Dystrophin-Deficient Muscular Dystrophy in a Family of Shiba Inu Dogs with a Complex Deletion Encompassing DMD Exon 5
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Clinical Examinations
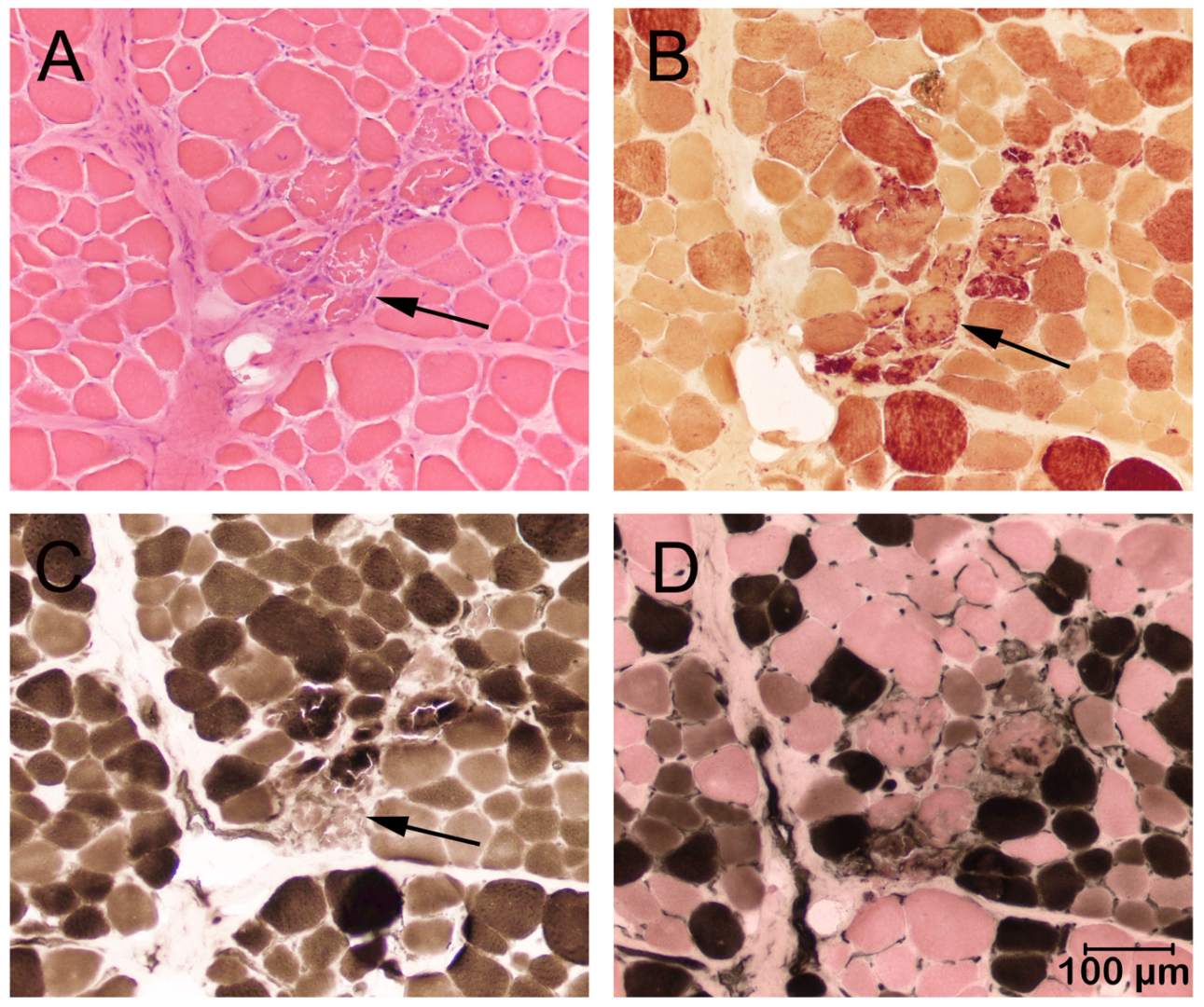
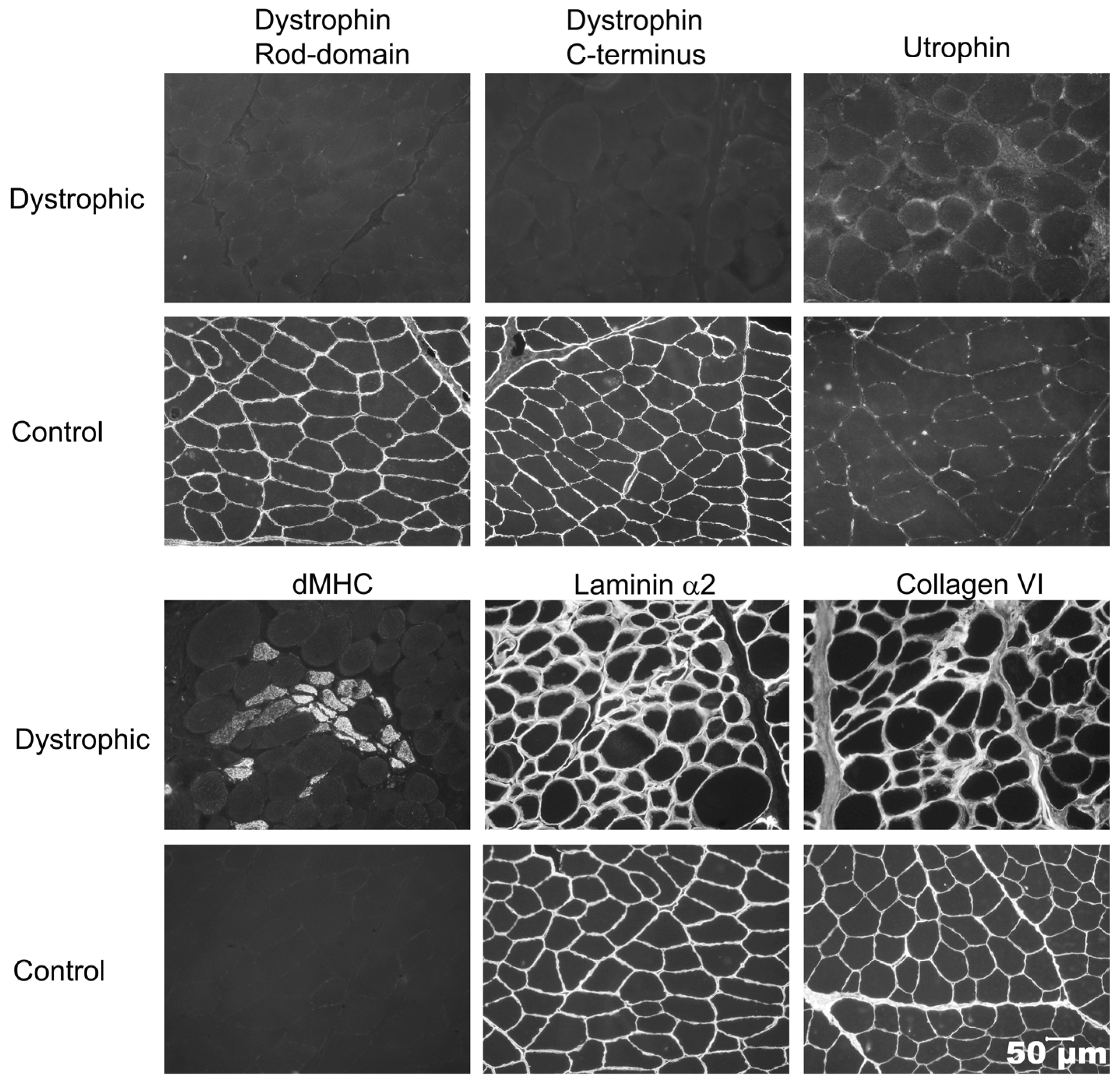
2.3. Histopathology and Immunostaining
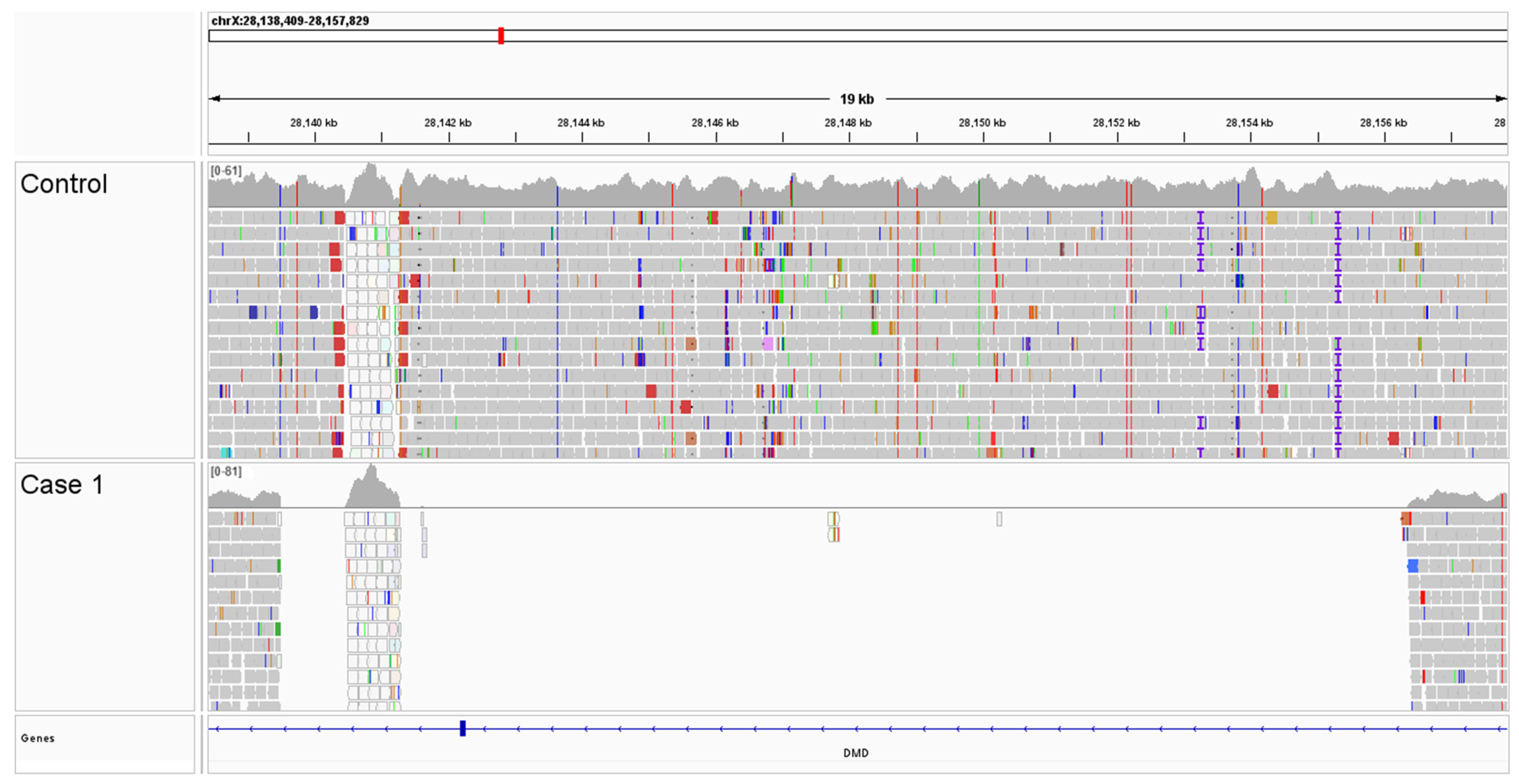
2.4. Genetic Investigations
2.5. Long-Read PCR Sequencing
3. Results
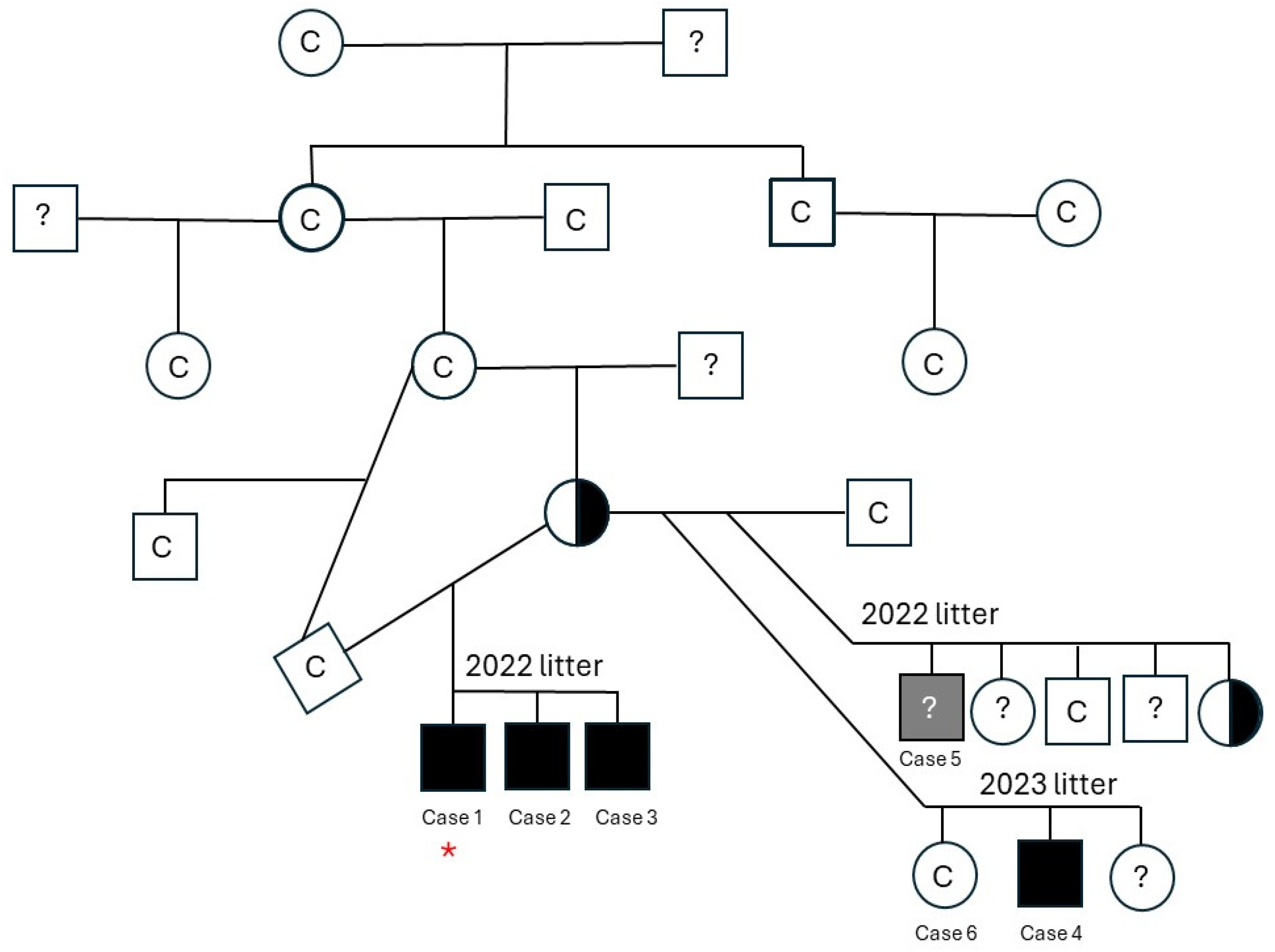
3.1. Animals/Clinical Evaluation
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
3.1.4. Case 4
3.1.5. Case 5
3.1.6. Case 6
3.2. Histopathology and Immunohistochemistry (Cases 1 and 2)
3.3. Genetic Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BMD | Becker muscular dystrophy |
| BCS | Body condition score |
| CK | Creatine kinase |
| dMHC | Developmental myosin heavy chain |
| DMD | Dystrophin deficient muscular dystrophy |
| EMG | Electromyogram |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MD | Muscular dystrophy |
| NGS | Next generation sequencing |
| WGS | Whole genome sequencing |
References
- Hoang, T.; Dowdy, R.A.E. A Review of Muscular Dystrophies. Anesth. Prog. 2024, 71, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, F.; Torelli, S.; Ferlini, A. Dystrophin and mutations: One gene, several proteins, multiple phenotypes. Lancet Neurol. 2003, 2, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Beltran, E.; Shelton, G.D.; Guo, L.T.; Dennis, R.; Sanchez-Masian, D.; Robinson, D.; De Risio, L. Dystrophin-deficient muscular dystrophy in a Norfolk terrier. J. Small Anim. Pract. 2015, 56, 351–354. [Google Scholar] [CrossRef]
