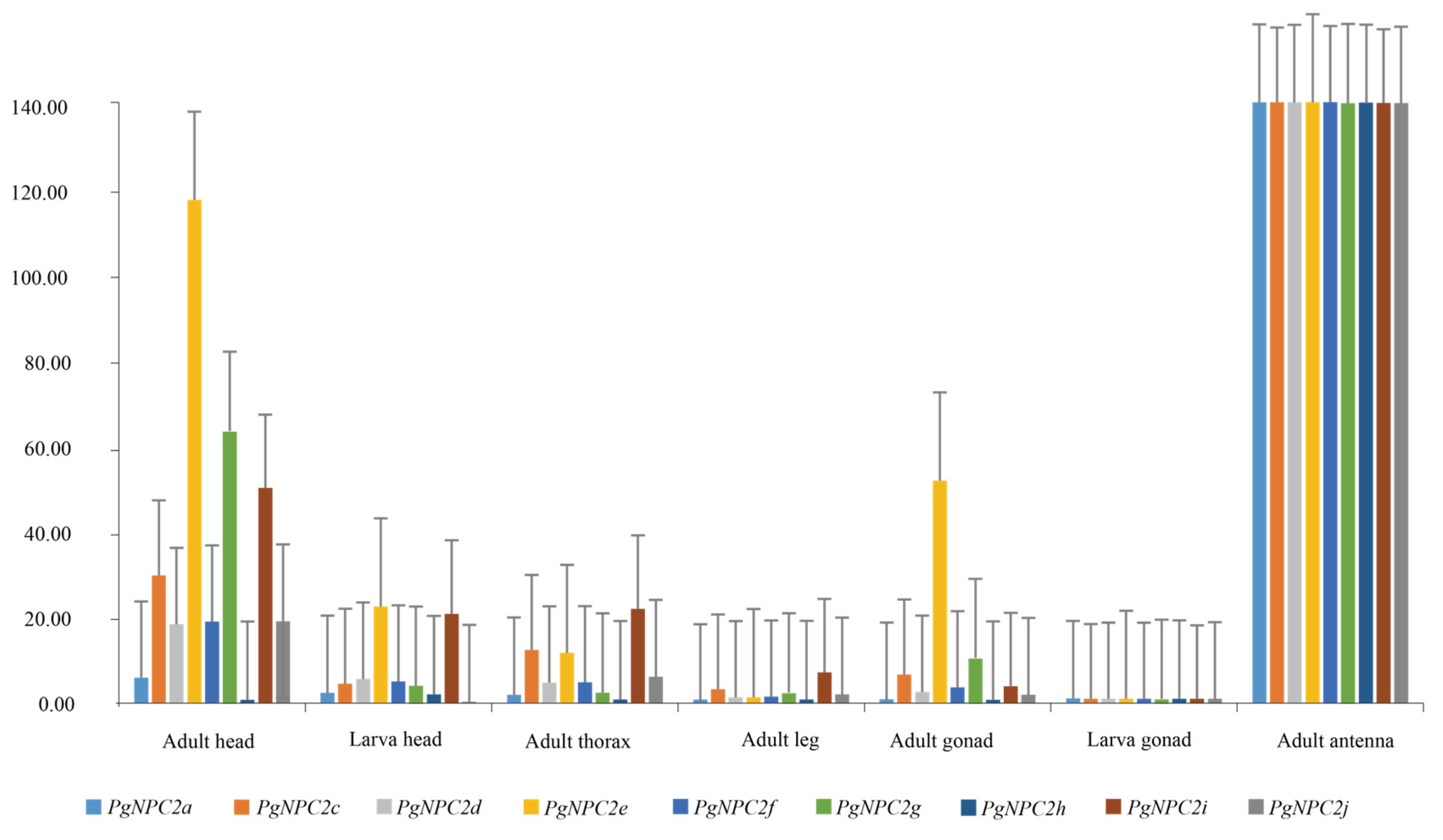
3.7. The Variance of NPC2 Gene Expression Levels in Different Tissues of P. glacialis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Dong, W.J.; Fu, T.T.; Gao, W.; Lu, C.Q.; Yan, F.; Wu, Y.H.; Jiang, K.; Jin, J.Q.; Chen, H.M.; et al. Herpetological phylogeographic analyses support a Miocene focal point of Himalayan uplift and biological diversification. Natl. Sci. Rev. 2021, 8, nwaa263. [Google Scholar] [CrossRef] [PubMed]
- Condamine, F.L.; Rolland, J.; Höhna, S.; Sperling, F.A.H.; Sanmartín, I. Testing the role of the Red Queen and Court Jester as drivers of the macroevolution of Apollo butterflies. Natl. Sci. Rev. 2018, 67, 940–964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.N.; Ren, Y.; Zhang, J.Q. Conservation and innovation: Plastome evolution during rapid radiation of Rhodiola on the Qinghai-Tibetan Plateau. Natl. Sci. Rev. 2020, 144, 106713. [Google Scholar] [CrossRef]
- Su, C.Y.; Ding, C.; Zhao, Y.J.; He, B.; Nie, R.E.; Hao, J.S. Diapause-Linked gene expression pattern and related candidate duplicated genes of the mountain butterfly Parnassius glacialis (Lepidoptera: Papilionidae) revealed by comprehensive transcriptome profiling. Int. J. Mol. Sci. 2023, 24, 5577. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Y.J.; Su, C.Y.; Lin, G.H.; Wang, Y.L.; Li, L.; Ma, J.Y.; Yang, Q.; Hao, J.S. Phylogenomics reveal extensive phylogenetic discordance due to incomplete lineage sorting following the rapid radiation of alpine butterflies (Papilionidae: Parnassius). Syst. Entomol. 2023, 48, 585–599. [Google Scholar] [CrossRef]
- Galetti, M.; Moleon, M.; Jordano, P.; Pires, M.M.; Guimaraes, P.R., Jr.; Pape, T.; Nichols, E.; Hansen, D.; Olesen, J.M.; Munk, M.; et al. Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 2018, 93, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.S.; Xu, C.; Wang, Y.L.; Sun, X.Y.; Li, C.X.; Ma, J.Y.; Hao, J.S.; Yang, Q. Spatiotemporal differentiation of alpine butterfly Parnassius glacialis (Papilionidae: Parnassiinae) in China: Evidence from mitochondrial DNA and nuclear single nucleotide polymorphisms. Genes 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Su, C.Y.; He, B.; Nie, R.E.; Wang, Y.; Ma, J.Y.; Song, J.Y.; Yang, Q.; Hao, J.S. Dispersal from the Qinghai-Tibet plateau by a high-altitude butterfly is associated with rapid expansion and reorganization of its genome. Nat. Commun. 2023, 14, 8190. [Google Scholar] [CrossRef]
- Ding, C.; Su, C.Y.; Li, Y.L.; Zhao, Y.J.; Wang, Y.L.; Wang, Y.; Nie, R.E.; He, B.; Ma, J.Y.; Hao, J.S. Interspecific and intraspecific transcriptomic variations unveil the potential high-altitude adaptation mechanisms of the Parnassius butterfly species. Genes 2024, 15, 1013. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef] [PubMed]
- Storch, J.; Xu, Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim. Biophys. Acta 2009, 1791, 671–678. [Google Scholar] [CrossRef]
