1. Introduction
The cytochrome P450 21A2 (
CYP21A2) gene encodes the steroid 21-hydroxylase enzyme, which is responsible for hydroxylating progesterone and 17-hydroxyprogesterone, as well as reducing molecular oxygen, as shown in humans [
1,
2].
Near the locus of
CYP21A2, its pseudogene (
CYP21A1P) is located, and in humans, they share 98% nucleotide sequence similarity in exons and 96% similarity in introns [
3]. It is assumed that due to a very high sequence similarity, the majority of the identified variants arose from gene conversion events or non-homologous recombination [
1,
4,
5,
6]. It is also known that approx. 1441 human pseudogenes can be transcribed [
7]. In humans, the transcription of the
CYP21A2 pseudogene was confirmed using the Northern blot method [
8]. To the best of our knowledge, no transcriptomic or genomic studies have demonstrated
CYP21A1P expression in cattle; therefore, our results provide the first evidence addressing this unexplored aspect of the bovine CYP21 locus.
Molecular knowledge on bovine
CYP21A2 and
CYP21A1P is scarce. It is known that both loci, similarly to the human genome, are located within the class III region of the major histocompatibility complex [
9]. According to the NCBI Gene database, there are several transcript variants of bovine
CYP21A2 (see NCBI Gene:
CYP21A2;
https://www.ncbi.nlm.nih.gov/gene/281741, accessed on 10 February 2023). In livestock, promoter polymorphisms of
CYP21 have been investigated in relation to milk production and growth traits, although results have been inconsistent, and in some studies, the nomenclature did not clearly specify whether the variants were located in the functional gene or in the pseudogene [
10,
11,
12]. While some studies have reported
CYP21A2 expression in tissues beyond the adrenal gland—for instance, in the ovary during early pregnancy [
13]—comprehensive analyses of its expression across a broader range of tissues, along with investigations of transcript variability, remain lacking.
Mutations in
CYP21A2 are well-known causes of human autosomal recessive disorders of adrenal steroidogenesis, known as congenital adrenal hyperplasia (CAH), resulting from 21-hydroxylase deficiency [
14]. The wide phenotypic spectrum, from classic to non-classical forms, illustrates how different mutations affecting CYP21A2 activity can lead to variable clinical outcomes. In a study by Carvalho et al. [
15], it was already shown that similarity between the
CYP21A2 gene and its pseudogene (
CYP21A1P) predisposes the region to genetic recombination events, leading to the transfer of pathogenic variants from the pseudogene to the functional gene. Approximately 75% of deleterious variants in the
CYP21A2 gene result from gene conversion during mitosis, where segments of DNA are transferred from the pseudogene to the active gene. Around 20% of mutations arise due to unequal crossing over during meiosis, leading to duplications or deletions within the
CYP21A2 gene [
15]. Although this wealth of insight exists in human endocrinology, no analogous clinical or molecular evidence for
CYP21A2-linked CAH has been reported in cattle.
Usually, the CAH is associated with disorders of sex development (DSDs) in girls, and it is the most common monogenic XX DSD in humans [
16]. In contrast, individuals with the non-classical type typically develop symptoms related to androgen excess, and due to partial retention of 21-hydroxylase activity, they usually exhibit a mild and variable phenotype [
1,
14]. Studies of DSD in cattle have been mainly focused on freemartinism in heifers, associated with leukocyte XX/XY chimerism, originating from heterosexual twins [
17]. On the contrary, XX DSD in cattle was reported very rarely [
18,
19], in spite of the fact that this form of DSD is not rare in goats, pigs, horses, and dogs [
20].
CYP21A2 has not yet been studied in domestic animals affected with DSD.
Given the essential role of
CYP21A2 in steroid hormone biosynthesis, investigating its expression in cattle may yield valuable insights into adrenal and gonadal steroidogenesis in livestock. Steroid 21-hydroxylase (the CYP21A2 enzyme) mediates key steps in cortisol and aldosterone synthesis, and alterations in its function are central to CAH in humans [
21]. Comparative analyses of
CYP21A2 expression patterns across species can help elucidate conserved regulatory mechanisms in reproductive endocrinology. In farm animals, adrenal and ovarian-derived steroid hormones (e.g., cortisol and progesterone) are fundamental regulators of reproductive performance, fertility, and adaptation to stress. Thus, dysregulation of CYP21A2 activity may influence both reproductive disorders and production efficiency. Indeed, stress–endocrine interactions have been shown to markedly impact reproductive efficiency in cattle via endocrine, paracrine, and neural pathways [
22].