- Sakai, K.; Motegi, T.; Chambers, J.K.; Uchida, K.; Nishida, H.; Shimamura, S.; Tani, H.; Shimada, T.; Furuya, M. Dystrophin-deficient muscular dystrophy in a Toy Poodle with a single base pair insertion in exon 45 of the Duchenne muscular dystrophy gene. J. Vet. Med. Sci. 2022, 84, 502–506. [Google Scholar] [CrossRef]
- Stevens, R.; Kanazono, S.; Petesch, S.; Guo, L.T.; Shelton, G.D. Dystrophin-Deficient Muscular Dystrophy in Two Male Juvenile Brittanys. J. Am. Anim. Hosp. Assoc. 2022, 58, 292–296. [Google Scholar] [CrossRef]
- Kornegay, J.N. The golden retriever model of Duchenne muscular dystrophy. Skelet. Muscle 2017, 7, 9. [Google Scholar] [CrossRef]
- Barthélémy, I.; Calmels, N.; Weiss, R.B.; Tiret, L.; Vulin, A.; Wein, N.; Peccate, C.; Drougard, C.; Beroud, C.; Deburgrave, N.; et al. X-linked muscular dystrophy in a Labrador Retriever strain: Phenotypic and molecular characterisation. Skelet. Muscle 2020, 10, 23. [Google Scholar] [CrossRef]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Shelton, G.D.; Minor, K.M.; Friedenberg, S.G.; Cullen, J.N.; Guo, L.T.; Mickelson, J.R. Current Classification of Canine Muscular Dystrophies and Identification of New Variants. Genes 2023, 14, 1557. [Google Scholar] [CrossRef]
- Shiga, T.; Okuno, S.; Uchida, K.; Chambers, J.K.; Nakayama, H. Electrophysiological and histopathological findings of muscular disease suspected as myotonic dystrophy in a Shiba dog. J. Vet. Med. Sci. 2018, 80, 480–484. [Google Scholar] [CrossRef]