- Qian, H.W.; Wu, X.L.; Du, X.M.; Yao, X.; Zhao, X.; Lee, J.C.; Yang, H.Y.; Yan, N. Structural basis of Low-pH-Dependent lysosomal cholesterol egress by NPC1 and NPC2. Cell 2020, 182, 98–111.e18. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Tan, L.; Xie, X.; Zhao, Y. The characteristics and biological significance of NPC2: Mutation and disease. Mutat. Res. Rev. Mutat. Res. 2019, 782, 108284. [Google Scholar] [CrossRef]
- Sleat, D.E.; Wiseman, J.A.; El-Banna, M.; Price, S.M.; Verot, L.; Shen, M.M.; Tint, G.S.; Vanier, M.T.; Walkley, S.U.; Lobel, P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA 2004, 101, 5886–5891. [Google Scholar] [CrossRef]
- Zhang, L.Z.N.; Jiang, H.; Wu, F.; Li, H. Molecular cloning and expression pattern analysis of NPC2 gene family of Apis cerana cerana. Sci. Agric. Sin. 2022, 55, 2461–2471. [Google Scholar]
- Huang, X.; Warren, J.T.; Buchanan, J.; Gilbert, L.I.; Scott, M.P. Drosophila Niemann-Pick type C-2 genes control sterol homeostasis and steroid biosynthesis: A model of human neurodegenerative disease. Development 2007, 134, 3733–3742. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Tsuchiya, W.; Fujii, T.; Fujimoto, Z.; Miyazawa, M.; Ishibashi, J.; Matsuyama, S.; Ishikawa, Y.; Yamazaki, T. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc. Natl. Acad. Sci. USA 2014, 111, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Zhong, X.; Yu, X.Q. Drosophila melanogaster NPC2 proteins bind bacterial cell wall components and may function in immune signal pathways. Insect Biochem. Mol. Biol. 2012, 42, 545–556. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.N.; Peng, Y.; Lu, Z.Y.; Shan, S.; Yang, Y.Q.; Li, R.J.; Zhang, Y.J.; Guo, Y.Y. Functional characterization of a Niemann-Pick type C2 protein in the parasitoid wasp Microplitis mediator. Insect. Sci. 2018, 25, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlowski, L.; Tunstrom, K.; Espeland, M.; Wheat, C.W. The genome assembly and annotation of the Apollo butterfly Parnassius apollo, a flagship species for conservation biology. Genome Biol. Evol. 2021, 13, evab122. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Zhang, R.; Yang, J.; Chang, Z.; Zhu, L.X.; Lu, S.H.; Xie, F.A.; Mao, J.L.; Dong, Z.W.; Liu, G.C.; et al. High-quality reference genomes of swallowtail butterflies provide insights into their coloration evolution. Zool. Res. 2022, 43, 367–379. [Google Scholar] [CrossRef]
- Lu, S.; Yang, J.; Dai, X.; Xie, F.; He, J.; Dong, Z.; Mao, J.; Liu, G.; Chang, Z.; Zhao, R.; et al. Chromosomal-level reference genome of Chinese peacock butterfly (Papilio bianor) based on third-generation DNA sequencing and Hi-C analysis. GigaScience 2019, 8, giz128. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.J.; van der Burg, K.R.L.; Mazo-Vargas, A.; Reed, R.D. ChIP-Seq-Annotated Heliconius erato genome highlights patterns of cis-regulatory evolution in Lepidoptera. Cell Rep. 2016, 16, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. TrimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.S.; He, F.M.; Zhang, X.Q.; Wang, J.Q.; Zhang, Z.L.; Jiang, H.R.; Zhao, B.A.; Du, C.; Che, Y.Z.; Feng, X.; et al. Genome-wide identification and expression profiling of Potato (Solanum tuberosum L.) universal stress proteins reveal essential roles in mechanical damage and deoxynivalenol stress. Int. J. Mol. Sci. 2024, 25, 1341. