Telomere Length and Mitochondrial Copy Number as Potential Biomarkers for Male Infertility in Iraqi Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
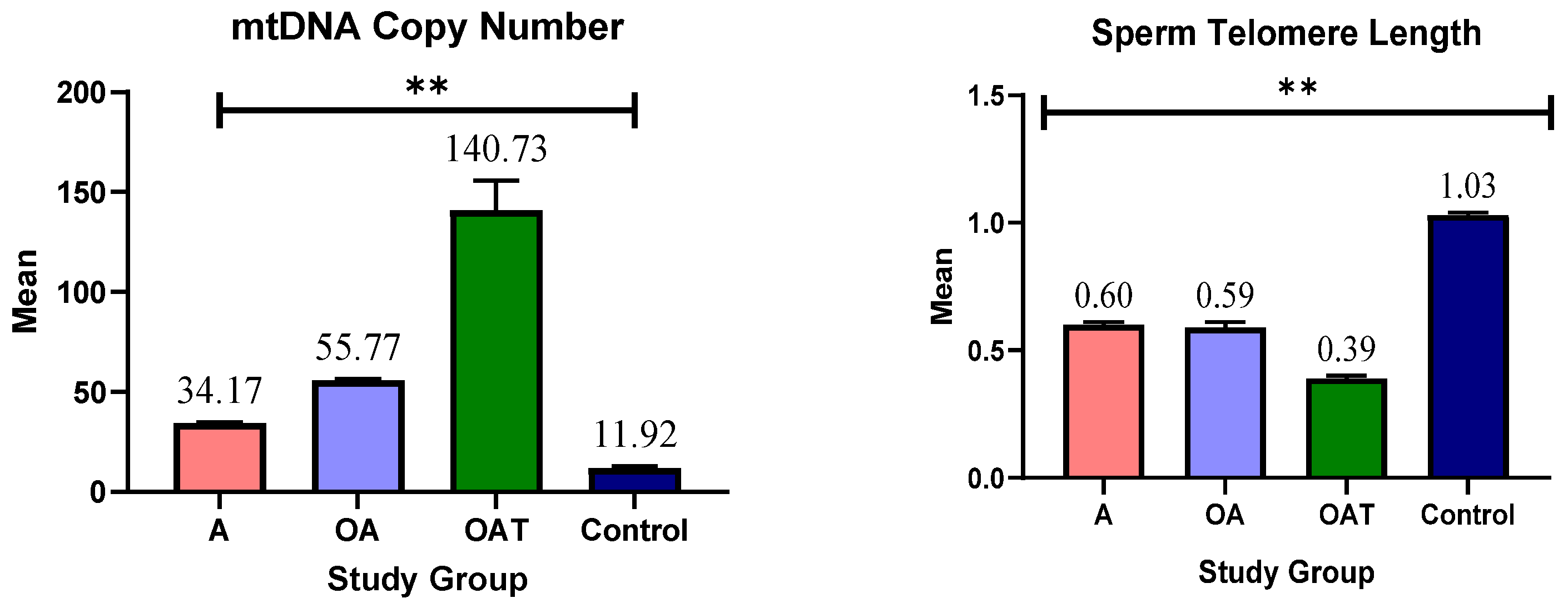
- Group 1 (A): 49 men with asthenozoospermia;
- Group 2 (OA): 44 men with oligoasthenozoospermia;
- Group 3 (OAT): 57 men with oligoasthenoteratozoospermia.
