Genetic Assessment and Clinical Correlates in Severe Hypertriglyceridemia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection, Data Extraction, and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Study Populations and Triglyceride Levels
3.3. Therapeutic Outcomes
3.4. Genotype–MASLD Relationship
4. Discussion
Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegele, R.A. Plasma lipoproteins: Genetic influences and clinical implications. Nat. Rev. Genet. 2009, 10, 109–121. [Google Scholar] [CrossRef]
- Karwatowska-Prokopczuk, E.; Lesogor, A.; Yan, J.H.; Hoenlinger, A.; Margolskee, A.; Li, L.; Tsimikas, S. Efficacy and safety of olezarsen in lowering apolipoprotein C-III and triglycerides in healthy Japanese Americans. Lipids Health Dis. 2024, 23, 329. [Google Scholar] [CrossRef] [PubMed]
- Moulin, P.; Dufour, R.; Averna, M.; Arca, M.; Cefalu, A.B.; Noto, D.; D’Erasmo, L.; Di Costanzo, A.; Marcais, C.; Alvarez-Sala Walther, L.A.; et al. Identification and diagnosis of patients with familial chylomicronaemia syndrome (FCS): Expert panel recommendations and proposal of an “FCS score”. Atherosclerosis 2018, 275, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Alexander, V.J.; Baker, B.F.; Brisson, D.; Tremblay, K.; Singleton, W.; Geary, R.S.; Hughes, S.G.; Viney, N.J.; Graham, M.J.; et al. Antisense Inhibition of Apolipoprotein C-III in Patients with Hypertriglyceridemia. N. Engl. J. Med. 2015, 373, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Ginsberg, H.N.; Chapman, M.J.; Nordestgaard, B.G.; Kuivenhoven, J.A.; Averna, M.; Boren, J.; Bruckert, E.; Catapano, A.L.; Descamps, O.S.; et al. The polygenic nature of hypertriglyceridaemia: Implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014, 2, 655–666. [Google Scholar] [CrossRef]
- Brunzell, J.D.; Schrott, H.G. The interaction of familial and secondary causes of hypertriglyceridemia: Role in pancreatitis. J. Clin. Lipidol. 2012, 6, 409–412. [Google Scholar] [CrossRef]