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.M.; Tian, S.C.; Huang, S.L.; Wei, G.B.; Han, D.M.; Li, J.G.; Guo, D.L.; Zhou, Y. Genome-wide identification of the longan R2R3-MYB gene family and its role in primary and lateral root. BMC Plant Biol. 2023, 23, 448. [Google Scholar] [CrossRef]
- Hu, K.; Ni, P.; Xu, M.H.; Zou, Y.; Chang, J.Y.; Gao, X.; Li, Y.H.; Ruan, J.; Hu, B.; Wang, J.X. HiTE: A fast and accurate dynamic boundary adjustment approach for full-length transposable element detection and annotation. Nat. Commun. 2024, 15, 5573. [Google Scholar] [CrossRef]
- Ginestet, C. ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2011, 174, 245–246. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Burge, C.; Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 1997, 268, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.; Blanco, E.; Guigó, R. GeneID in Drosophila. Genome Res. 2000, 10, 511–515. [Google Scholar] [CrossRef]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef]
- Majoros, W.H.; Pertea, M.; Salzberg, S.L. TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene-finders. Bioinformatics 2004, 20, 2878–2879. [Google Scholar] [CrossRef] [PubMed]
- Stanke, M.; Keller, O.; Gunduz, I.; Hayes, A.; Waack, S.; Morgenstern, B. AUGUSTUS: A web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005, 33, W465–W467. [Google Scholar] [CrossRef]
- Keilwagen, J.; Hartung, F.; Grau, J. GeMoMa: Homology-based gene prediction utilizing intron position conservation and RNA-seq data. Methods Mol. Biol. 2019, 1962, 161–177. [Google Scholar]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Ishiguro-Watanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar]
- Zhang, Y.H.; Chu, C.; Wang, S.; Chen, L.; Lu, J.; Kong, X.; Huang, T.; Li, H.; Cai, Y.D. The use of Gene Ontology term and KEGG pathway enrichment for analysis of drug half-life. PLoS ONE 2016, 11, e0165496. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Oudelaar, A.M.; Higgs, D.R. The relationship between genome structure and function. Nat. Rev. Genet. 2021, 22, 154–168. [Google Scholar] [CrossRef]
- Fan, K.; Shen, H.; Bibi, N.; Li, F.; Yuan, S.; Wang, M.; Wang, X. Molecular evolution and species-specific expansion of the NAP members in plants. J. Integr. Plant. Biol. 2015, 57, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Thornton, K. Gene duplication and evolution. Science 2001, 293, 1551. [Google Scholar] [CrossRef] [PubMed]
