A Complex Case of Retinoblastoma Solved by the Combined Approach of Humor/Plasma cfDNA-NGS and LR-WGS
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Genomic DNA Isolation and Analysis
2.3. Clinical Exome Sequencing (CES)
2.4. Methylation-Specific Multiplex Ligation-Dependent Probe Amplification (MS-MLPA) Analysis
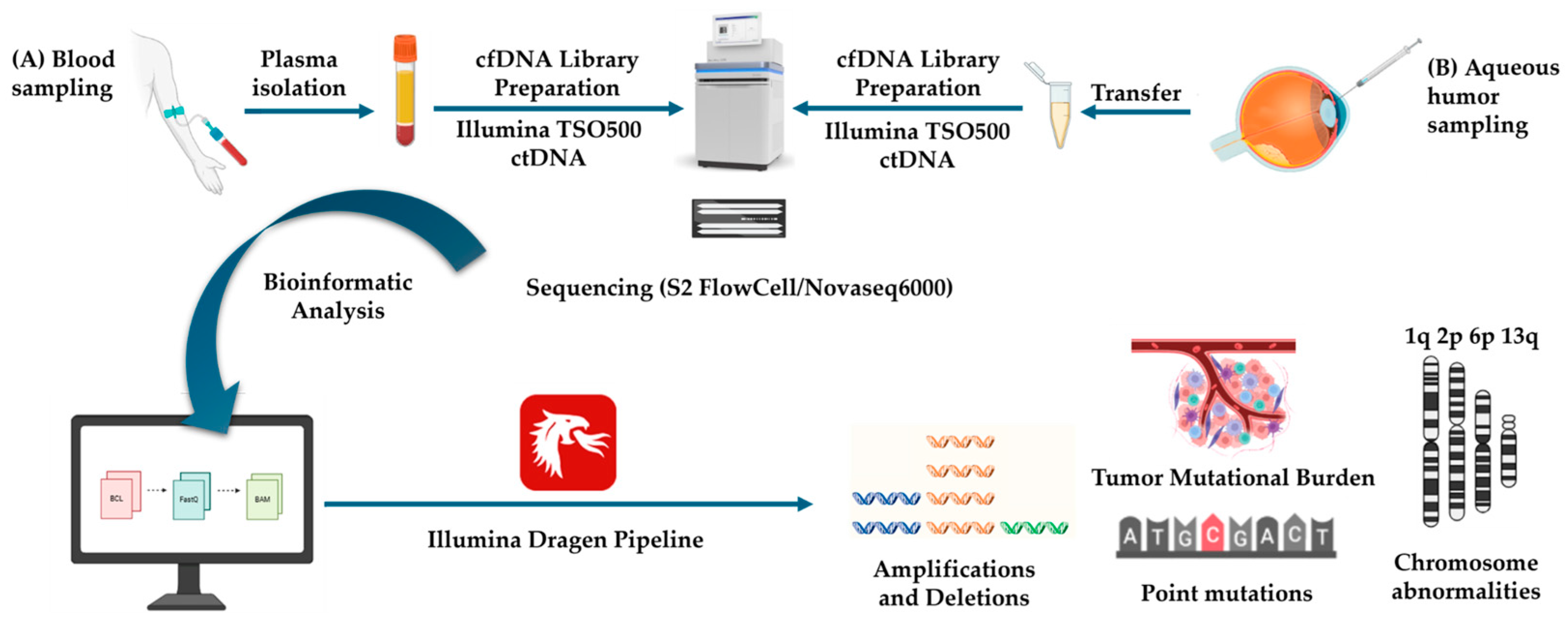
2.5. Cell-Free fDNA (cf-DNA) Isolation and Next-Generation Sequencing (NGS) Liquid Biopsy Analysis
2.6. Long-Read–Whole-Genome Sequencing (LR-WGS)
- Clair3 for single-nucleotide variants (SNVs) and small indel calling,
- Sniffles2 for structural variant (SV) detection,
- modkit for the extraction and quantification of methylation (5-mC and 5-hmC) from modBAM files,
- Straglr for the identification of short tandem repeat (STR) expansions,
- Spectre for genome-wide copy number variation (CNV) profiling.