- Lindberg, D.A. Acute pancreatitis and hypertriglyceridemia. Gastroenterol. Nurs. 2009, 32, 75–82; quiz 83-74. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Sikora Kessler, A.; Baum, S.J.; Kutrieb, E.; Vera Llonch, M.; Lonshteyn, A.; Weycker, D.; Soffer, D.E. Rates of acute pancreatitis and cardiovascular events among adults with severe or extreme hypertriglyceridemia in US clinical practice. Lipids Health Dis. 2025, 24, 252. [Google Scholar] [CrossRef]
- Gouni-Berthold, I. The role of antisense oligonucleotide therapy against apolipoprotein-CIII in hypertriglyceridemia. Atherosclerosis. Suppl. 2017, 30, 19–27. [Google Scholar] [CrossRef]
- Ooi, E.M.; Barrett, P.H.; Chan, D.C.; Watts, G.F. Apolipoprotein C-III: Understanding an emerging cardiovascular risk factor. Clin. Sci. 2008, 114, 611–624. [Google Scholar] [CrossRef]
- Warden, B.A.; Duell, P.B. Volanesorsen for treatment of patients with familial chylomicronemia syndrome. Drugs Today 2018, 54, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Laranjeira, F.; Correia-da-Silva, G. Understanding Hypertriglyceridemia: Integrating Genetic Insights. Genes. 2024, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Dron, J.S.; Hegele, R.A. Genetics of Hypertriglyceridemia. Front. Endocrinol. 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, I.; Lupoli, R.; Di Minno, A.; Di Minno, M.N.D. Volanesorsen to treat severe hypertriglyceridaemia: A pooled analysis of randomized controlled trials. Eur. J. Clin. Investig. 2022, 52, e13841. [Google Scholar] [CrossRef]
- Malmendier, C.L.; Lontie, J.F.; Grutman, G.A.; Delcroix, C. Metabolism of apolipoprotein C-III in normolipemic human subjects. Atherosclerosis 1988, 69, 51–59. [Google Scholar] [CrossRef]
- Gordts, P.L.; Nock, R.; Son, N.H.; Ramms, B.; Lew, I.; Gonzales, J.C.; Thacker, B.E.; Basu, D.; Lee, R.G.; Mullick, A.E.; et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Investig. 2016, 126, 2855–2866. [Google Scholar] [CrossRef]
- Jorgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G.; Tybjaerg-Hansen, A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014, 371, 32–41. [Google Scholar] [CrossRef]
- The TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar] [CrossRef]
- Boren, J.; Packard, C.J.; Taskinen, M.R. The Roles of ApoC-III on the Metabolism of Triglyceride-Rich Lipoproteins in Humans. Front. Endocrinol. 2020, 11, 474. [Google Scholar] [CrossRef]