- Demuth, J.P.; De Bie, T.; Stajich, J.E.; Cristianini, N.; Hahn, M.W. The evolution of mammalian gene families. PLoS ONE 2006, 1, e85. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Canestro, C.; Albalat, R.; Irimia, M.; Garcia-Fernandez, J. Impact of gene gains, losses and duplication modes on the origin and diversification of vertebrates. Cell Dev. Biol. 2013, 24, 83–94. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ma, H.; Wang, J.; Wang, M.; Wang, M.; Yin, H.; Zhang, Y.; Zhang, X.; Shen, J.; Wang, D.; et al. DNA transposons mediate duplications via transposition-independent and dependent mechanisms in metazoans. Nat. Commun. 2021, 12, 4280. [Google Scholar] [CrossRef]
- Yu, J.; Tehrim, S.; Wang, L.; Dossa, K.; Zhang, X.; Ke, T.; Liao, B. Evolutionary history and functional divergence of the cytochrome P450 gene superfamily between Arabidopsis thaliana and Brassica species uncover effects of whole genome and tandem duplications. BMC Genom. 2017, 18, 733. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lenkov, K.; Lipatov, M.; Macpherson, J.M.; Petrov, D.A. High rate of recent transposable element-induced adaptation in Drosophila melanogaster. PLoS Biol. 2008, 6, e251. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.R.; Greene, W.K. Transposable elements: Powerful facilitators of evolution. Bioessays 2009, 31, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Brunner, S.; Pea, G.; Fengler, K.; Zuccolo, A.; Rafalski, A. Gene duplication and exon shuffling by helitron-like transposons generate intraspecies diversity in maize. Nat. Genet. 2005, 37, 997–1002. [Google Scholar] [CrossRef]
- Grabundzija, I.; Messing, S.A.; Thomas, J.; Cosby, R.L.; Bilic, I.; Miskey, C.; Gogol-Doring, A.; Kapitonov, V.; Diem, T.; Dalda, A.; et al. A Helitron transposon reconstructed from bats reveals a novel mechanism of genome shuffling in eukaryotes. Nat. Commun. 2016, 7, 10716. [Google Scholar] [CrossRef]
- Casola, C.; Betran, E. The genomic impact of gene retrocopies: What have we learned from comparative genomics, population genomics, and transcriptomic analyses? Genome Biol. Evol. 2017, 9, 1351–1373. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.S. Dormancy, diapause, and the role of the circadian system insect photoperiodism. Annu. Rev. Entomol. 2020, 65, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Pandey, S.P.; Singh, P.; Pradhan, L.; Pande, V.; Sane, A.P. Early wound-responsive cues regulate the expression of WRKY family genes in chickpea differently under wounded and unwounded conditions. Physiol. Mol. Biol. Plants 2022, 28, 719–735. [Google Scholar] [CrossRef]


| Species | Gene Name | Gene ID | Amino Acid Number/aa | Molecular Weight /Da | Theoretical Isoelectric | Instability | Stability | Lipolysis Indices | Total Mean Hydrophilic Value |
|---|---|---|---|---|---|---|---|---|---|
| P. apollo | PaNPC2a | Papo004992.1 | 154 | 17,122.9 | 5.73 | 50.96 | unstable | 99.29 | −0.023 |
| PaNPC2b | Papo004994.1 | 110 | 12,421.3 | 5.38 | 53.96 | unstable | 87.64 | −0.093 | |
| PaNPC2c | Papo004995.1 | 167 | 18,299.3 | 8.63 | 38.97 | stable | 93.89 | 0.116 | |
| PaNPC2d | Papo023738.1 | 170 | 18,628.9 | 8.97 | 37.31 | stable | 98.12 | 0.094 | |
| P. glacialis | PgNPC2a | evm.model.CTG_193.50 | 174 | 19,187.2 | 4.6 | 49.94 | unstable | 98.45 | 0.326 |