The aim of this study was to identify the CYP21A2 transcript variants in cow adrenal glands and ovaries, as well as to detect the potential transcription of its pseudogene (CYP21A2).
2. Materials and Methods
2.1. Ethics Statement
The samples in this project were collected during routine commercial slaughter. This case is covered by Polish law and did not require the approval of the local Bioethical Commission for Animal Care and Use in Poznan, Poland. Animals were not subjected to any experimental procedures prior to slaughter, and tissue sampling was performed exclusively from post-mortem by-products, in accordance with animal welfare regulations (including Directive 2010/63/EU).
2.2. Animals and Sample Collection
Ovarian (n = 6) and adrenal gland (n = 6) samples were collected from 12 adult Holstein-Friesian cows during routine commercial slaughter at an abattoir located in the Wielkopolska Province, Poland. Animals were euthanized using percussive stunning (headshot), which involved a single impact to the head with a captive bolt pistol, resulting in immediate loss of consciousness without the use of any chemicals. Following euthanasia, tissue samples (post-mortem by-products) were collected, rapidly frozen in liquid nitrogen, and stored at −80 °C for further RNA isolation.
2.3. RNA Extraction and cDNA Synthesis
Total RNA was isolated using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Germantown, MD, USA) from 12 cows (6 samples of ovaries and 6 samples of adrenal gland). Approximately 30 mg of tissue from each sample was homogenized in the RTL buffer provided with the kit, utilizing the TissueLyser LT (Qiagen, Germantown, MD, USA). Subsequent steps followed the manufacturer’s protocol (including the DNA-se digestion step). RNA concentration and purity were measured with a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), based on absorbance at 260/280 nm (
Supplementary Materials, Table S1).
For cDNA synthesis, roughly 1 µg of RNA per sample was reverse-transcribed using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Mannheim, Germany) in a final reaction volume of 20 µL, according to the manufacturer’s instructions. The obtained cDNA was evaluated again with the NanoDrop ND-2000 spectrophotometer and diluted with nuclease-free water.