- Guan, Y.; Hou, X.; Tian, P.; Ren, L.; Tang, Y.; Song, A.; Zhao, J.; Gao, L.; Song, G. Elevated Levels of Apolipoprotein CIII Increase the Risk of Postprandial Hypertriglyceridemia. Front. Endocrinol. 2021, 12, 646185. [Google Scholar] [CrossRef] [PubMed]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.E.; Attie, A.D.; Biddinger, S.B. The regulation of ApoB metabolism by insulin. Trends Endocrinol. Metab. 2013, 24, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Gunasekaran, G.; Bekki, Y.; Lourdusamy, V.; Schwartz, M. Surgical Treatments of Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. S1), 128–136. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus, P. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014 e1991. [Google Scholar] [CrossRef]
- Larouche, M.; Watts, G.F.; Ballantyne, C.; Gaudet, D. An overview of persistent chylomicronemia: Much more than meets the eye. Curr. Opin. Endocrinol. Diabetes Obes. 2025, 32, 75–88. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, D.; Mao, R.; Yao, Z.; Wu, Q.; Lv, W. Global burden of MAFLD, MAFLD related cirrhosis and MASH related liver cancer from 1990 to 2021. Sci. Rep. 2025, 15, 7083. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Schwarz, J.; Berthold, H.K. Updates in Drug Treatment of Severe Hypertriglyceridemia. Curr. Atheroscler. Rep. 2023, 25, 701–709. [Google Scholar] [CrossRef]
- Graham, M.J.; Lee, R.G.; Brandt, T.A.; Tai, L.J.; Fu, W.; Peralta, R.; Yu, R.; Hurh, E.; Paz, E.; McEvoy, B.W.; et al. Cardiovascular and Metabolic Effects of ANGPTL3 Antisense Oligonucleotides. N. Engl. J. Med. 2017, 377, 222–232. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Ostergaard, L.H.; Long, M.T.; Kjaer, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Abdelmalek, M.F.; Armstrong, M.J.; Jara, M.; Kjaer, M.S.; Krarup, N.; Lawitz, E.; Ratziu, V.; Sanyal, A.J.; Schattenberg, J.M.; et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, Z.; Nagy, A.; Jahner, K.; Szabados, E. Impact of Selected Glucagon-like Peptide-1 Receptor Agonists on Serum Lipids, Adipose Tissue, and Muscle Metabolism—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 8214. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Nejadghaderi, S.A.; Balibegloo, M.; Rezaei, N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci. Rep. 2024, 7, e2165. [Google Scholar] [CrossRef]