- Dubowitz, V.; Sewry, C.A.; Oldfors, A. Histological and histochemical stains and reactions. In Muscle Biopsy: A Practical Approach, 5th ed.; Elsevier: St. Louis, MO, USA, 2021; pp. 14–23. [Google Scholar]
- Guo, L.T.; Moore, S.A.; Forcales, S.; Engvall, E.; Shelton, G.D. Evaluation of commercial dysferlin antibodies on canine, mouse and human skeletal muscle. Neuromuscul. Disord. 2010, 20, 820–825. [Google Scholar] [CrossRef]
- Liu, L.A.; Engvall, E. Sarcoglycan isoforms in skeletal muscle. J. Biol. Chem. 1999, 274, 38171–38176. [Google Scholar] [CrossRef] [PubMed]
- Leivo, I.; Engvall, E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc. Natl. Acad. Sci. USA 1988, 85, 1544–1548. [Google Scholar] [CrossRef]
- Hessle, H.; Engvall, E. Type VI collagen. Studies on its localization, structure and biosynthetic form with monoclonal antibodies. J. Biol. Chem. 1984, 259, 3955–3961. [Google Scholar] [CrossRef]
- Wang, C.; Wallerman, O.; Arendt, M.-L.; Sundström, E.; Karlsson, Å.; Nordin, J.; Mäkeläinen, S.; Pielberg, G.R.; Hanson, J.; Ohlsson, Å.; et al. A novel canine reference genome resolves genomic architecture and uncovers transcript complexity. Commun. Biol. 2021, 4, 185. [Google Scholar] [CrossRef]
- Meadows, J.R.; Kidd, J.M.; Wang, G.D.; Parker, H.G.; Schall, P.Z.; Bianchi, M.; Christmas, M.J.; Bougiouri, K.; Buckley, R.M.; Hitte, C.; et al. Genome sequencing of 2000 canids by the Dog10K consortium advances the understanding of demography, genome function and architecture. Genome Biol. 2023, 24, 187. [Google Scholar]