2.4. Semi-Quantitative Real-Time PCR
cDNA served as a template for real-time PCR performed on the LightCycler
® 480 II system (Roche, Mannheim, Germany). The expression level of the
CYP21A2 gene was assessed in both ovarian and adrenal gland tissues. Primers were designed using the Primer3Plus tool (
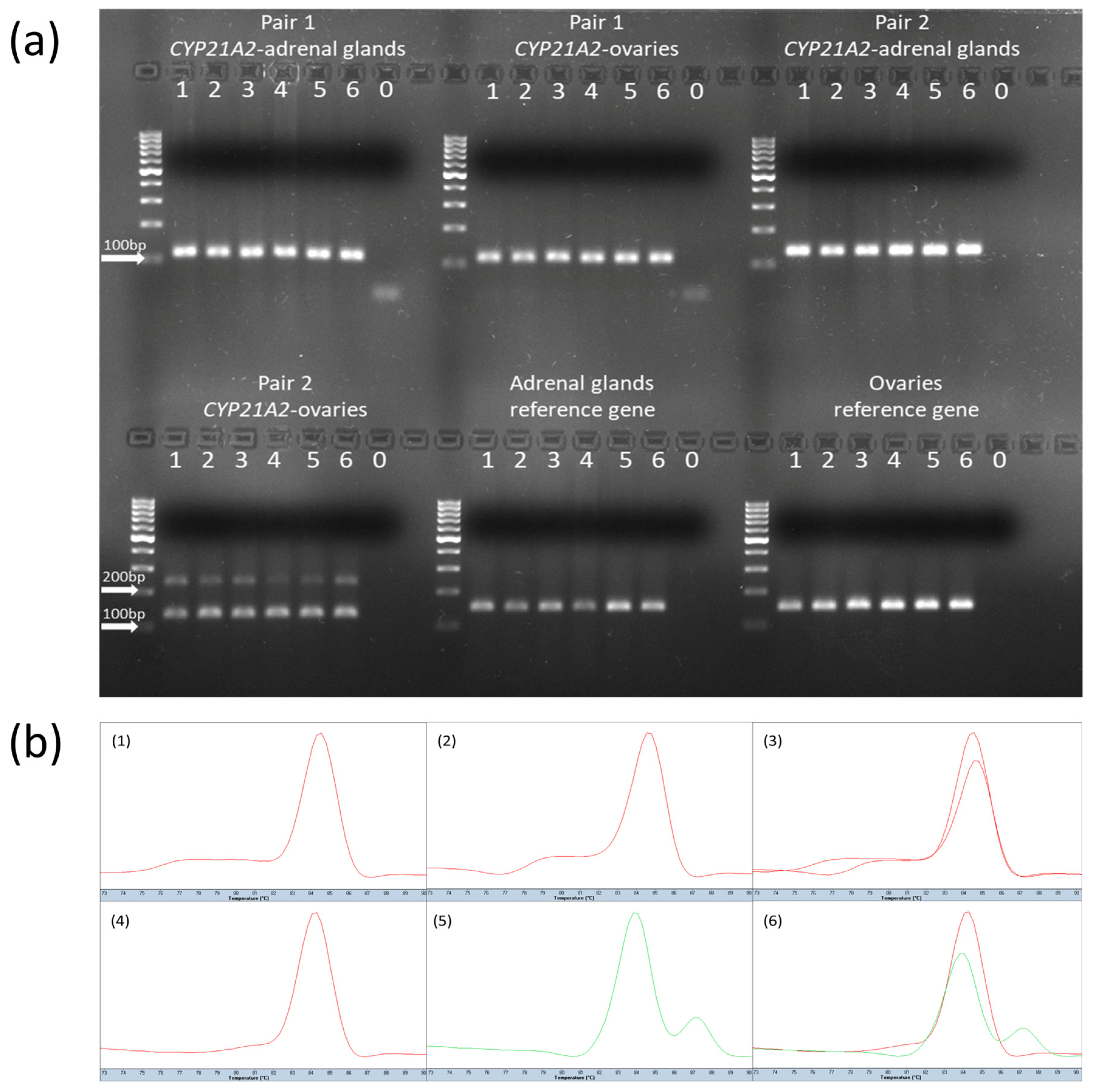
https://www.primer3plus.com, accessed on 1 March 2023). To verify the presence of specific transcript variants, the identity of semi-qPCR products was assessed by combining melting curve analysis and agarose gel electrophoresis. To distinguish between four annotated transcript variants of the
CYP21A2 gene (XM_015459900.3, XM_024983378.2, XM_024983377.2, and NM_001013596.1), two primer pairs were designed: pair 1 and pair 2 (
Supplementary Material, Table S2). Pair 1 generates PCR products of the same length for variants XM_015459900.3 and XM_024983377.2 (222 bp) and a shorter product of identical length for XM_024983378.2 and NM_001013596.1 (113 bp). Pair 2 produces PCR products of the same length for XM_015459900.3 and XM_024983378.2 (232 bp) and a shorter product of identical length for XM_024983377.2 and NM_001013596.1 (130 bp), as shown in
Figure 1. By combining results from both primer pairs, it was possible to reliably discriminate among all four transcript variants based on product size and melting temperature profiles.
All semi-qPCR reactions were conducted in duplicate, using the LightCycler® 480 SYBR Green I Master mix (Roche, Mannheim, Germany), with a total reaction volume of 10 μL per well, following the manufacturer’s instructions. The thermal cycling conditions included an initial denaturation step at 95 °C for 10 min, followed by 45 amplification cycles comprising denaturation at 95 °C for 10 s, primer annealing at 62 °C for 5 s, and extension at 72 °C for 5 s. To confirm the specificity of the amplified products, a melting curve analysis was performed after each semi-qPCR run. Next, the amplicon lengths were evaluated using 2% agarose gel electrophoresis, with visualization on the Chemi Doc MP Imagine System (Bio-Rad, Hercules, CA, USA).