- Dron, J.S.; Wang, J.; Cao, H.; McIntyre, A.D.; Iacocca, M.A.; Menard, J.R.; Movsesyan, I.; Malloy, M.J.; Pullinger, C.R.; Kane, J.P.; et al. Severe hypertriglyceridemia is primarily polygenic. J. Clin. Lipidol. 2019, 13, 80–88. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Hadley, T.D.; Roth, M.; Agha, A.M.; Pulipati, V.P.; Nugent, A.K.; Virani, S.S.; Nambi, V.; Moriarty, P.M.; Davidson, M.H.; et al. Genetic Testing for Hypertriglyceridemia in Academic Lipid Clinics: Implications for Precision Medicine-Brief Report. Arter. Thromb. Vasc. Biol. 2022, 42, 1461–1467. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Bergmark, B.A.; Marston, N.A.; Prohaska, T.A.; Alexander, V.J.; Zimerman, A.; Moura, F.A.; Murphy, S.A.; Goodrich, E.L.; Zhang, S.; Gaudet, D.; et al. Olezarsen for Hypertriglyceridemia in Patients at High Cardiovascular Risk. N. Engl. J. Med. 2024, 390, 1770–1780. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liang, X.; Zhang, X.; Li, Y. The effects of GLP-1 receptor agonists on visceral fat and liver ectopic fat in an adult population with or without diabetes and nonalcoholic fatty liver disease: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0289616. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; McGinniss, J.; Gaudet, D.; Pordy, R. Longer-Term Efficacy and Safety of Evinacumab in Patients with Refractory Hypercholesterolemia. JAMA Cardiol. 2023, 8, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Saddique, M.N.; Thapa, R.; Khan, Y.; Javaid, H.; Khawar, M.M.; Rehman, A.; Dave, R.; Raval, R.; Hejmadi, S.; Singh, P.; et al. fficacy and safety of Olezarsen in treating hypertriglyceridemia: A systematic review and meta-analysis of randomized controlled trials. Discov. Med. 2025, 2, 117. [Google Scholar] [CrossRef]
