Curing Sickle Cell Disease by Allogeneic Hematopoietic Stem Cell (HSC) Transplantation Toward In Vivo HSC Gene Therapy
Abstract
1. Introduction
2. Curing Sickle Cell Disease: Allogeneic Hematopoietic Stem Cell Transplantation or Gene Therapies, Lentiviral-Based or Nonviral CRISPR-Cas9-Based
2.1. Hematopoietic Stem Cell Transplantation
2.2. Allogeneic Hematopoietic Stem Cell Transplantation in Sickle Cell Disease
2.2.1. Risk of Developing a Secondary Malignancy After an Allogeneic Hematopoietic Stem Cell Transplant
2.3. Autologous Hematopoietic Stem Cell Transplantation Using Genetically Modified Autologous Hematopoietic Stem and Progenitor Cells, i.e., Gene Therapy in Sickle Cell Disease
2.3.1. Lentiviral-Based Gene Therapy Approved for Treating Sickle Cell Disease
2.3.2. Causation of Malignancy After Treatment of Sickle Cell Disease with the Lentiviral-Based Gene Therapy
2.4. Comparing Allogeneic Hematopoietic Stem Cell Transplantation with Autologous Hematopoietic Stem Cell Ex Vivo Gene Therapy in Sickle Cell Disease
| Allogeneic Hematopoietic Stem Cell Transplantation | Autologous Hematopoietic Stem Cell Ex Vivo Gene Therapy | |
|---|---|---|
| Source of transplanted HSPCs | Donor, preferred HLA-matched | Self (autologous HSPCs); no donor |
| Availability of donor | Only up to 30% of patients requiring a transplant have an HLA-matched donor | Not applicable |
| Genome editing of HSPCs performed | None | Yes, via Lentiviral vector or Nonviral CRISPR-Cas9-single guide RNA |
| Risks of treatment | ||
| Immunological complications | Yes | None |
| Yes | No risk |
| Yes | No risk |
| Due to myeloablative conditioning (chemotherapy) | Yes, including treatment-related mortality, infertility, and increased risk of cancer | Yes, including treatment-related mortality, infertility, and increased risk of cancer |
| Graft failure | Yes, possible | Not applicable |
| Donor-derived leukemia | Yes | No risk |
| Secondary malignancy reported | Yes; 3 cases per 1000 person-years [84] | Yes, reported in lentiviral-based therapy, not in CRISPR-Cas9-based therapy |
| Off-target toxicity risk present | Not applicable | Yes |
| Costs of therapy | Variable, up to about $0.5 million | $2.2 to $3.1 million |
| Efficacy of therapy | Event-free survival depends on patient age (better in <12 years), donor type, and conditioning [77]; overall 3-year survival 91% and 95% in CIBMTR and EBMT registries [82] | High chance (>95%) of eliminating vaso-occlusive crises and disease symptoms |
| Accessibility | Also available for ages under 12 years | Only available for ages 12 years and older with recurrent vaso-occlusive events |
| Only available in specialized centers with access to expertise in HSC transplantation | Only available in specialized centers with access to expertise in HSC transplantation | |
| Follow-up | Established treatment for decades | Extensive follow-up required for long-term evaluation of efficacy and safety |
3. Ex Vivo CRISPR-Cas9-Based Gene Therapy Approved for the Treatment of Severe Sickle Cell Disease