2.2. Ethical Approval
2.3. Semen Analysis
2.3.1. Macroscopic Examination
2.3.2. Microscopic Examination
2.4. Sperm DNA Extraction, Purity, and Concentration
2.5. Quantification of Sperm mtDNA Copy Number by Real-Time PCR
2.5.1. Principle
2.5.2. Procedure
2.6. Quantification of Sperm Telomere Length (STL) Expression by qPCR
2.6.1. Principles
2.6.2. Procedure and Reaction Components
2.7. Statistical Analysis
3. Results
3.1. Age of the Study Groups
3.2. Demographic Characteristics
3.3. Seminal Fluid Parameters
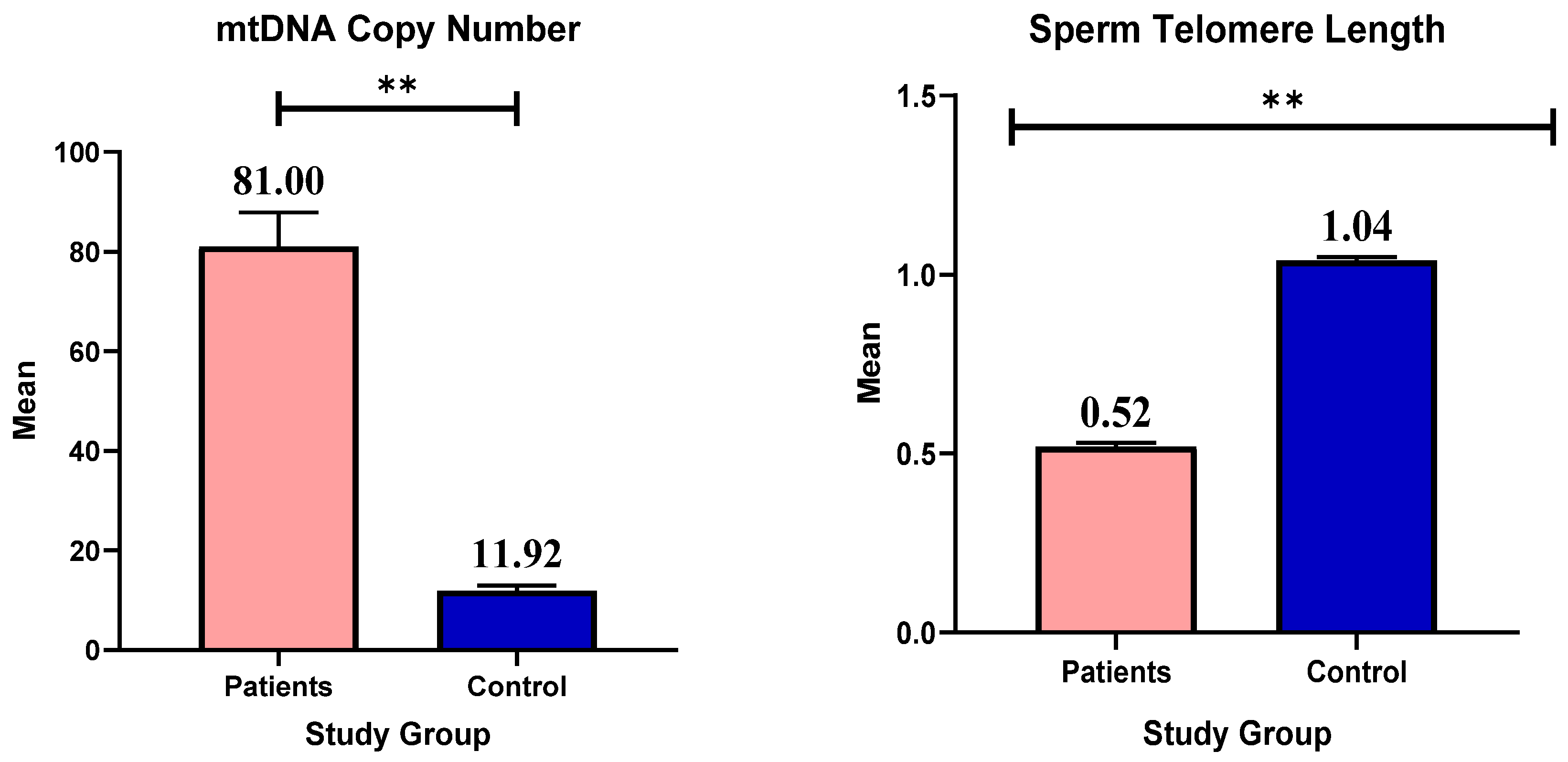
3.4. mtDNA Copy Number and Telomere Length
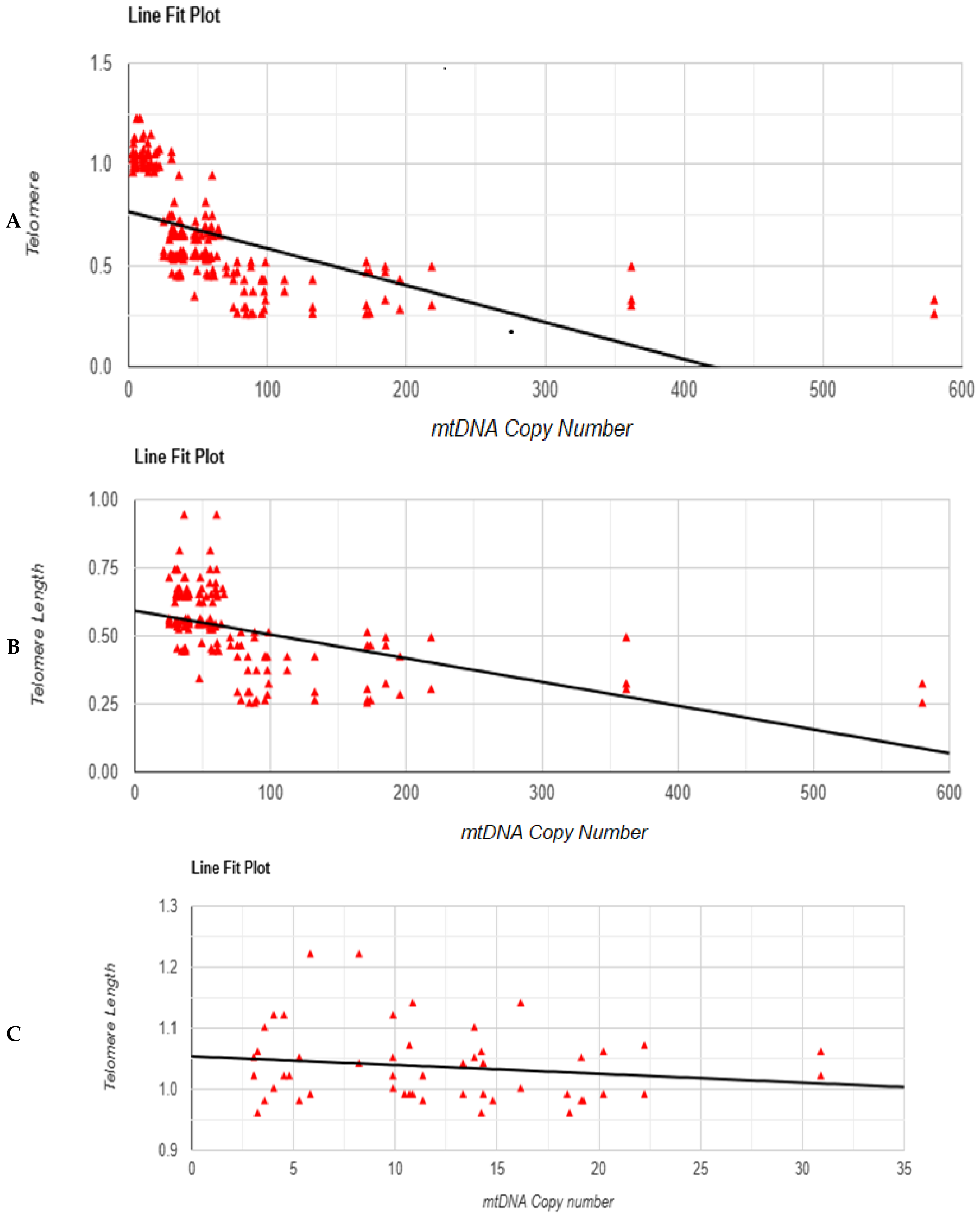
3.5. Correlation Test Between mtDNA Copy Number and Telomere Length
3.6. Linear Regression Test Between mtDNA Copy Number and Telomere Length
3.7. Receiver Operating Characteristic (ROC) Curve Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dourou, P.; Gourounti, K.; Lykeridou, A.; Gaitanou, K.; Petrogiannis, N.; Sarantaki, A. Quality of life among couples with a fertility related diagnosis. Clin. Pract. 2023, 13, 251–263. [Google Scholar] [CrossRef]
- Jaiswal, A.; Baliu-Souza, T.; Turner, K.; Nadiminty, N.; Rambhatla, A.; Agarwal, A.; Krawetz, S.A.; Dupree, J.M.; Saltzman, B.; Schon, S.B.; et al. Sperm centriole assessment identifies male factor infertility in couples with unexplained infertility—A pilot study. Eur. J. Cell Biol. 2022, 101, 151243. [Google Scholar] [CrossRef]
- Legese, N.; Tura, A.K.; Roba, K.T.; Demeke, H. The prevalence of infertility and factors associated with infertility in Ethiopia: Analysis of Ethiopian Demographic and Health Survey (EDHS). PLoS ONE 2023, 18, e0291912. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.S.; Gupta, A.S. Causes and prevalence of factors causing infertility in a public health facility. J. Hum. Reprod. Sci. 2019, 12, 287–293. [Google Scholar] [CrossRef]
- Jha, P. The hazards of smoking and the benefits of cessation: A critical summation of the epidemiological evidence in high-income countries. eLife 2020, 9, e49979. [Google Scholar] [CrossRef] [PubMed]
- Hussam, F.; AbdulhameedKhudair, S.; Alkhafaje, W.K.; Alnassar, Y.S.; Kaoud, R.M.; Najm Abed, A.; Jabbar, H.S.; Numan, H.A. A cross-sectional study regarding infertility among women in Iraq. J. Obstet. Gynecol. Cancer Res. 2022, 8, 47–52. [Google Scholar] [CrossRef]
- Mohammed, T.; Burhan, S.; Ali Hamza, N.; Kathem, A. Ratio of male to female infertility in Baghdad Al-Karkh (2015–2020). Teikyo Med. J. 2021, 44, 2197–2207. [Google Scholar]
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial function in development and diease. Dis. Model. Mech. 2021, 14, dmm048912. [Google Scholar] [CrossRef]