- Ariza, M.J.; Coca-Prieto, I.; Rioja, J.; Muniz-Grijalvo, O.; Zambon-Rados, D.; Blanco-Echevarria, A.; Arrobas-Velilla, T.; Delgado-Lista, J.; Leon-Jimenez, D.; Casanas-Martinez, M.; et al. Pathogenicity assessment of genetic variants identified in patients with severe hypertriglyceridemia: Novel cases of familial chylomicronemia syndrome from the Dyslipidemia Registry of the Spanish Atherosclerosis Society. Genet. Med. 2025, 27, 101365. [Google Scholar] [CrossRef]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef]
- Dixon, D.L.; Khaddage, S.; Bhagat, S.; Koenig, R.A.; Salgado, T.M.; Baker, W.L. Effect of pharmacist interventions on reducing low-density lipoprotein cholesterol (LDL-C) levels: A systematic review and meta-analysis. J. Clin. Lipidol. 2020, 14, 282–292 e284. [Google Scholar] [CrossRef]
- Verrijken, A.; Beckers, S.; Francque, S.; Hilden, H.; Caron, S.; Zegers, D.; Ruppert, M.; Hubens, G.; Van Marck, E.; Michielsen, P.; et al. A gene variant of PNPLA3, but not of APOC3, is associated with histological parameters of NAFLD in an obese population. Obesity 2013, 21, 2138–2145. [Google Scholar] [CrossRef]
- Nagle, C.A.; Klett, E.L.; Coleman, R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009, 50, S74–S79. [Google Scholar] [CrossRef]
- Gagnon, E.; Gill, D.; Burgess, S.; Arsenault, B.J. Remnant cholesterol concentrations best explain the cardiovascular benefit of APOC3 genetic inhibition: A drug target Mendelian randomization study. Eur. Heart J. Open 2025, 5, oeaf018. [Google Scholar] [CrossRef]
- Peloso, G.M.; Nomura, A.; Khera, A.V.; Chaffin, M.; Won, H.H.; Ardissino, D.; Danesh, J.; Schunkert, H.; Wilson, J.G.; Samani, N.; et al. Rare Protein-Truncating Variants in APOB, Lower Low-Density Lipoprotein Cholesterol, and Protection Against Coronary Heart Disease. Circ. Genom. Precis. Med. 2019, 12, e002376. [Google Scholar] [CrossRef]
- Giammanco, A.; Spina, R.; Cefalu, A.B.; Averna, M. APOC-III: A Gatekeeper in Controlling Triglyceride Metabolism. Curr. Atheroscler. Rep. 2023, 25, 67–76. [Google Scholar] [CrossRef]
- Jianu, N.; Nitu, E.T.; Merlan, C.; Nour, A.; Buda, S.; Suciu, M.; Luca, S.A.; Sbarcea, L.; Andor, M.; Buda, V. A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II. Pharmaceuticals 2025, 18, 1150. [Google Scholar] [CrossRef]