- Cullen, J.N.; Friedenberg, S.G. Whole animal genome sequencing: User-friendly, rapid, containerized pipelines for processing, variant discovery, and annotation of short-read whole genome sequencing data. G3 2023, 13, jkad117. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef]
- Toh, Z.Y.; Thandar Aung-Htut, M.; Pinniger, G.; Adams, A.M.; Krishnaswarmy, S.; Wong, B.L.; Fletcher, S.; Wilton, S.D. Deletion of Dystrophin In-Frame Exon 5 Leads to a Severe Phenotype: Guidance for Exon Skipping Strategies. PLoS ONE 2016, 11, e0145620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedenberg, S.G.; Vansteenkiste, D.; Yost, O.; Treeful, A.E.; Meurs, K.M.; Tokarz, D.A.; Olby, N.J. A de novo mutation in the EXT2 gene associated with osteochondromatosis in a litter of American Staffordshire Terriers. J. Vet. Intern. Med. 2018, 32, 986–992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shelton, G.D.; Minor, K.M.; Vieira, N.M.; Kunkel, L.M.; Friedenberg, S.G.; Cullen, J.N.; Guo, L.T.; Zatz, M.; Mickelson, J.R. Tandem duplication within the DMD gene in Labrador retrievers with a mild clinical phenotype. Neuromuscul. Disord. 2022, 32, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.N.; Spurney, C.F.; Nghiem, P.P.; Brinkmeyer-Langford, C.L.; Hoffman, E.P.; Nagaraju, K. Pharmacologic management of Duchenne muscular dystrophy: Target identification and preclinical trials. ILAR J. 2014, 55, 119–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giliberto, F.; Radic, C.P.; Luce, L.; Ferreiro, V.; de