- Ferreira, T.; Rodriguez, S. Mitochondrial DNA: Inherent complexities relevant to genetic analyses. Genes 2024, 15, 617. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I.; et al. Sperm mitochondrial content and mitochondrial DNA to nuclear DNA ratio are associated with body mass index and progressive motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef]
- Rosati, A.J.; Whitcomb, B.W.; Brandon, N.; Louis, G.M.B.; Mumford, S.L.; Schisterman, E.F.; Pilsner, J.R. Sperm mitochondrial DNA biomarkers and couple fecundity. Hum. Reprod. 2020, 35, 2619–2625. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, J.; Wang, G. Mitochondria, telomeres and telomerase subunits. Front. Cell Dev. Biol. 2019, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Ale-Agha, N.; Jakobs, P.; Goy, C.; Zurek, M.; Rosen, J.; Dyballa-Rukes, N.; Metzger, S.; Greulich, J.; von Ameln, F.; Eckermann, O.; et al. Mitochondrial telomerase reverse transcriptase protects from myocardial ischemia/reperfusion injury by improving complex I composition and function. Circulation 2021, 144, 1876–1890. [Google Scholar] [CrossRef]
- Smith, E.M.; Pendlebury, D.F.; Nandakumar, J. Structural biology of telomeres and telomerase. Cell Mol. Life Sci. 2020, 77, 61–79. [Google Scholar] [CrossRef]
- Kohlrausch, F.B.; Wang, F.; Chamani, I.; Keefe, D.L. Telomere shortening and fusions: A link to aneuploidy in early human embryo development. Obstet. Gynecol. Surv. 2021, 76, 429–436. [Google Scholar] [CrossRef]
- Thilagavathi, J.; Kumar, M.; Mishra, S.S.; Venkatesh, S.; Kumar, R.; Dada, R. Analysis of sperm telomere length in men with idiopathic infertility. Arch. Gynecol. Obstet. 2013, 287, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Berneau, S.C.; Shackleton, J.; Nevin, C.; Altakroni, B.; Papadopoulos, G.; Horne, G.; Brison, D.R.; Murgatroyd, C.; Povey, A.C.; Carroll, M. Associations of sperm telomere length with semen parameters, clinical outcomes and lifestyle factors in human normozoospermic samples. Andrology 2020, 8, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Kurjanowicz, P.; Moskovtsev, S.; Librach, C. Genomic fragmentation and extrachromosomal telomeric repeats impact assessment of telomere length in human spermatozoa: Quantitative experiments and systematic review. Hum. Reprod. 2017, 32, 2170–2177. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Alwash, Z.F.; Hassain, A.S.; Mohammed, M.Q.; Al-Saedi, M.K.A.; Al-Tameemi, A.K.A. The role of CD279 and CD274 gene polymorphisms in Iraqi patients with multiple sclerosis using real-time qPCR HRM technique. Res. J. Biotechnol. 2024, 19, 237–243. [Google Scholar] [CrossRef]