| PgNPC2b | evm.model.CTG_193.68 | 170 | 18,719.6 | 8.28 | 40.11 | unstable | 92.29 | 0.039 | |
| PgNPC2c | evm.model.CTG_193.70 | 170 | 18,671.6 | 8.63 | 40.36 | unstable | 94.59 | 0.066 | |
| PgNPC2d | evm.model.CTG_193.71 | 170 | 18,699.6 | 8.28 | 39.11 | stable | 94.59 | 0.05 | |
| PgNPC2e | evm.model.CTG_54.223 | 169 | 18,632.5 | 8.28 | 42.1 | unstable | 92.84 | 0.027 | |
| PgNPC2f | evm.model.CTG_54.222 | 170 | 18,699.6 | 8.28 | 39.11 | stable | 94.59 | 0.05 | |
| PgNPC2g | evm.model.CTG_54.221 | 170 | 18,607.4 | 8.27 | 48.95 | unstable | 86 | −0.002 | |
| PgNPC2h | evm.model.CTG_54.220 | 175 | 19,299.3 | 9.02 | 41.31 | unstable | 89.09 | −0.11 | |
| PgNPC2i | evm.model.CTG_162.121 | 114 | 12,533.5 | 5.17 | 58.35 | unstable | 105.96 | 0.32 | |
| PgNPC2j | evm.model.CTG_162.120 | 154 | 16,928.6 | 5.63 | 51.2 | unstable | 94.87 | −0.008 | |
| P. orleans | PoNPC2a | evm.model.ctg18.150 | 154 | 16,968.6 | 6.07 | 39.09 | stable | 92.34 | −0.103 |
| PoNPC2b | evm.model.ctg18.151 | 103 | 11,132.8 | 5.45 | 56.63 | unstable | 97.38 | 0.256 | |
| Pi. napi | PnNPC2a | Pnap002095.1 | 175 | 19,309.5 | 8.03 | 42.62 | unstable | 86.17 | −0.065 |
| PnNPC2b | Pnap002017.1 | 165 | 18,105 | 8.39 | 37.3 | stable | 81.52 | −0.108 | |
| Pi. rapae | PrNPC2a | Prap002064.1 | 165 | 18,129 | 7.5 | 42.05 | unstable | 86.24 | −0.127 |
| PrNPC2b | Prap002333.1 | 156 | 17,008 | 8.07 | 27.47 | stable | 99.23 | 0.053 | |
| Pa. machaon | PmNPC2a | Pmac012693.1 | 166 | 18,221.1 | 6.72 | 33.02 | stable | 94.4 | 0.051 |
| Pa. bianor | PbNPC2a | Pbia004755.1 | 121 | 13,646.9 | 8.58 | 45.37 | unstable | 82.07 | −0.086 |
| PbNPC2b | Pbia004754.1 | 166 | 18,211 | 6.71 | 33.5 | stable | 91.51 | 0.017 | |
| F. adippe | FaNPC2a | Fadi023545.1 | 165 | 18,367.3 | 8.21 | 35.33 | stable | 92.67 | −0.096 |
| FaNPC2b | Fadi023544.1 | 190 | 21,707.5 | 8.9 | 50.38 | unstable | 89.11 | −0.056 | |
| H. erato | HeNPC2a | Hera002910.1 | 157 | 17,543.6 | 5.26 | 38.23 | stable | 114.78 | 0.526 |
| H. comma | HcNPC2a | Hcom020357.1 | 192 | 21,082.2 | 8.8 | 38.06 | stable | 76.67 | −0.084 |
| HcNPC2b | Hcom020358.1 | 133 | 14,664 | 7.62 | 38.45 | stable | 82.03 | −0.095 | |
| C. argiolus | CaNPC2a | Carg014702.1 | 156 | 17,534.2 | 8.48 | 38.82 | stable | 85.51 | −0.28 |
| CaNPC2b | Carg004219.1 | 163 | 17,910.6 | 6.41 | 29.3 | stable | 89.02 | −0.064 |
| Gene ID | Chromosome | Position Relation | Start | End | Transposon Type |
|---|---|---|---|---|---|
| PgNPC2a | Hic_asm_2 | gene contains TE | 1335330 | 1335554 | RC/Helitron |
| PgNPC2a | Hic_asm_2 | gene close to TE | 1338311 | 1338500 | LTR/Gypsy |
| PgNPC2b | Hic_asm_2 | gene contains TE | 2517825 | 2518233 | RC/Helitron |
| PgNPC2b | Hic_asm_2 | gene contains TE | 2519529 | 2519773 | LINE/RTE |
| PgNPC2b | Hic_asm_2 | gene close to TE | 2520625 | 2520760 | LTR/Gypsy |
| PgNPC2c | Hic_asm_2 | gene contains TE | 2592201 | 2592612 | RC/Helitron |
| PgNPC2c | Hic_asm_2 | gene contains TE | 2593177 | 2593421 | DNA/hAT |
| PgNPC2c | Hic_asm_2 | gene contains TE | 2593755 | 2594059 | LINE/RTE |