Brasi, C.; Szijan, I. Symptomatic female carriers of Duchenne muscular dystrophy (DMD): Genetic and clinical characterization. J. Neurol. Sci. 2014, 336, 36–41. [Google Scholar] [CrossRef]
- Lechner, A.; Herzig, J.J.; Kientsch, J.G.; Kohler, M.; Bloch, K.E.; Ulrich, S.; Schwarz, E.I. Cardiomyopathy as cause of death in Duchenne muscular dystrophy: A longitudinal observational study. ERJ Open Res. 2023, 9, 00176–02023. [Google Scholar] [CrossRef]
- D’Amario, D.; Arcudi, A.; Narducci, M.L.; Novelli, V.; Canonico, F.; Parodi, A.; Dell’eRa, G.; Di Francesco, M.; Laborante, R.; Borovac, J.A.; et al. Arrhythmic Risk Stratification and Sudden Cardiac Death Prevention in Duchenne Muscular Dystrophy: A Critical Appraisal. Rev. Cardiovasc. Med. 2025, 26, 27089. [Google Scholar] [CrossRef]
- Guo, L.J.; Soslow, J.H.; Bettis, A.K.; Nghiem, P.P.; Cummings, K.J.; Lenox, M.W.; Miller, M.W.; Kornegay, J.N.; Spurney, C.F. Natural History of Cardiomyopathy in Adult Dogs with Golden Retriever Muscular Dystrophy. J. Am. Heart Assoc. 2019, 8, e012443. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Awano, H.; Yamamoto, T.; Nambu, Y.; Iijima, K. Serum cardiac troponin I is a candidate biomarker for cardiomyopathy in Duchenne and Becker muscular dystrophies. Muscle Nerve 2022, 65, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Maede, Y.; Amano, Y.; Nishida, A.; Murase, T.; Sasaki, A.; Inaba, M. Hereditary high-potassium erythrocytes with high Na, K-ATPase activity in Japanese