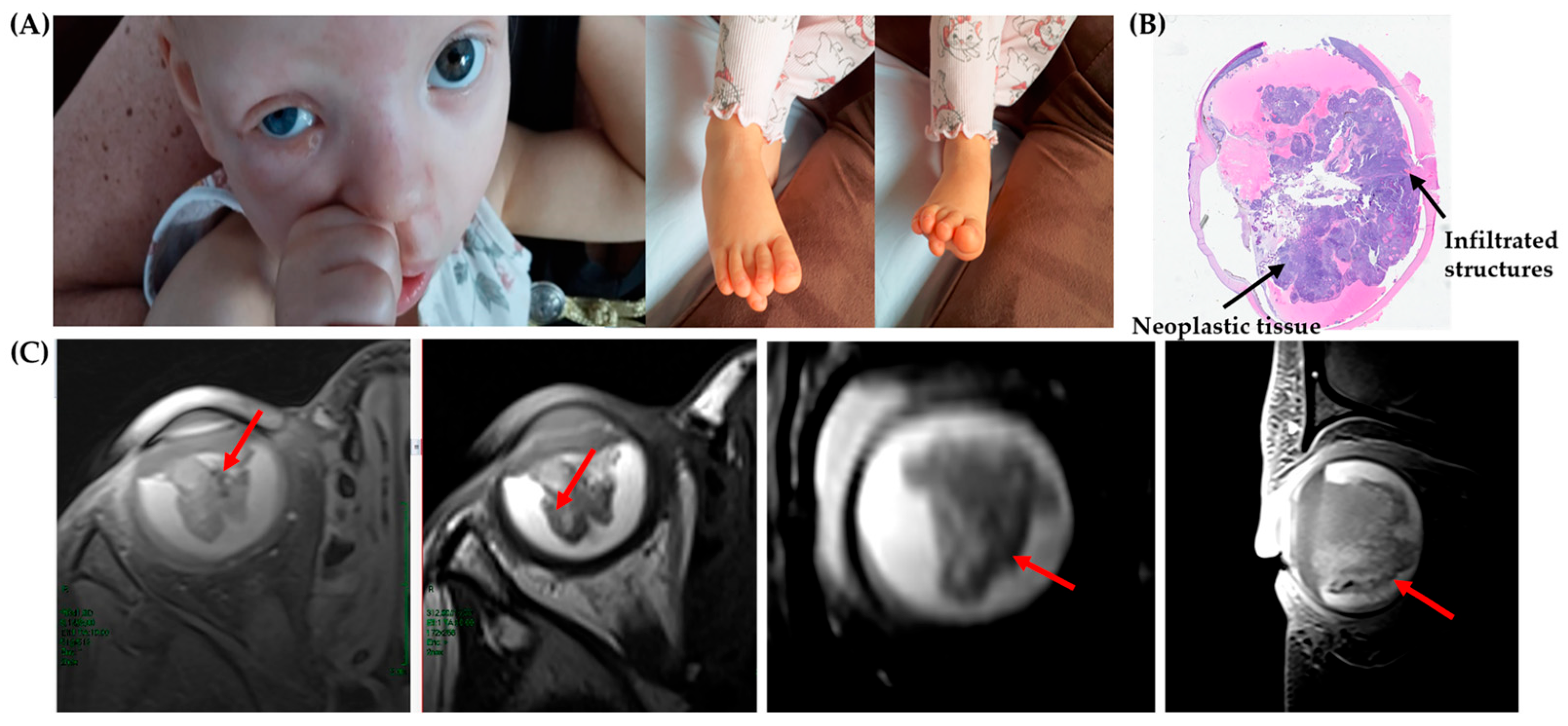
3. Case Description
3.1. Results
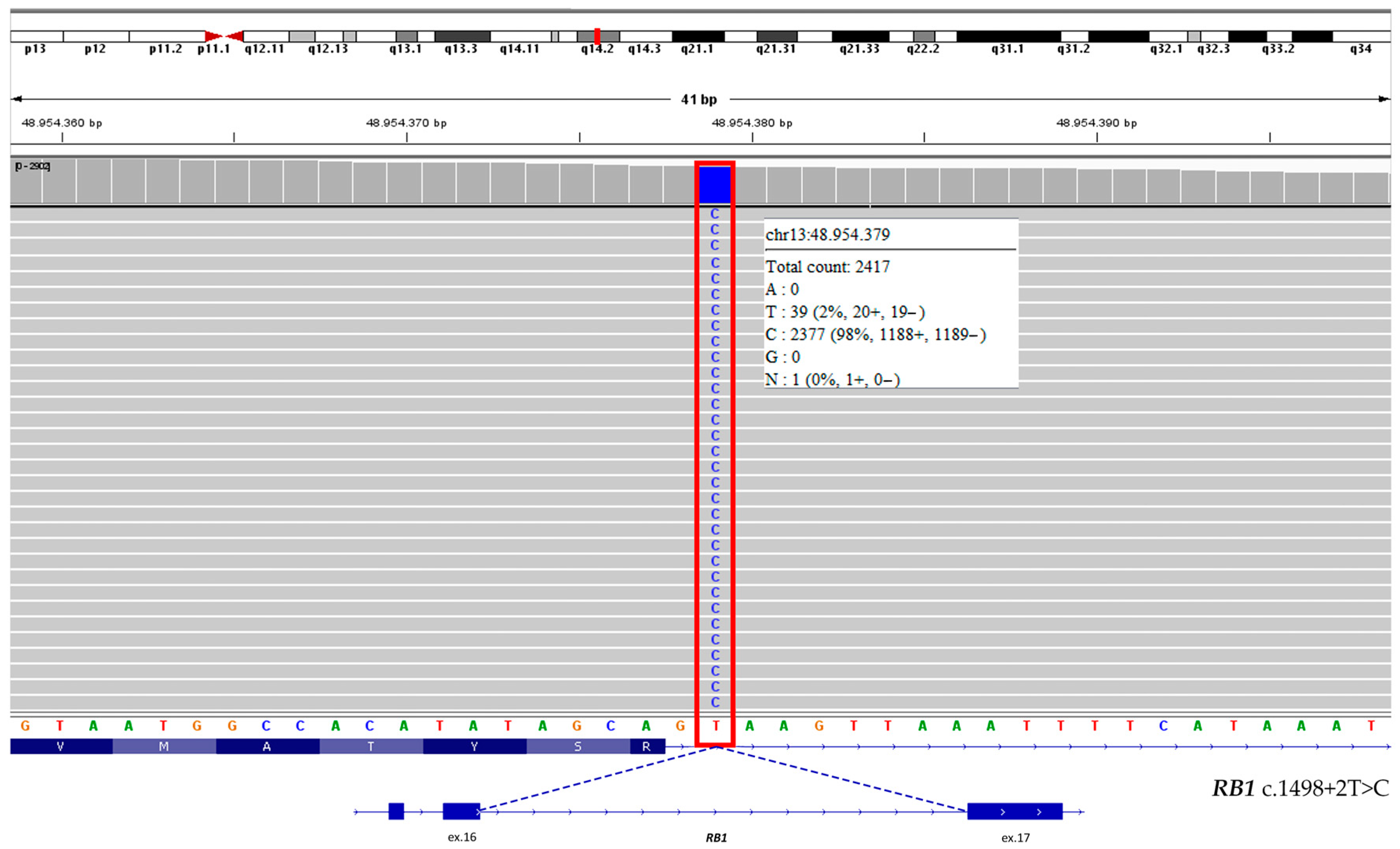
3.1.1. Clinical Exome Sequencing (CES) and MS-MLPA Results
3.1.2. Liquid Biopsy Results
3.1.3. Long-Read–Whole-Genome Sequencing (LR-WGS) Results
4. Discussion
4.1. Molecular Findings and RB1 Pathogenic Variant
4.2. Somatic Copy Number Alterations (SCNAs) and Oncogenic Drivers
4.3. Additional Molecular Findings
4.4. Chromosomal Duplication and Rare Genetic Events
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | Aqueous humor |
| ACMG | American College of Medical Genetics and Genomics |
| AMP | Association for Molecular Pathology |
| ART | Assisted reproductive technology |
| AURKA | Aurora kinase A |
| cfDNA | Cell-free DNA |
| ctDNA | Circulating tumor DNA |
| CNV | Copy number variation |
| HAC | High accuracy |
| HBV | Hepatitis B Virus |
| HE | Hematoxylin/Eosin |
| IGV | Integrative Genomics Viewer |
| INDEL | Insertion/deletion |
| lnRNA | Long non-coding RNA |
| LR | Long read |
| MRI | Magnetic resonance image |
| MSI | Microsatellite instability |
| MS-MLPA | Methylation-Specific Multiplex ligation probe amplification |
| NGS | Next-generation sequencing |
| ONT | Oxford Nanopore Technology |
| pRB | Retinoblastoma Protein |
| RB | Retinoblastoma |
| SCNA | Somatic copy number alteration |
| SNV | Single-nucleotide variant |
| STR | Short tandem repeat |
| SV | Structural variant |
| TGS | Third-generation sequencing |
| TMB | Tumor mutational burden |
| TSO | TruSight Oncology |
| VAF | Variant allele frequency |
| VUS | Variant of uncertain significance |
| WES | Whole-exome sequencing |
| WGS | Whole-genome sequencing |
References