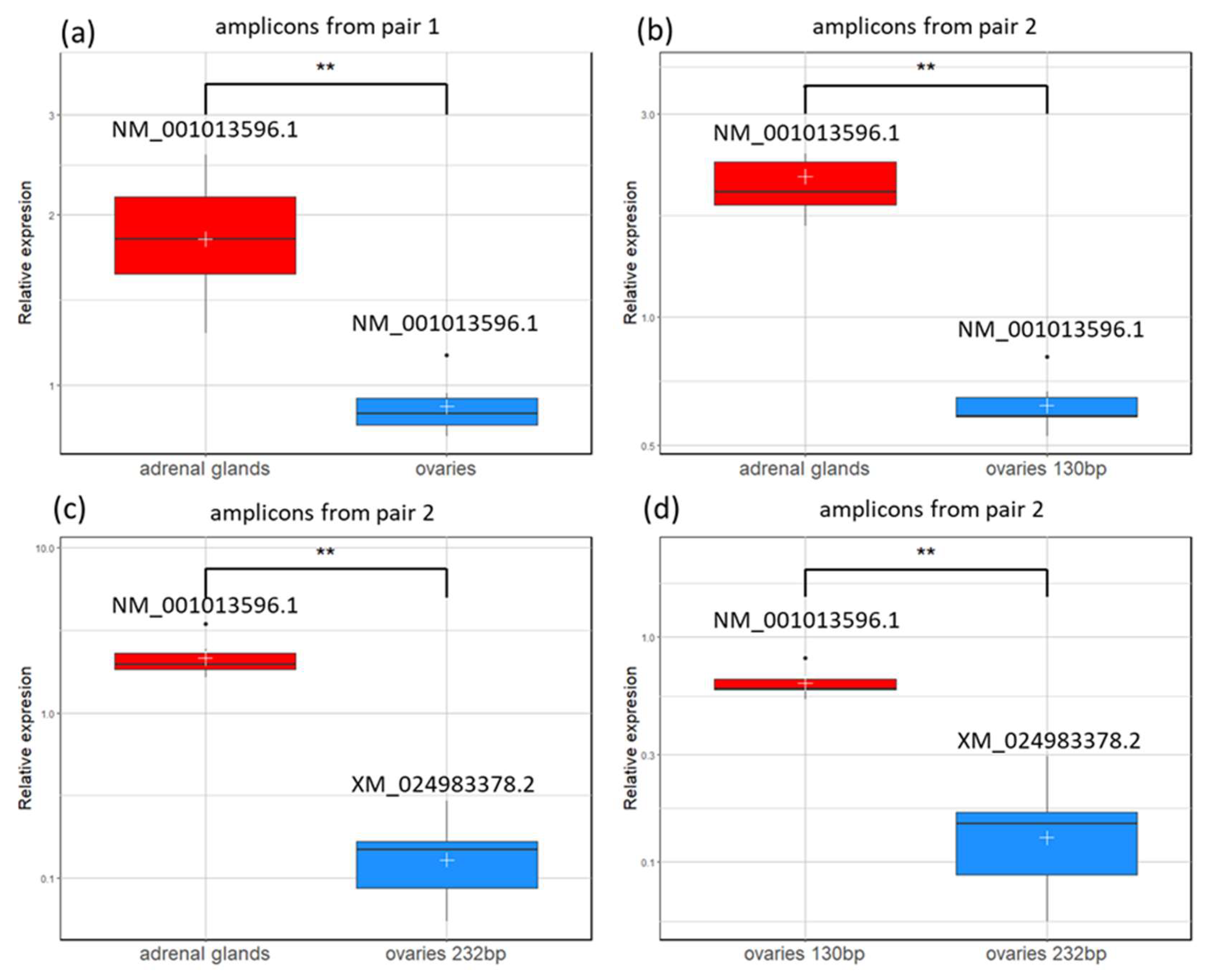
The relative semi-quantitative transcript levels were quantified based on band insensitivity measurements (Image Lab, version 6.0.1 build 34, Bio-Rad, Hercules, CA, USA) after agarose electrophoresis. The results from each
CYP21A2 transcript band were normalized to the reference
TATA-Box Binding Protein gene. The obtained mean values were statistically analyzed. The densitometry values of amplicons are presented in
Supplementary Materials, Table S3.