shiba dogs. Res. Vet. Sci. 1991, 50, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Segura, L.G.; Lorenz, J.D.; Weingarten, T.N.; Scavonetto, F.; Bojanić, K.; Selcen, D.; Sprung, J. Anesthesia and Duchenne or Becker muscular dystrophy: Review of 117 anesthetic exposures. Paediatr. Anesth. 2013, 23, 855–864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mcleay, L.; Hardinge, S.; Minor, K.M.; Friedenberg, S.G.; Cullen, J.N.; Guo, L.T.; Shelton, G.D. Dystrophin-Deficient Muscular Dystrophy in a Family of Shiba Inu Dogs with a Complex Deletion Encompassing DMD Exon 5. Genes 2025, 16, 1369. https://doi.org/10.3390/genes16111369
Mcleay L, Hardinge S, Minor KM, Friedenberg SG, Cullen JN, Guo LT, Shelton GD. Dystrophin-Deficient Muscular Dystrophy in a Family of Shiba Inu Dogs with a Complex Deletion Encompassing DMD Exon 5. Genes. 2025; 16(11):1369. https://doi.org/10.3390/genes16111369
Chicago/Turabian StyleMcleay, Laura, Simone Hardinge, Katie M. Minor, Steven G. Friedenberg, Jonah N. Cullen, Ling T. Guo, and G. Diane Shelton. 2025. "Dystrophin-Deficient Muscular Dystrophy in a Family of Shiba Inu Dogs with a Complex Deletion Encompassing DMD Exon 5" Genes 16, no. 11: 1369. https://doi.org/10.3390/genes16111369
APA StyleMcleay, L., Hardinge, S., Minor, K. M., Friedenberg, S. G., Cullen, J. N., Guo, L. T., & Shelton, G. D. (2025). Dystrophin-Deficient Muscular Dystrophy in a Family of Shiba Inu Dogs with a Complex Deletion Encompassing DMD Exon 5. Genes, 16(11), 1369. https://doi.org/10.3390/genes16111369