3.1. The Normal Hemoglobin Switch at Birth and the Protective Effect of Fetal Hemoglobin in Sickle Cell Disease
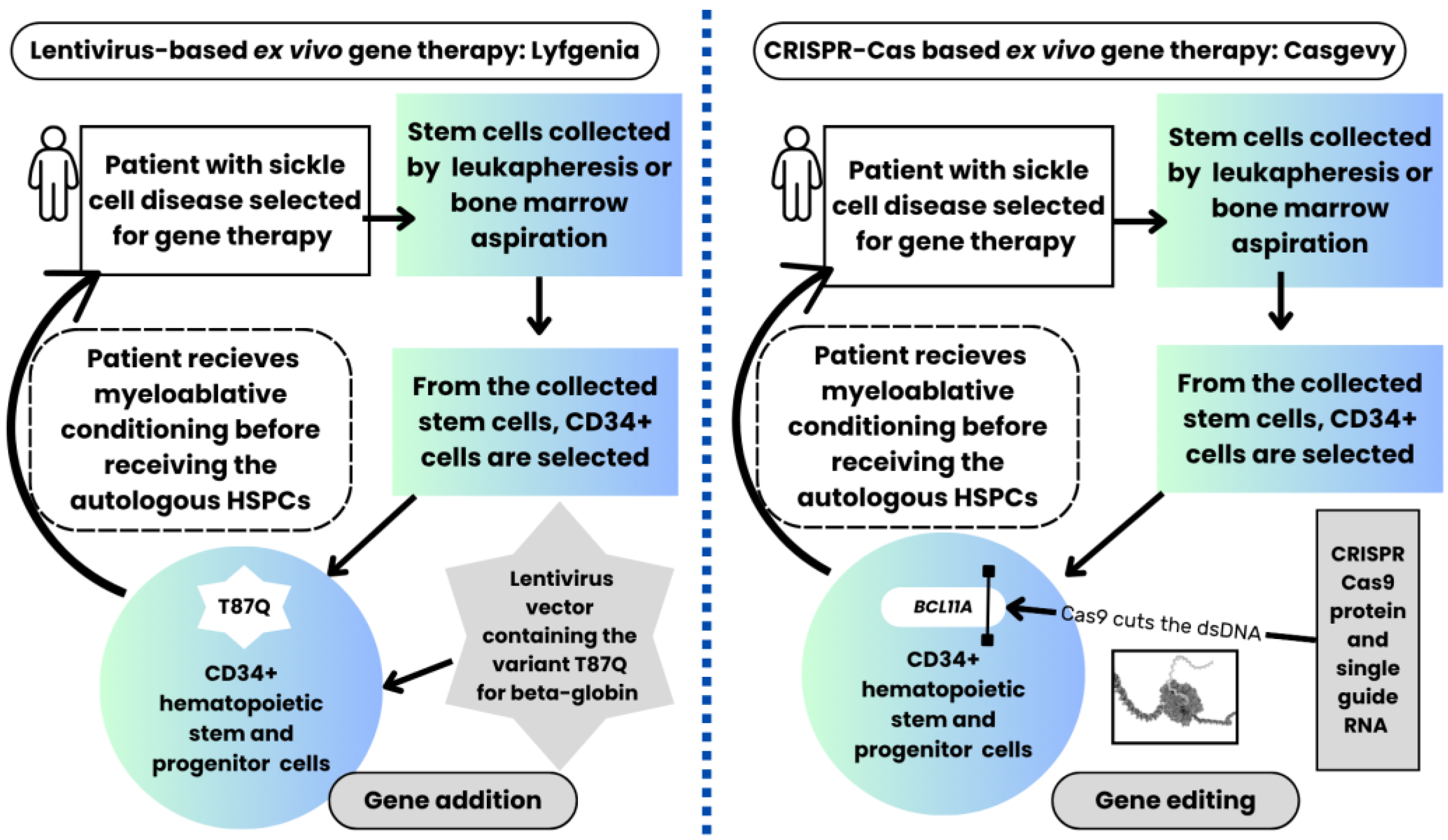
3.2. The Mechanisms of Action of the FDA-Approved Lentiviral-Based Gene Therapy Lovotibeglogene Autotemcel (Lyfgenia TM) and the CRISPR-Cas9-Based Non-Viral Gene Therapy Exagamglogene Autotemcel (Casgevy TM) for Sickle Cell Anemia
3.3. Comparing the FDA-Approved Lentiviral-Based Gene Therapy (Lyfgenia TM) with the CRISPR-Cas9-Based Non-Viral Gene Therapy (Casgevy TM) for Sickle Cell Disease, Including Potential Risks of Both Gene Therapies
| Lentiviral-Based Gene Therapy | CRISPR-Cas9-Based Gene Therapy | |
|---|---|---|
| Gene Therapy Name | Lyfgenia TM | Casgevy TM |
| Regulatory approval | 2023 | 2023 |
| Genome editing tool | Zinc finger nucleases | CRISPR-Cas9 with single guide RNA |
| Viral-based | Yes, lentiviral | Nonviral |
| Risks of gene therapy | ||
| Yes, present, including treatment-related mortality, infertility, and increased risk of cancer | Yes, present, including treatment-related mortality, infertility, and increased risk of cancer |
| Yes, acute myeloid leukemia was reported in two patients after gene therapy (see Section 2.3.2); FDA black box warning | No secondary malignancy has yet been reported |
| Yes, due to possible viral vector insertion at any off-target site | Yes, due to genome edits at off-target sites |
| Costs of therapy | $3.1 million | $2.2 million |
| Efficacy of therapy | High chance of eliminating vaso-occlusive crises and disease symptoms | High chance of eliminating vaso-occlusive crises and disease symptoms |
| Accessibility | Only available for ages 12 years and older with recurrent vaso-occlusive events | Only available for ages 12 years and older with recurrent vaso-occlusive events |
| Only available in specialized centers with access to expertise in hematopoietic stem cell transplantation | Only available in specialized centers with access to expertise in hematopoietic stem cell transplantation | |
| Follow-up | Extensive follow-up required to evaluate long-term efficacy and safety | Extensive follow-up required to evaluate long-term efficacy and safety |
4. The Cost-Effectiveness of Gene Therapy Compared with Standard-of-Care Treatment for Sickle Cell Disease
5. Examples of Other Ex Vivo Gene Therapy Approaches for the Treatment of Severe Sickle Cell Disease
5.1. Ex Vivo Lentiviral-Based Approaches Studied or in Clinical Trials for Gene Therapy in Sickle Cell Disease
5.2. Other Ex Vivo CRISPR-Cas9 Single Guide RNA-Based Approaches Studied for Gene Therapy in Sickle Cell Disease
5.2.1. Preclinical Studies to Mimic Hereditary Persistence of Fetal Hemoglobin (HPFH) or Introduce HPFH-like Mutations in CD34+ HSPCs
5.2.2. Phase 1/2 Clinical Trial for Targeted Disruption of the HBG1 and HBG2 (γ-globin) Gene Promoters
5.2.3. Converting the Sickle Mutation to a Non-Sickling Globin Variant by Base Editing
5.2.4. FDA-Approved Phase I/II Clinical Trials to Correct the Sickle Mutation in HSPCs by CRISPR-Cas9 Editing Using a Single-Stranded Oligonucleotide Donor by Electroporation or by an Adeno-Associated Virus (AAV) Vector