- Katz-Wise, S.L.; Priess, H.A.; Hyde, J.S. Gender-role attitudes and behavior across the transition to parenthood. Dev. Psychol. 2010, 46, 18–28. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Maggo, S.; North, L.Y.; Ozuna, A.; Ostrow, D.; Grajeda, Y.R.; Hakimjavadi, H.; Cotter, J.A.; Judkins, A.R.; Levitt, P.; Gai, X. A method for measuring mitochondrial DNA copy number in pediatric populations. Front. Pediatr. 2024, 12, 1401737. [Google Scholar] [CrossRef] [PubMed]
- Alshattawi, S.; Taleb, R.M.; Shanyoor, G.J.; Al-Saedi, M.K.A.; Mohammed, M.Q. Linking inflammation to gene expression: Insights from GPR65, NUAK2, and OPG in ankylosing spondylitis. Gene Rep. 2025, 41, 102315. [Google Scholar] [CrossRef]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere length measurement by qPCR—Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 2019, 99, 271–278. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Riedel, G.; Rüdrich, U.; Fekete-Drimusz, N.; Manns, M.P.; Vondran, F.W.R.; Bock, M. An extended ΔCT-method facilitating normalisation with multiple reference genes suited for quantitative RT-PCR analyses of human hepatocyte-like cells. PLoS ONE 2014, 9, e93031. [Google Scholar] [CrossRef]
- Shi, W.H.; Ye, M.J.; Qin, N.X.; Zhou, Z.Y.; Zhou, X.Y.; Xu, N.X.; Chen, S.C.; Li, S.Y.; Xu, C.M. Associations of sperm mtDNA copy number, DNA fragmentation index, and reactive oxygen species with clinical outcomes in ART treatments. Front. Endocrinol. 2022, 13, 849534. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Kopecka, V.; Sipek, J.; Rubes, J. Association between sperm mitochondrial DNA copy number and deletion rate and industrial air pollution dynamics. Sci. Rep. 2022, 12, 8324. [Google Scholar] [CrossRef]
- Moazamian, A.; Gharagozloo, P.; Aitken, R.J.; Drevet, J.R. Oxidative stress and reproductive function: Sperm telomeres, oxidative stress, and infertility. Reproduction 2022, 164, F125–F133. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Cui, H.; Chen, Q.; Yang, W.; Zou, P.; Yang, H.; Zhou, N.; Deng, J.; Liu, J.; Cao, J.; et al. Sperm telomere length is associated with sperm nuclear DNA integrity and mitochondrial DNA abnormalities among healthy male college students in Chongqing, China. Hum. Reprod. 2023, 38, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Sakaloglou, P.; Bouba, I.; Sofikitis, N.; Georgiou, I. Functional association between telomeres, oxidation and mitochondria. Front. Reprod. Health 2023, 5, 1107215. [Google Scholar] [CrossRef]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef]