- Chen, J.; Cao, X.; Xu, S.; Chen, X.; Xie, R.; Ye, G.; Zhang, Y.; Huang, S.; Shen, X.; Xiao, Y.; et al. Global, Regional, and National Burden of Retinoblastoma in Infants and Young Children: Findings from the Global Burden of Disease Study 1990–2021. EClinicalMedicine 2024, 76, 102860. [Google Scholar] [CrossRef] [PubMed]
- Dimaras, H.; Corson, T.W.; Cobrinik, D.; White, A.; Zhao, J.; Munier, F.L.; Abramson, D.H.; Shields, C.L.; Chantada, G.L.; Njuguna, F.; et al. Retinoblastoma. Nat. Rev. Dis. Primers 2015, 1, 15021. [Google Scholar] [CrossRef]
- Dimaras, H.; Khetan, V.; Halliday, W.; Orlic, M.; Prigoda, N.L.; Piovesan, B.; Marrano, P.; Corson, T.W.; Eagle, R.C., Jr.; Squire, J.A.; et al. Loss of RB1 induces non-proliferative retinoma: Increasing genomic instability correlates with progression to retinoblastoma. Hum. Mol. Genet. 2008, 17, 1363–1372. [Google Scholar] [CrossRef]
- Guzmán, F.; Fazeli, Y.; Khuu, M.; Salcido, K.; Singh, S.; Benavente, C.A. Retinoblastoma Tumor Suppressor Protein Roles in Epigenetic Regulation. Cancers 2020, 12, 2807. [Google Scholar] [CrossRef]
- Iacovacci, J.; Brough, R.; Moughari, F.A.; Alexander, J.; Kemp, H.; Tutt, A.N.J.; Natrajan, R.; Lord, C.J.; Haider, S. Proteogenomic discovery of RB1-defective phenocopy in cancer predicts disease outcome, response to treatment, and therapeutic targets. Sci. Adv. 2025, 11, eadq9495. [Google Scholar] [CrossRef]
- Flores, M.; Goodrich, D.W. Retinoblastoma Protein Paralogs and Tumor Suppression. Front. Genet. 2022, 13, 818719. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, E.J.; Zhang, B.; Wu, C.; Pardeshi, L.; Shi, C.; Mou, P.K.; Liu, Y.; Tan, K.; Shim, J.S. Synthetic lethality of RB1 and aurora A is driven by stathmin-mediated disruption of microtubule dynamics. Nat. Commun. 2020, 11, 5105. [Google Scholar] [CrossRef]