2.5. PCR Amplification and Gel Purification of cDNA Fragments
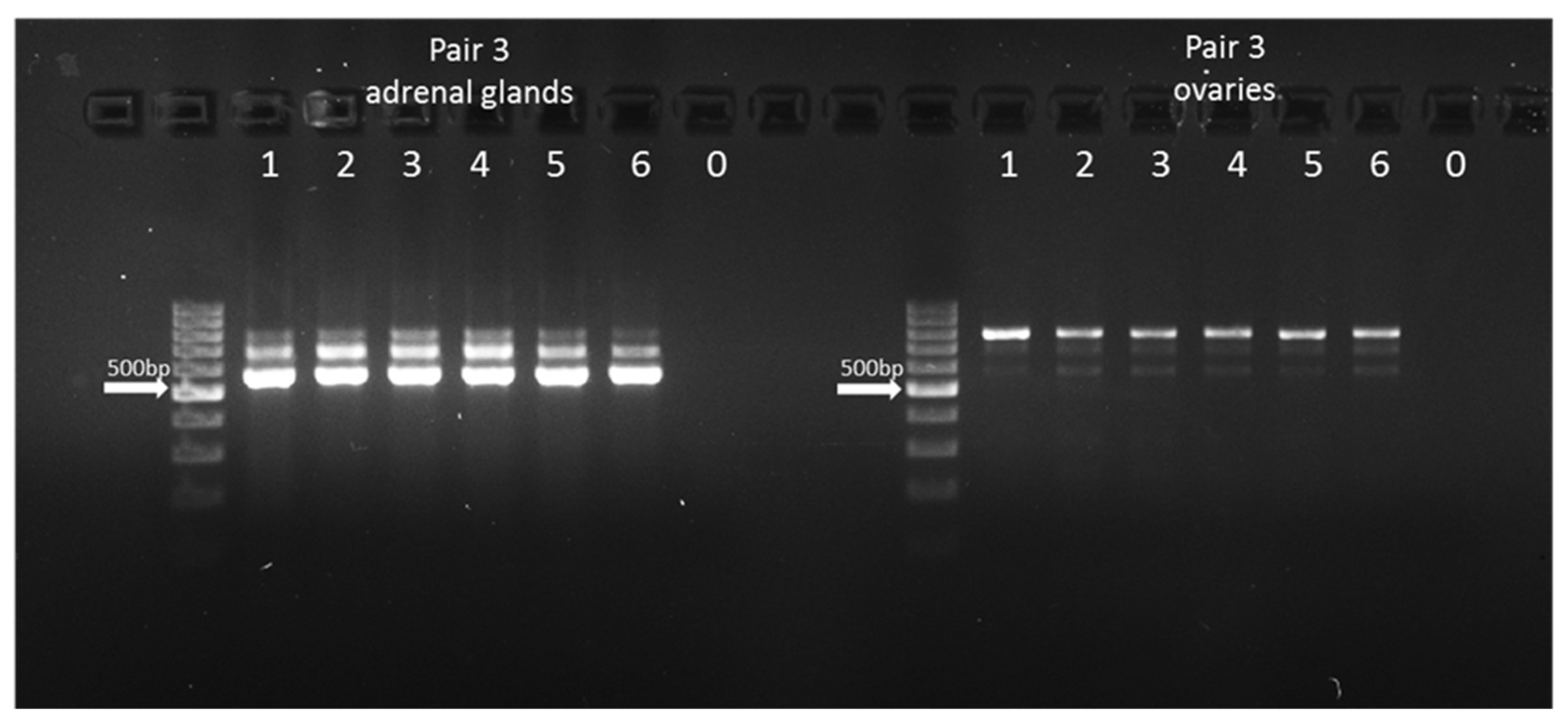
Since pairs 1 and 2 used for semi-qPCR did not distinguish between amplicons from
CYP21A2 and its pseudogene, the additional primer pair was designed to amplify a longer fragment spanning exons 1-3 of the gene and its pseudogene (
Supplementary Material, Table S2). The expected PCR product lengths were as follows: 667 bp (for XM_015459900.3 and XM_024983377.2), 559 bp (for XM_024983378.2 and NM_001013596.1), and 762 bp for the pseudogene. Polymerase chain reactions were carried out using a Bio-Rad thermocycler. Each reaction mixture (20 µL) contained 10 ng of cDNA, 10× reaction buffer B (EURx), a mix of ultrapure dNTPs (1.25 nM each, EURx), 5 nM of each primer, 1 unit of Taq DNA Polymerase (EURx, Gdansk, Poland), and nuclease-free water. The PCR program included an initial denaturation at 95 °C for 10 min, followed by 38 cycles consisting of denaturation at 95 °C for 40 s, primer annealing at 63 °C, and elongation at 72 °C for 40 s. The final extension was performed at 72 °C for 10 min, after which samples were held at 4 °C. Negative controls without the cDNA template were included.