- Rahman Aisyah, C.; Mizuno, Y.; Masuda, M.; Iwamoto, T.; Yamasaki, K.; Uchida, M.; Kariya, F.; Higaki, S.; Konishi, S. Association between sperm mitochondrial DNA copy number and concentrations of urinary cadmium and selenium. Biol. Trace Elem. Res. 2024, 202, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- VahediRaad, M.; Firouzabadi, A.M.; TofighiNiaki, M.; Henkel, R.; Fesahat, F. The impact of mitochondrial impairments on sperm function and male fertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria quality control and male fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Mens Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wai, T.; Ao, A.; Zhang, X.; Cyr, D.; Dufort, D.; Shoubridge, E.A. The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 2010, 83, 52–62. [Google Scholar] [CrossRef]
- Mai, Z.; Yang, D.; Wang, D.; Zhang, J.; Zhou, Q.; Han, B.; Sun, Z. A narrative review of mitochondrial dysfunction and male infertility. Transl. Androl. Urol. 2024, 13, 2134–2145. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
- Wu, H.; Whitcomb, B.W.; Huffman, A.; Brandon, N.; Labrie, S.; Tougias, E.; Lynch, K.; Rahil, T.; Sites, C.K.; Pilsner, J.R. Associations of sperm mitochondrial DNA copy number and deletion rate with fertilization and embryo development in a clinical setting. Hum. Reprod. 2019, 34, 163–170. [Google Scholar] [CrossRef]
- Jiang, M.; Kauppila, T.E.S.; Motori, E.; Li, X.; Atanassov, I.; Folz-Donahue, K.; Bonekamp, N.A.; Albarran-Gutierrez, S.; Stewart, J.B.; Larsson, N.-G. Increased total mtDNA copy number cures male infertility despite unaltered mtDNA mutation load. Cell Metab. 2017, 26, 429–436.e4. [Google Scholar] [CrossRef]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G.; et al. The association of female and male infertility with telomere length (review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef]
- Darmishonnejad, Z.; Tavalaee, M.; ZareiKheirabadi, M.; Zohrabi, D.; Nasr-Esfahani, M.H. Relationship between sperm telomere length and sperm quality in infertile men. Andrologia 2020, 52, e13546. [Google Scholar] [CrossRef]
- Amir, S.; Vakonaki, E.; Tsiminikaki, K.; Tzatzarakis, M.N.; Michopoulou, V.; Flamourakis, M.; Kalliantasi, K.; Karzi, V.; Fragkiadaki, P.; Renieri, E.A.; et al. Sperm telomere length: Diagnostic and prognostic biomarker in male infertility (review). World Acad. Sci. J. 2019, 1, 259–263. [Google Scholar] [CrossRef]