5.2.5. Reverting the Sickle Cell Allele to a Wild Type Allele by Prime Editing [141]
6. Why Would In Vivo Gene Therapy for Sickle Cell Disease Transform the Treatment of Patients with Sickle Cell Disease Worldwide?
6.1. Preclinical Studies Using an Adenoviral Vector System Toward In Vivo Hematopoietic Stem Cell Gene Therapy in Sickle Cell Disease
6.2. Lipid-Based Nanoparticles for In Vivo CRISPR-Based Gene Editing, Including for Hematopoietic Stem Cells
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| ABE | Adenine base editor |
| BCNU | 1,3-bis(2-chloroethyl)-1-nitroso-urea |
| Cas | CRISPR-associated |
| CI | Confidence interval |
| CIBMTR | Center for International Blood and Marrow Transplant Research |
| CRISPR | Clustered, regularly interspaced, short, palindromic repeats |
| EBMT | European Blood and Marrow Transplant |
| FDA | Food and Drug Administration |
| G-CSF | Granulocyte colony-stimulating factor |
| GVHD | Graft versus host disease |
| HbS | Hemoglobin S |
| HDAd | Helper-dependent adenovirus |
| HLA | Human leukocyte antigen |
| HSCs | Hematopoietic stem cells |
| HSCT | Hematopoietic stem cell transplant |
| HSPCs | Hematopoietic stem and progenitor cells |
| HPFH | Hereditary persistence of fetal hemoglobin |
| LNPs | Lipid-based nanoparticles |
| NBS | Newborn screening |
| O6-BG | O6-benzylguanine |
| PBMCs | Peripheral blood mononuclear cells |
| sgRNA | Single-guide RNA |
| TALE | Transcription activator-like effector |
| TALEN | Transcription activator-like effector (TALE) nuclease |
| ZFNs | Zinc finger nucleases |
References
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Hegemann, I.; Medici, M.; Birkegård, C. Systematic Literature Review Shows Gaps in Data on Global Prevalence and Birth Prevalence of Sickle Cell Disease and Sickle Cell Trait: Call for Action to Scale Up and Harmonize Data Collection. J. Clin. Med. 2023, 12, 5538. [Google Scholar] [CrossRef]
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of β-thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Benz, E.J., Jr. Introduction to the Thalassemia Syndromes: Molecular Medicine’s Index Case. Hematol. Oncol. Clin. N. Am. 2023, 37, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Tesio, N.; Bauer, D.E. Molecular Basis and Genetic Modifiers of Thalassemia. Hematol. Oncol. Clin. N. Am. 2023, 37, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. beta-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, A.; Kwiatkowski, J.L.; Aydinok, Y. Thalassaemia. Lancet 2022, 399, 2310–2324. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Pecker, L.H.; Naik, R.P. The current state of sickle cell trait: Implications for reproductive and genetic counseling. Blood 2018, 132, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Sickle Cell Disease Collaborators. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef] [PubMed]
- United States Centers for Disease Control and Prevention. Data and Statistics on Sickle Cell Disease. Available online: https://www.cdc.gov/sickle-cell/data/index.html (accessed on 14 August 2025).