The amplicon lengths were evaluated using 2% agarose gel electrophoresis, with visualization on the Chemi Doc MP Imagine System (Bio-Rad, Hercules, CA, USA).
2.6. Sanger Sequencing Analysis of cDNA
Individual PCR products (three separate bands) were cut out of the agarose gel and purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA, USA), followed by sequencing reactions using the BigDye Terminator v3.1 Cycle Sequencing Kit (Life Technologies, Waltham, MA, USA) according to the manufacturer’s guidelines. After sequencing amplification, products were purified through Sephadex G50 columns (Sigma, Darmstadt, Germany). Capillary electrophoresis was conducted using the Genetic Analyzer 3500 (Applied Biosystems, Waltham, MA, USA). Sequencing data were analyzed with DNASTAR software (DNASTAR, Madison, WI, USA), and the obtained sequences were aligned to reference sequences from the GenBank database: NC_037350 for CYP21A2 and XR_003032199.2 for CYP21A1P.
2.7. Statistical Analysis
Statistical analysis was carried out in the R software using the stats package [
23]. The Shapiro–Wilk test was used to test the normality of the data. Subsequently, a nonparametric two-tailed Mann–Whitney U-test was performed for results from semi-qPCR.
4. Discussion
Our results demonstrate that
CYP21A2 is actively transcribed in both bovine tissues—adrenal glands and ovaries—with a predominant presence of NM_001013596.1 in adrenal tissue and a dual expression of NM_001013596.1 and XM_024983378.2 transcripts in ovarian samples. In addition, the detection of a pseudogene-derived transcript (762 bp) suggests an additional layer of regulation, for instance, by acting as a competing endogenous RNA that modulates
CYP21A2 transcript stability, or by serving as a source of small regulatory RNAs. Ovarian expression of
CYP21A2 may have been underestimated, yet evidence indicates that
CYP21A2 is expressed in granulosa cells and in the corpus luteum (CL), highlighting its role not only as a key adrenal enzyme but also as a potentially important player in local ovarian steroid metabolism. In particular,
CYP21A2 activity may contribute to mineralocorticoid synthesis, which has been linked to follicular maturation and CL function [
24]. Moreover, decreased expression of this gene was observed in CL of cows during early pregnancy [
13]. The differences observed here in the
CYP21A2 expression levels between the adrenal gland and ovaries are in agreement with basic knowledge that its high expression in the adrenal cortex reflects the need for the production of corticosteroids, which are essential for electrolyte balance, blood pressure regulation, and stress responses. In the ovary,
CYP21A2 expression is much lower but important for local steroid metabolism [
24].
The suggestive transcriptional activity of the
CYP21A1P pseudogene is an interesting finding. While pseudogenes were long considered non-functional genome elements, recent evidence supports their regulatory potential through coding-independent mechanisms. For instance, the bovine aromatase pseudogene
CYP19P1 is expressed in the placenta and has been suggested to modulate
CYP19A1 expression [
25]. By analogy,
CYP21A1P may exert a similar modulatory effect on
CYP21A2, particularly when co-expressed in steroidogenic tissues.