- Margiana, R.; Gupta, R.; Al-Jewari, W.M.; Hjazi, A.; Alsaab, H.O.; Mustafa, Y.F.; Singh, R.; Thaibt, R.; Alkhayyat, S.; Ibrahim, A.J. Evaluation of telomere length, reactive oxygen species, and apoptosis in spermatozoa of patients with oligospermia. Cell Biochem. Funct. 2024, 42, e3935. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, V.S.; Shahid, M.; Deo, P.; Fenech, M. Reduced SIRT1 and SIRT3 and lower antioxidant capacity of seminal plasma is associated with shorter sperm telomere length in oligospermic men. Int. J. Mol. Sci. 2024, 25, 718. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Vatsalaswamy, P.; Manvikar, P. The association of male infertility with telomere length: A case control study. Indian. J. Clin. Anat. Physiol. 2021, 8, 325–332. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Barragán, M.; Bellu, E.; Oliva, R.; Rodríguez, A.; Vassena, R. Sperm telomere length in donor samples is not related to ICSI outcome. J. Assist. Reprod. Genet. 2018, 35, 649–657. [Google Scholar] [CrossRef]
- Yan, X.; Yang, P.; Li, Y.; Liu, T.; Zha, Y.; Wang, T.; Zhang, J.; Feng, Z.; Li, M. New insights from bidirectional Mendelian randomization: Causal relationships between telomere length and mitochondrial DNA copy number in aging biomarkers. Aging 2024, 16, 7387–7404. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Carpenter, L.L.; Kao, H.-T.; Porton, B.; Philip, N.S.; Ridout, S.J.; Ridout, K.K.; Price, L.H. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp. Gerontol. 2015, 66, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Dafopoulos, S.; Stavros, S.; Dafopoulos, K.; Drakakis, P.; Georgiou, I.; Zachariou, A. Association of obesity with telomere length in human sperm. J. Clin. Med. 2024, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Pang, M.-G. Mitochondrial functionality in male fertility: From spermatogenesis to fertilization. Antioxidants 2021, 10, 10010098. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence (5′→3′ Direction) | Size bp | Ref. |
|---|---|---|---|
| ND1 for copy number using qPCR (58 °C) | |||
| Forward | ATTCGATGTTGAAGCCTGAGACT | 108 | [13] |
| Reverse | TGACCCTTGGCCATAATATGATT | ||
| GAPDH for copy number using qPCR (58 °C) | |||
| Forward | TGAGAAGTATGACAACAGCC | 120 | [14] |
| Reverse | TCCTTCCACGATACCAAAG | ||
| Telomere primer (55 °C) | |||
| Forward | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT | [15] | |
| Reverse | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT | ||
| Single-Copy gene Reaction (SCR) primers (55 °C) | |||
| Forward | CAGCAAGTGGGAAGGTGTAATCC | [15] | |
| Reverse | CCCATTCTATCATCAACGGGTACAA | ||
| Parameters | Group | Chi-Square | p-Value | ||