- Liao, Q.; Yang, J.; Shi, H.; Mengjiang, R.; Li, Y.; Zhang, Q.; Wen, X.; Ge, S.; Chai, P.; Fan, X.; et al. Aurora A Kinase Inhibition Is Synthetic Lethal With the Activation of MYCN in Retinoblastoma. Investig. Ophthalmol. Vis. Sci. 2025, 66, 20. [Google Scholar] [CrossRef]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Corson, T.W.; Gallie, B.L. One hit, two hits, three hits, more? Genomic changes in the development of retinoblastoma. Genes Chromosomes Cancer 2007, 46, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Rushlow, D.E.; Mol, B.M.; Kennett, J.Y.; Yee, S.; Pajovic, S.; Thériault, B.L.; Prigoda-Lee, N.L.; Spencer, C.; Dimaras, H.; Corson, T.W.; et al. Characterisation of retinoblastomas without RB1 mutations: Genomic, gene expression, and clinical studies. Lancet Oncol. 2013, 14, 327–334. [Google Scholar] [CrossRef]
- McEvoy, J.; Nagahawatte, P.; Finkelstein, D.; Richards-Yutz, J.; Valentine, M.; Ma, J.; Mullighan, C.; Song, G.; Chen, X.; Wilson, M.; et al. RB1 gene inactivation by chromothripsis in human retinoblastoma. Oncotarget 2014, 5, 438–450. [Google Scholar] [CrossRef]
- Berry, J.L.; Xu, L.; Murphree, A.L.; Krishnan, S.; Stachelek, K.; Zolfaghari, E.; McGovern, K.; Lee, T.C.; Carlsson, A.; Kuhn, P.; et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol. 2017, 135, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.L.; Xu, L.; Polski, A.; Jubran, R.; Kuhn, P.; Kim, J.W.; Hicks, J. Aqueous humor is superior to blood as a liquid biopsy for retinoblastoma. Ophthalmology 2020, 127, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Gerrish, A.; Jenkinson, H.; Cole, T. The impact of cell-free DNA analysis on the management of retinoblastoma. Cancers 2021, 13, 1570. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Polski, A.; Prabakar, R.K.; Reid, M.W.; Chevez-Barrios, P.; Jubran, R.; Kim, J.W.; Kuhn, P.; Cobrinik, D.; Hicks, J.; et al. Chromosome 6p amplification in aqueous humor cell-free DNA is a prognostic biomarker for retinoblastoma ocular survival. Mol. Cancer Res. 2020, 18, 1166–1175. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef]
- Gerrish, A.; Stone, E.; Clokie, S.; Ainsworth, J.R.; Jenkinson, H.; McCalla, M.; Hitchcott, C.; Colmenero, I.; Allen, S.; Parulekar, M.; et al. Non-invasive diagnosis of retinoblastoma using cell-free DNA from aqueous humour. Br. J. Ophthalmol. 2019, 103, 721–724. [Google Scholar] [CrossRef]
- Laurie, N.A.; Donovan, S.L.; Shih, C.S.; Zhang, J.; Mills, N.; Fuller, C.; Teunisse, A.; Lam, S.; Ramos, Y.; Mohan, A.; et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006, 444, 61–66. [Google Scholar] [CrossRef]
- McEvoy, J.; Flores-Otero, J.; Zhang, J.; Nemeth, K.; Brennan, R.; Bradley, C.; Krafcik, F.; Rodriguez-Galindo, C.; Wilson, M.; Xiong, S.; et al. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell 2011, 20, 260–275. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.; Ulyanov, A.; Brennan, R.; Wu, G.; Pounds, S.; Zhang, J.; Dyer, M.A. Analysis of MDM2 and MDM4 single nucleotide polymorphisms, mRNA splicing and protein expression in retinoblastoma. PLoS ONE 2012, 7, e42739. [Google Scholar] [CrossRef]
- Jeyaprakash, K.; Kumaran, M.; Kim, U.; Santhi, R.; Muthukkaruppan, V.; Devarajan, B.; Vanniarajan, A. Investigating druggable kinases for targeted therapy in retinoblastoma. J. Hum. Genet. 2024, 69, 467–474. [Google Scholar] [CrossRef]
- Ioan, D.M.; Vermeesch, J.; Fryns, J.P. Terminal distal 13q trisomy due to de novo dup(13)(q32→qter). Genet. Couns. 2005, 16, 435–436. Available online: https://pubmed.ncbi.nlm.nih.gov/16440891/ (accessed on 18 November 2025).