In humans,
CYP21A1P shares 98% sequence identity with
CYP21A2 and is transcribed in the adrenal gland, albeit at lower levels (10–20% compared with
CYP21A2) [
8]. While such data have not yet been confirmed in cattle, the detected 762 bp amplicon exhibited pseudogene-specific features, suggesting its transcriptional activity. Moreover, the observed differences in band intensity between studied tissues indicate relatively higher amplification from
CYP21A2 gene transcripts than from its
CYP21A1P pseudogene in the adrenal gland, and the reverse situation is found in the ovaries. It should be noted, however, that the appearance of two unexpected bands (559 and 667 bp—potential transcript variants of
CYP21A2) in adrenal samples contrasts with the pattern obtained for the shorter amplicons used for the semi-quantitative approach, where only one transcript variant was identified. This discrepancy may result from higher primer specificity and amplification efficiency for shorter fragments, suggesting that in the bovine adrenal gland, only one transcript (NM_001013596.1) is predominantly expressed. As a limitation of this work, we need to point out that the observed double peaks at four specific positions may reflect the presence of mixed PCR products derived from both the gene and the pseudogene, which indicates technical difficulties with clear separation and purification after agarose gel electrophoresis. However, the presence of the longest PCR product, confirmed by the Sanger method, being sequence-specific for
CYP21A1P, suggests the transcriptional activity of this pseudogene. Nevertheless, several methodological limitations must be acknowledged. The semi-quantitative nature of the PCR applied in this study only allows for approximate comparisons of transcript abundance and does not reflect absolute expression levels. Therefore, differences in band intensity should be interpreted with caution, as they may also reflect variations in amplification efficiency or template composition. Additionally, due to the high sequence similarity between
CYP21A2 and its pseudogene
CYP21A1P, the possibility of cross-amplification cannot be excluded, highlighting technical challenges related to achieving complete separation and purification of closely related products after agarose gel electrophoresis. Despite these limitations, the longest PCR product exhibited a sequence confirmed by Sanger sequencing as
CYP21A1P-specific, providing molecular evidence that this pseudogene may be transcriptionally active in cattle.
Pseudogenes may influence their parental gene by acting as RNA decoys, modulating transcript stability, or serving as competing endogenous RNAs. In humans, pseudogene-derived mutations transferred to
CYP21A2 by gene conversion or promoter alterations can result in non-classical congenital adrenal hyperplasia (NC-CAH), with measurable effects on transcriptional efficiency [
26]. Thus, it is plausible that bovine
CYP21A1P transcription contributes to regulatory crosstalk with
CYP21A2. From an evolutionary perspective, the persistence of pseudogene transcription suggests that these elements may provide selective advantages through regulatory functions rather than protein-coding capacity. Although initially regarded as non-functional by-products of genome evolution, increasing evidence indicates that pseudogenes can contribute to gene regulatory networks in diverse ways. For example, some pseudogene transcripts have been shown to act as competing endogenous RNAs (ceRNAs), serving as decoys for microRNAs and thereby modulating the expression of their parental genes. A well-known case is the pseudogene
PTENP1, which regulates the tumor suppressor
PTEN by sequestering specific miRNAs such as miR-499-5p. This mechanism shows how pseudogene-derived transcripts, even if noncoding, may exert significant post-transcriptional regulatory effects [
27]. In cattle, if
CYP21A1P is indeed transcribed, analogous ceRNA-like functions could be considered, whereby pseudogene transcripts influence
CYP21A2 or other components of steroidogenic pathways. Such a role has been proposed for pseudogenes in other systems, for example, in breast cancer [
28]. Although speculative at this stage, such regulatory interactions could add an additional layer of complexity to the control of steroid biosynthesis and reproductive physiology in cattle. Others give rise to small interfering RNAs that participate in post-transcriptional silencing, or they may influence chromatin organization and transcriptional activity. The retention of pseudogenes in mammalian genomes is therefore not simply a reflection of neutral drift but may represent adaptive value associated with their noncoding regulatory roles [
29]. Our identification of pseudogene transcripts in the bovine tissues suggests a possible role in diversifying local steroid metabolism.