- Tramontano, D.; Bini, S.; D’Erasmo, L.; Arca, M. Recent Apolipoprotein CIII trials. Curr. Opin. Lipidol. 2022, 33, 309–318. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, J.; Liu, J.; Ma, H. Associations Between GGT/HDL and MAFLD: A Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2022, 15, 383–394. [Google Scholar] [CrossRef]
- Hu, W.; Gong, W.; Yang, F.; Cheng, R.; Zhang, G.; Gan, L.; Zhu, Y.; Qin, W.; Gao, Y.; Li, X.; et al. Dual GIP and GLP-1 receptor agonist tirzepatide alleviates hepatic steatosis and modulates gut microbiota and bile acid metabolism in diabetic mice. Int. Immunopharmacol. 2025, 147, 113937. [Google Scholar] [CrossRef]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef] [PubMed]
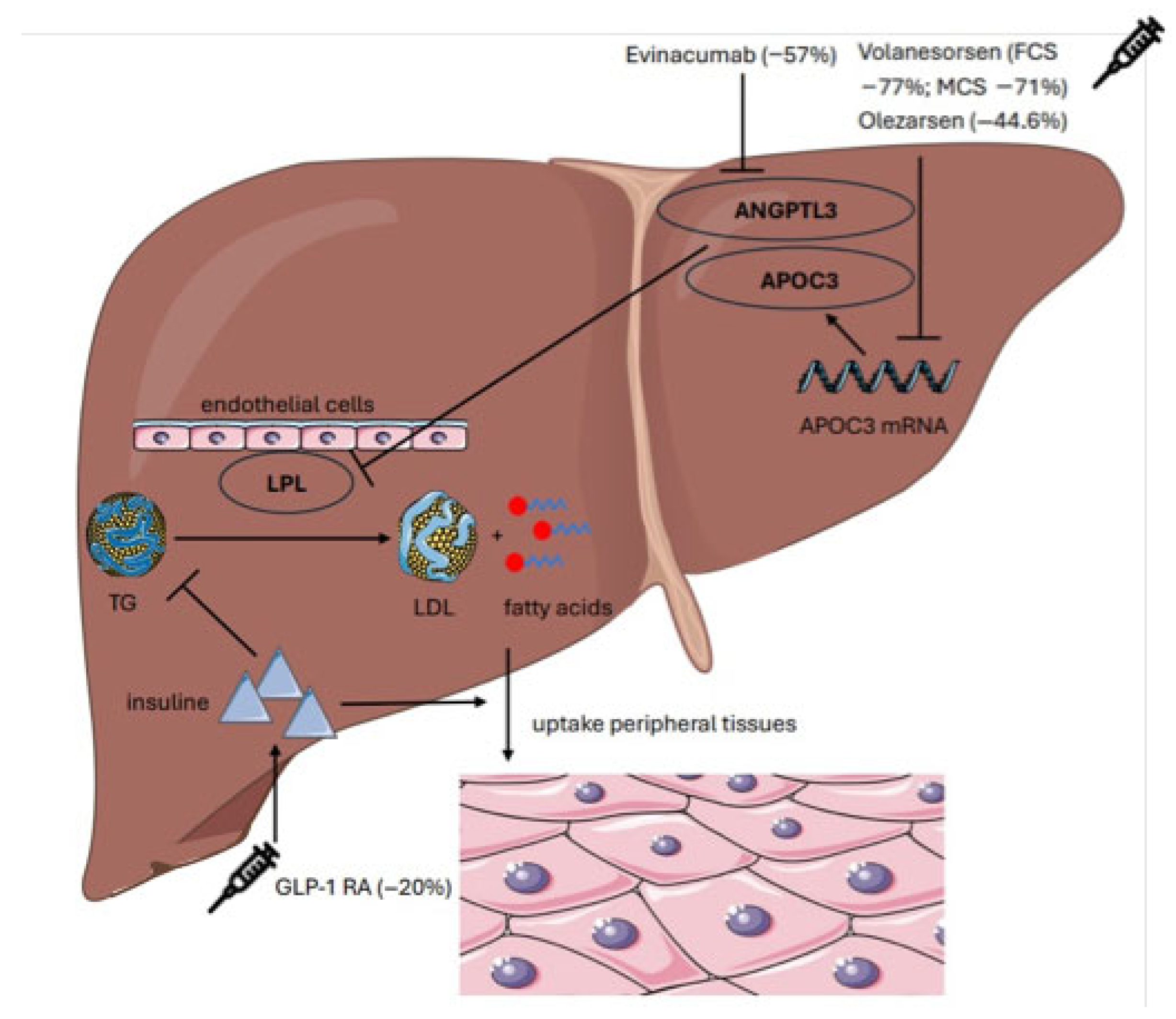
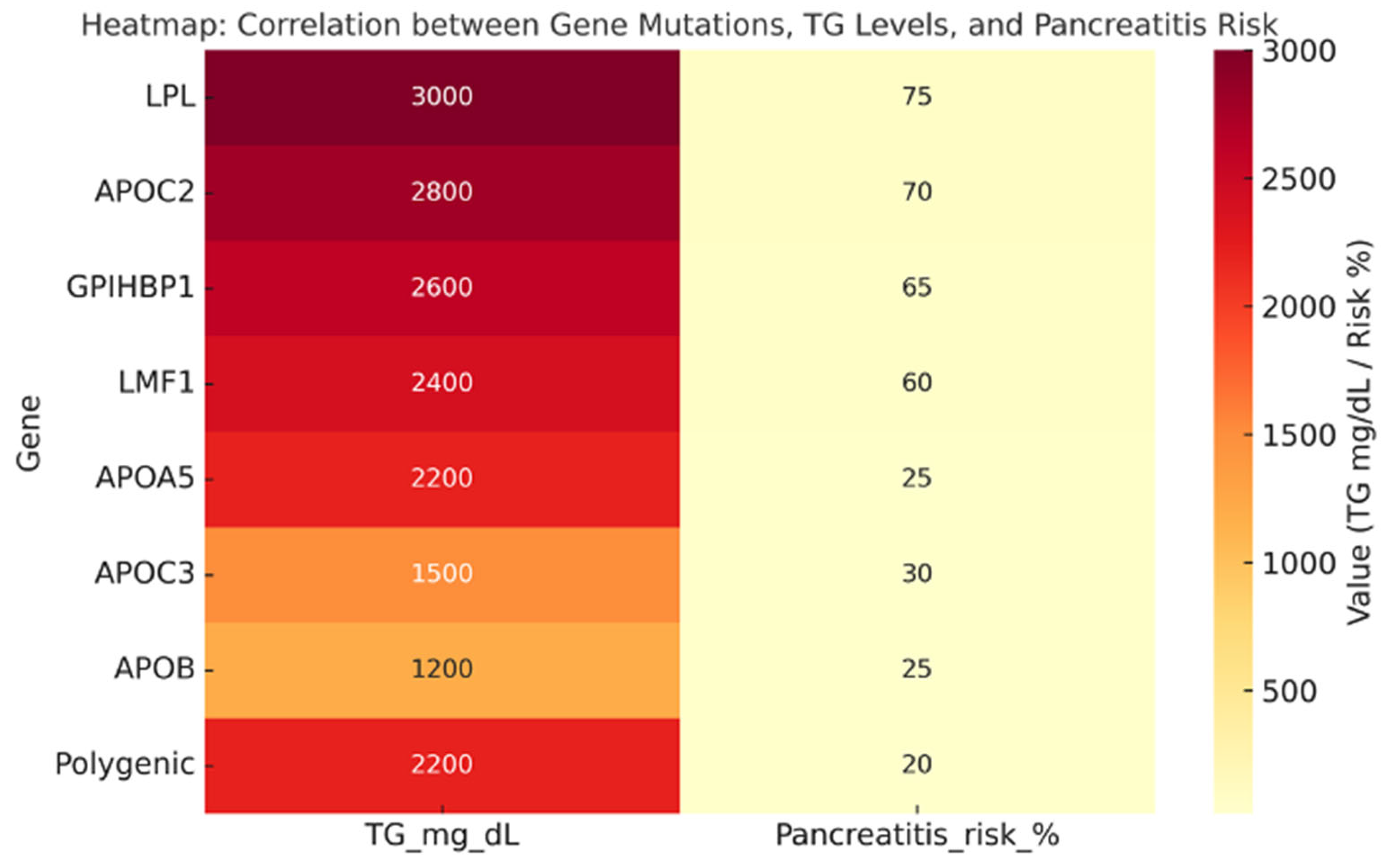
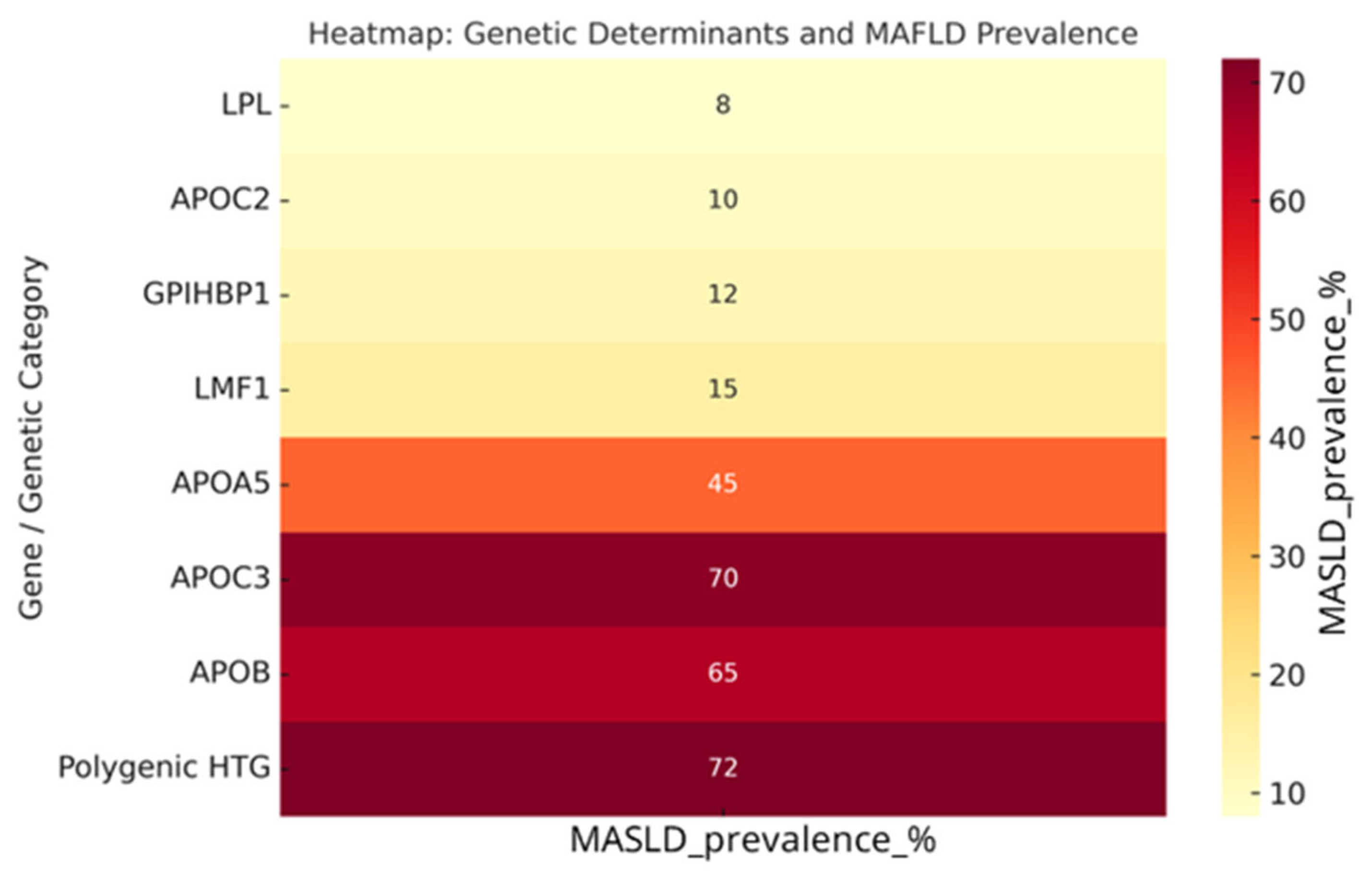
| Study | Type of Study | Intervention | Population | TG Reduction (%) | Clinical Outcomes |
|---|---|---|---|---|---|
| Calcaterra et al. (2022) [15] | Meta-analysis | Volanesorsen | 66 | −77% | No pancreatitis |
| Witzum et al. (2019) [39] | RCT | Volanesorsen, | 114 | −71% | QoL improvement |
| Bergmark et al. (2023) [40] | RCT | Olezarsen | 202 | −44.6% | ↑ HDL + 39% ↓ ApoC-III − 65% |
| Rosenson et al. (2022) [43] | Meta-analysis | Evinacumab | 96 | −57% | LDL-C − 45% ApoCIII − 71%, |
| Newsome et al. (2021) [41] | RCT | Semaglutide | 320 | −27% | 59% NASH resolution |
| Saddique et al. (2023) [44] | Meta-analysis | Olezarsen | 202 | −44% | Consistent TG lowering; improved tolerability |
| Liao et al. (2023) [42] | Meta-analysis | GLP-1R | 859 | n.r. | Liver fat reduction 30–35%. |
| Deshotels et al. (2022) [38] | Observational | n.a. | 79 | n.a. | Pancreatitis risk assessment by genotype. |
| Dron et al. (2019) [37] | Observational | n.a. | 563 | n.a. | Genetic landscape of severe HTG. |
| Karwatowska-Prokopczuk et al. (2024) [2] | RCT | Olezarsen | 20 | −73.8% | ApoC-III − 81.6% TG − 73.8% |
| Genotype | Prevalence | TG Levels (mg/dL) | Phenotype |
|---|---|---|---|
| LPL (biallelic) | 1.1% of SHTG (FCS) | 3000 | Classic FCS, chylomicronemia, recurrent pancreatitis |