- Rogers, J.F. Clinical delineation of proximal and distal partial 13q trisomy. Clin. Genet. 1984, 25, 221–229. [Google Scholar] [CrossRef]
- Yan, J.; Deng, Y.X.; Cai, Y.L.; Cong, W.D. LncRNA MIR17HG promotes the proliferation, migration, and invasion of retinoblastoma cells by up-regulating HIF-1α expression via sponging miR-155-5p. Kaohsiung J. Med. Sci. 2022, 38, 554–564. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, X.; Yi, G.; Wu, T.; Sun, Y.; Zhang, Z.; Fu, M. Bioinformatics analysis proposes a possible role for long noncoding RNA MIR17HG in retinoblastoma. Cancer Rep. 2024, 7, e1933. [Google Scholar] [CrossRef]
- Kandalam, M.M.; Beta, M.; Maheswari, U.K.; Swaminathan, S.; Krishnakumar, S. Oncogenic microRNA 17-92 cluster is regulated by epithelial cell adhesion molecule and could be a potential therapeutic target in retinoblastoma. Mol. Vis. 2012, 18, 2279–2287. [Google Scholar]
- Bolger, G.B.; Stamberg, J.; Kirsch, I.R.; Hollis, G.F.; Schwarz, D.F.; Thomas, G.H. Chromosomal Translocation t(14;22) and Oncogene (c-sis) Variant in a Pedigree with Familial Meningioma. N. Engl. J. Med. 1985, 312, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Smidt, M.; Kirsch, I.; Ratner, L. Deletion of Alu Sequences in the Fifth c-sis Intron in Individuals with Meningiomas. J. Clin. Invest. 1990, 86, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Kim, M.E.; Polski, A.; Prabakar, R.K.; Shen, L.; Peng, C.C.; Reid, M.W.; Chévez-Barrios, P.; Kim, J.W.; Shah, R.; et al. Establishing the Clinical Utility of ctDNA Analysis for Diagnosis, Prognosis, and Treatment Monitoring of Retinoblastoma: The Aqueous Humor Liquid Biopsy. Cancers 2021, 13, 1282. [Google Scholar] [CrossRef] [PubMed]
- Mendes, T.B.; Oliveira, I.D.; Gamba, F.T.; Lima, F.T.; Morales, B.F.S.C.; Macedo, C.R.D.; Teixeira, L.F.; de Toledo, S.R.C. Retinoblastoma: Molecular Evaluation of Tumor Samples, Aqueous Humor, and Peripheral Blood Using a Next-Generation Sequence Panel. Int. J. Mol. Sci. 2025, 26, 3523. [Google Scholar] [CrossRef] [PubMed]


| HUMOR-cfDNA NGS Liquid Biopsy | PLASMA-cfDNA NGS Liquid Biopsy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CNV | CNV | ||||||||
| Gene | Chr | Cytoband | Interval | Fold Change | Gene | Chr | Cytoband | Interval | Fold Change |
| MDM4 | 1 | 1q32.1 | 204,485,505–204,526,342 | 1.4 | MDM4 | 1 | 1q32.1 | / | / |
| ALK | 2 | 2p23.2 | 29,416,088–30,143,527 | 1.3 | ALK | 2 | 2p23.2 | / | / |
| TFRC | 3 | 3q29 | 195,776,752–195,806,640 | 0.5 | TFRC | 3 | 3q29 | / | / |
| CCND3 | 6 | 6p21.2 | 41,903,676–42,014,927 | 1.4 | CCND3 | 6 | 6p21.2 | / | / |
| BRAF | 7 | 7q34 | 140,434,395–140,624,505 | 1.1 | BRAF | 7 | 7q34 | / | / |
| FGF3 | 11 | 11q13.3 | 69,623,035–69,635,441 | 0.8 | FGF3 | 11 | 11q13.3 | / | / |