- Sickle Cell Disease Association of America, Inc. Sickle Cell Disease FAQs. Available online: https://www.sicklecelldisease.org/sickle-cell-health-and-disease/faqs/ (accessed on 14 August 2025).
- Grosse, S.D.; Odame, I.; Atrash, H.K.; Amendah, D.D.; Piel, F.B.; Williams, T.N. Sickle cell disease in Africa: A neglected cause of early childhood mortality. Am. J. Prev. Med. 2011, 41 (Suppl. 4), S398–S405. [Google Scholar] [CrossRef] [PubMed]
- Scourfield, L.E.A.; Nardo-Marino, A.; Williams, T.N.; Rees, D.C. Infections in sickle cell disease. Haematologica 2025, 110, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Samuels-Reid, J.H. Pneumococcal sepsis in children with sickle cell disease. J. Natl. Med. Assoc. 1984, 76, 289–292. [Google Scholar] [PubMed]
- Battersby, A.J.; Knox-Macaulay, H.H.; Carrol, E.D. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr. Blood Cancer 2010, 55, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Brousse, V.; Piel, F.B.; Menzel, S.; Rees, D.C. Environmental determinants of severity in sickle cell disease. Haematologica 2015, 100, 1108–1116. [Google Scholar] [CrossRef]
- Tubman, V.N.; Mohandas, N.; Abrams, C.S. New ASH initiatives to improve patient care in the long-overlooked sickle cell disease. Blood 2023, 142, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Lubeck, D.; Agodoa, I.; Bhakta, N.; Danese, M.; Pappu, K.; Howard, R.; Gleeson, M.; Halperin, M.; Lanzkron, S. Estimated Life Expectancy and Income of Patients With Sickle Cell Disease Compared With Those Without Sickle Cell Disease. JAMA Netw. Open 2019, 2, e1915374. [Google Scholar] [CrossRef] [PubMed]
- Jiao, B.; Johnson, K.M.; Ramsey, S.D.; Bender, M.A.; Devine, B.; Basu, A. Long-term survival with sickle cell disease: A nationwide cohort study of Medicare and Medicaid beneficiaries. Blood Adv. 2023, 7, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- DeBaun, M.R.; Ghafuri, D.L.; Rodeghier, M.; Maitra, P.; Chaturvedi, S.; Kassim, A.; Ataga, K.I. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: A pooled analysis. Blood 2019, 133, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; San, B.J.; Yeo, Y.H.; Chan, K.H.; Shaaban, H.S.; Ezekwudo, D.E.; Idowu, M. Social Vulnerability and Sickle Cell Disease Mortality in the US. JAMA Netw. Open 2024, 7, e2440599. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Rees, D.C.; DeBaun, M.R.; Nnodu, O.; Ranque, B.; A Thompson, A.; E Ware, R.; Abboud, M.R.; Abraham, A.; E Ambrose, E.; et al. Defining global strategies to improve outcomes in sickle cell disease: A Lancet Haematology Commission. Lancet Haematol. 2023, 10, e633–e686. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. Sickle Cell Disease. Available online: https://www.afro.who.int/health-topics/sickle-cell-disease (accessed on 17 August 2025).