| PgNPC2c | Hic_asm_2 | gene close to TE | 2594490 | 2594684 | LTR/Gypsy |
| PgNPC2d | Hic_asm_2 | gene contains TE | 2609212 | 2609717 | RC/Helitron |
| PgNPC2d | Hic_asm_2 | gene contains TE | 2610207 | 2610263 | DNA/hAT |
| PgNPC2d | Hic_asm_2 | gene contains TE | 2612034 | 2612351 | LINE/RTE |
| PgNPC2e | Hic_asm_2 | gene contains TE | 2660198 | 2660703 | RC/Helitron |
| PgNPC2e | Hic_asm_2 | gene contains TE | 2660910 | 2661154 | DNA/hAT |
| PgNPC2e | Hic_asm_2 | gene contains TE | 2661489 | 2661741 | LINE/RTE |
| PgNPC2e | Hic_asm_2 | gene close to TE | 2662201 | 2662395 | LTR/Gypsy |
| PgNPC2f | Hic_asm_2 | gene contains TE | 2701489 | 2701971 | RC/Helitron |
| PgNPC2f | Hic_asm_2 | gene contains TE | 2702201 | 2702445 | DNA/hAT |
| PgNPC2f | Hic_asm_2 | gene contains TE | 2704393 | 2704618 | LINE/RTE |
| PgNPC2g | Hic_asm_2 | gene contains TE | 2712171 | 2712725 | RC/Helitron |
| PgNPC2g | Hic_asm_2 | gene contains TE | 2712845 | 2713124 | LINE/L1 |
| PgNPC2h | Hic_asm_2 | gene contains TE | 2722118 | 2722514 | LTR/Gypsy |
| PgNPC2h | Hic_asm_2 | gene contains TE | 2723163 | 2723709 | RC/Helitron |
| PgNPC2h | Hic_asm_2 | gene contains TE | 2729218 | 2729589 | LTR/ERV |
| PgNPC2h | Hic_asm_2 | gene contains TE | 2729769 | 2730136 | DNA/hAT |
| PgNPC2i | Hic_asm_11 | gene overlaps TE | 17857018 | 17857217 | DNA/PIF-Harbinger |
| PgNPC2i | Hic_asm_11 | gene close to TE | 17858425 | 17858553 | LTR/Gypsy |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17875148 | 17878336 | LINE/RTE |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17866674 | 17867025 | DNA/TcMar |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17871065 | 17871268 | RC/Helitron |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17873433 | 17875156 | RC/Helitron |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17879907 | 17880988 | DNA/hAT |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17891108 | 17893662 | DNA/P |
| PgNPC2j | Hic_asm_11 | gene contains TE | 17894246 | 17894618 | DNA/TcMar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Su, C.; Guo, X.; Zhao, Y.; Nie, R.; He, B.; Hao, J. Genome-Wide Identification, Gene Duplication, and Expression Pattern of NPC2 Gene Family in Parnassius glacialis. Genes 2025, 16, 249. https://doi.org/10.3390/genes16030249
Zhu Z, Su C, Guo X, Zhao Y, Nie R, He B, Hao J. Genome-Wide Identification, Gene Duplication, and Expression Pattern of NPC2 Gene Family in Parnassius glacialis. Genes. 2025; 16(3):249. https://doi.org/10.3390/genes16030249
Chicago/Turabian StyleZhu, Zhenyao, Chengyong Su, Xuejie Guo, Youjie Zhao, Ruie Nie, Bo He, and Jiasheng Hao. 2025. "Genome-Wide Identification, Gene Duplication, and Expression Pattern of NPC2 Gene Family in Parnassius glacialis" Genes 16, no. 3: 249. https://doi.org/10.3390/genes16030249
APA StyleZhu, Z., Su, C., Guo, X., Zhao, Y., Nie, R., He, B., & Hao, J. (2025). Genome-Wide Identification, Gene Duplication, and Expression Pattern of NPC2 Gene Family in Parnassius glacialis. Genes, 16(3), 249. https://doi.org/10.3390/genes16030249