| APOC2 (biallelic) | <1% (very rare) | 2500–3000 | FCS phenotype, poor response to fibrates/omega-3 |
| GPIHBP1 | <2% | >2500 | Chylomicronemia, xanthomas, lipemia retinalis |
| LMF1 | <1% | >2000 | Severe HTG with variable penetrance |
| APOA5 | 10–15% (heterozygotes common) | 1800–2500 | Intermediate phenotype, often with secondary triggers |
| APOC3 | Rare (<5%) | 500–1500 | Associated with hepatic steatosis, insulin resistance |
| APOB | Rare (<5%) | 800–1500 | Variable phenotype, altered VLDL distribution |
| Polygenic HTG (PRS ≥ 90° percentile) | ≈32–47% | 2200 | Most common cause of SHTG, influenced by environment |
| Isolated heterozygotes | 15–20% of Polygenic HTG patients | 500–1500 mg/dL | Pancreatitis in 10–15% |
| Genetically uncharacterized | ≈50% (no rare SNV/CNV, PRS not extreme) | 2300 | Likely environmental/epigenetic drivers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, C.; Ciciola, P.; D’Errico, G.; Di Taranto, M.D.; Fortunato, G.; Gross, C.; Garn, J.; Iannuzzo, G.; Di Minno, M.; Calcaterra, I. Genetic Assessment and Clinical Correlates in Severe Hypertriglyceridemia: A Systematic Review. Genes 2025, 16, 1377. https://doi.org/10.3390/genes16111377
De Luca C, Ciciola P, D’Errico G, Di Taranto MD, Fortunato G, Gross C, Garn J, Iannuzzo G, Di Minno M, Calcaterra I. Genetic Assessment and Clinical Correlates in Severe Hypertriglyceridemia: A Systematic Review. Genes. 2025; 16(11):1377. https://doi.org/10.3390/genes16111377
Chicago/Turabian StyleDe Luca, Carmine, Paola Ciciola, Guido D’Errico, Maria Donata Di Taranto, Giuliana Fortunato, Carina Gross, Jonathan Garn, Gabriella Iannuzzo, Matteo Di Minno, and Ilenia Calcaterra. 2025. "Genetic Assessment and Clinical Correlates in Severe Hypertriglyceridemia: A Systematic Review" Genes 16, no. 11: 1377. https://doi.org/10.3390/genes16111377
APA StyleDe Luca, C., Ciciola, P., D’Errico, G., Di Taranto, M. D., Fortunato, G., Gross, C., Garn, J., Iannuzzo, G., Di Minno, M., & Calcaterra, I. (2025). Genetic Assessment and Clinical Correlates in Severe Hypertriglyceridemia: A Systematic Review. Genes, 16(11), 1377. https://doi.org/10.3390/genes16111377