| FGF9 | 13 | 13q12.11 | 22,245,512–22,278,213 | 0.6 | FGF9 | 13 | 13q12.11 | / | / |
| BRCA2 | 13 | 13q13.1 | 32,890,596–32,972,909 | 0.8 | BRCA2 | 13 | 13q13.1 | / | / |
| HUMOR-cfDNA NGS Liquid Biopsy | PLASMA-cfDNA NGS Liquid Biopsy | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNV | SNV | ||||||||||||
| Gene | Chr | Variant | CADD | VAF % | Read Depth | ACMG Class | Gene | Chr | Variant | CADD | VAF % | Read Depth | ACMG Class |
| FANCD2 | 3 | c.573C>G p.(Ser191Arg) | 9.7 | 49 | 1181 | VUS | FANCD2 | 3 | c.573C>G p.(Ser191Arg) | 9.7 | 49 | 604 | VUS |
| FGFR4 | 5 | c.1162G>A p.(Gly388Arg) | 23.6 | 48 | 1942 | Benign | FGFR4 | 5 | c.1162G>A p.(Gly388Arg) | 23.6 | 48 | 602 | Benign |
| PMS2 | 7 | c.20C>G p.(Ser7Trp) | 13.38 | 0.8 | 891 | VUS | PMS2 | 7 | / | / | / | / | / |
| BRCA2 | 13 | c.7871A>G p.(Tyr2624Cys) | 26.7 | 1.3 | 1051 | VUS | BRCA2 | 13 | c.7871A>G p.(Tyr2624Cys) | 26.7 | 45 | 596 | VUS |
| RB1 | 13 | c.1498+2T>C | 32 | 98.5 | 1177 | Pathogenic | RB1 | 13 | / | / | / | / | / |
| STK11 | 19 | c.1069G>A p.(Glu357Lys) | 24 | 3.9 | 1294 | VUS | STK11 | 19 | / | / | / | / | / |
| CHEK2 | 22 | c.1117A>G p.(Lys373Glu) | 27.7 | 3.5 | 1888 | VUS | CHEK2 | 22 | / | / | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innamorato, S.; Basso, S.L.; Belakhdar, O.; Bruttini, M.; Fallerini, C.; Huseynli, H.; Caccialupi, G.; Pasquinelli, E.; Adduci, M.; Signori, G.; et al. A Complex Case of Retinoblastoma Solved by the Combined Approach of Humor/Plasma cfDNA-NGS and LR-WGS. Genes 2025, 16, 1399. https://doi.org/10.3390/genes16121399
Innamorato S, Basso SL, Belakhdar O, Bruttini M, Fallerini C, Huseynli H, Caccialupi G, Pasquinelli E, Adduci M, Signori G, et al. A Complex Case of Retinoblastoma Solved by the Combined Approach of Humor/Plasma cfDNA-NGS and LR-WGS. Genes. 2025; 16(12):1399. https://doi.org/10.3390/genes16121399
Chicago/Turabian StyleInnamorato, Simona, Simona L. Basso, Omaima Belakhdar, Mirella Bruttini, Chiara Fallerini, Heyran Huseynli, Giulia Caccialupi, Elena Pasquinelli, Mariarosaria Adduci, Giorgio Signori, and et al. 2025. "A Complex Case of Retinoblastoma Solved by the Combined Approach of Humor/Plasma cfDNA-NGS and LR-WGS" Genes 16, no. 12: 1399. https://doi.org/10.3390/genes16121399
APA StyleInnamorato, S., Basso, S. L., Belakhdar, O., Bruttini, M., Fallerini, C., Huseynli, H., Caccialupi, G., Pasquinelli, E., Adduci, M., Signori, G., Arcuri, F., Malagnino, V., Siciliano, M. C., Lazzi, S., Pesaresi, S., Galimberti, D., Galluzzi, P., De Francesco, S., Hadijstillanou, T., ... Ariani, F. (2025). A Complex Case of Retinoblastoma Solved by the Combined Approach of Humor/Plasma cfDNA-NGS and LR-WGS. Genes, 16(12), 1399. https://doi.org/10.3390/genes16121399