|---|---|---|---|---|---|
| Patients | Control | ||||
| Family History | Yes | 67 (44.67%) | 1 (2%) | 30.42 | 0.001 ** |
| No | 83 (55.33%) | 49 (98%) | |||
| Alcohol | Yes | 66 (44%) | 6 (12%) | 16.67 | 0.001 ** |
| No | 84 (56%) | 44 (88%) | |||
| Smoking | Yes | 48 (32%) | 21 (42%) | 1.66 | 0.2. |
| No | 102 (68%) | 29 (58%) | |||
| Treatment | Yes | 102 (68%) | 12 (24%) | 29.62 | 0.001 ** |
| No | 48 (32%) | 38 (76%) | |||
| Wife Miscarriage | Yes | 25 (16.67%) | 0 | 9.52 | 0.002 |
| No | 125 (83.33%) | 50 (100%) | |||
| Education Level | No. Edu * | 62 (41.33%) | 7 (14%) | 84.46 | 0.001 ** |
| P.S * | 11 (7.33%) | 0 | |||
| S.S * | 57 (38%) | 2 (4%) | |||
| University | 20 (13.33%) | 41 (82%) | |||
| Infertility Duration | 1–5 Years | 74 (49.33%) | 0 | 200.00 | 0.001 ** |
| 6–10 Years | 32 (21.33%) | 0 | |||
| ˃10 years | 44 (29.33%) | 0 | |||
| No | 0 | 50 | |||
| Parameters | Group | Mean | Std. Error Mean | p-Value |
|---|---|---|---|---|
| Volume (mL) | Patients | 3.110 | 0.14 | 0.001 ** |
| Control | 4.590 | 0.12 | ||
| Sperm Count (million/mL) | Patients | 15.947 | 1.59 | 0.001 ** |
| Control | 39.800 | 2.44 | ||
| Character | Patients | Control | ||
| Morphology of Sperm | Normal | 100 (66.67%) | 50 | 0.001 ** |
| Abnormal | 50 (33.33%) | 0 | ||
| Sperm Motility | PR | 89 (59.33%) | 50 | 0.001 ** |
| NP | 30 (20%) | 0 | ||
| IM | 31 (20.67%) | 0 |
| Parameters | Area | Cutoff | Explanation | p-Value | Sensitivity% | Specificity% |
|---|---|---|---|---|---|---|
| mtDNA Copy Number | 0.954 | 25.24 | Excellent | 0.001 ** | 95% | 92% |
| Sperm Telomere Length | 0.89 | 0.95 | Very Good | 0.001 ** | 95% | 88% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadhim, M.F.; Samawi, F.T.; Gargouri, A. Telomere Length and Mitochondrial Copy Number as Potential Biomarkers for Male Infertility in Iraqi Men. Genes 2025, 16, 1402. https://doi.org/10.3390/genes16121402
Kadhim MF, Samawi FT, Gargouri A. Telomere Length and Mitochondrial Copy Number as Potential Biomarkers for Male Infertility in Iraqi Men. Genes. 2025; 16(12):1402. https://doi.org/10.3390/genes16121402
Chicago/Turabian StyleKadhim, Mustafa Faeq, Farah Thamer Samawi, and Ali Gargouri. 2025. "Telomere Length and Mitochondrial Copy Number as Potential Biomarkers for Male Infertility in Iraqi Men" Genes 16, no. 12: 1402. https://doi.org/10.3390/genes16121402
APA StyleKadhim, M. F., Samawi, F. T., & Gargouri, A. (2025). Telomere Length and Mitochondrial Copy Number as Potential Biomarkers for Male Infertility in Iraqi Men. Genes, 16(12), 1402. https://doi.org/10.3390/genes16121402