- Quarmyne, M.O.; Bock, F.; Lakshmanan, S.; Attell, B.K.; Snyder, A.; Boudreaux, J.; Sheth, S.; Bender, M.A.; Lal, A. Newborn Screening for Sickle Cell Disease and Thalassemia. JAMA Health Forum. 2025, 6, e250064. [Google Scholar] [CrossRef] [PubMed]
- Lobitz, S.; Telfer, P.; Cela, E.; Allaf, B.; Angastiniotis, M.; Backman Johansson, C.; Badens, C.; Bento, C.; Bouva, M.J.; Canatan, D.; et al. with the endorsement of EuroBloodNet, the European Reference Network in Rare Haematological Diseases. Newborn screening for sickle cell disease in Europe: Recommendations from a Pan-European Consensus Conference. Br. J. Haematol. 2018, 183, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Nnodu, O.E.; Okeke, C.O.; Isa, H.A. Newborn screening initiatives for sickle cell disease in Africa. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Okeke, C.O.; Okeke, C.; Asala, S.; Ofakunrin, A.O.D.; Ufelle, S.; Nnodu, O.E. Sustainability of newborn screening for sickle cell disease in resource-poor countries: A systematic review. PLoS ONE 2024, 19, e0305110. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Kaple, M.; Ambad, R.S.; Dhok, A.; Anjankar, A. The Neonatal Screening for Sickle Cell Disease, Thalassemia, and G6PD Deficiency in Central India. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 4), S4026–S4029. [Google Scholar] [CrossRef] [PubMed]
- Surve, S.; Thakor, M.; Madkaikar, M.; Kaur, H.; Desai, S.; Shanmugam, R.; Mohanty, S.S.; Pandey, A.; Salomi Kerketta, A.; Dave, K.; et al. Protocol for a Multicentric Cohort Study on Neonatal Screening and Early Interventions for Sickle Cell Disease Among High-Prevalence States of India. Diagnostics 2025, 15, 730. [Google Scholar] [CrossRef] [PubMed]
- El-Haj, N.; Hoppe, C.C. Newborn Screening for SCD in the USA and Canada. Int. J. Neonatal Screen. 2018, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; Martinez, R.M. (Eds.) Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action; National Academies Press (US): Washington, DC, USA, 2020. [Google Scholar] [CrossRef] [PubMed]
- Galadanci, N.; Phillips, S.; Schlenz, A.; Ivankova, N.; Kanter, J. Current Methods of Newborn Screening Follow-Up for Sickle Cell Disease Are Highly Variable and without Quality Assurance: Results from the ENHANCE Study. Int. J. Neonatal Screen. 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- González de Aledo-Castillo, J.M.; Argudo-Ramírez, A.; Beneitez-Pastor, D.; Collado-Gimbert, A.; Almazán Castro, F.; Roig-Bosch, S.; Andrés-Masó, A.; Ruiz-Llobet, A.; Pedrals-Portabella, G.; Medina-Santamaria, D.; et al. Newborn Screening for Sickle Cell Disease in Catalonia between 2015-Epidemiology and Impact on Clinical Events. Int. J. Neonatal Screen. 2024, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Petigas, L.; Seck, N.; Doupa, D.; Diagne, I.; Roth-Kleiner, M. Findings supporting neonatal screening for sickle cell disease: An observational study in Senegal. Front. Pediatr. 2025, 13, 1578570. [Google Scholar] [CrossRef] [PubMed]
- Kazadi, C.; Ducruet, T.; Forté, S.; Robitaille, N.; Pastore, Y. Positive impacts of universal newborn screening on the outcome of children with sickle cell disease in the province of Quebec: A retrospective cohort study. EJHaem 2024, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, P.L.; Fasipe, T.A.; Wun, T. Sickle Cell Disease: A Review. JAMA 2022, 328, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yahouédéhou, S.C.M.A.; Adorno, E.V.; da Guarda, C.C.; Ndidi, U.S.; Carvalho, S.P.; Santiago, R.P.; Aleluia, M.M.; de Oliveira, R.M.; Gonçalves, M.S. Hydroxyurea in the management of sickle cell disease: Pharmacogenomics and enzymatic metabolism. Pharmacogenom. J. 2018, 18, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.; Kraft, W.K.; Miller, R. Hydroxyurea in the sickle cell disease modern era. Expert. Rev. Clin. Pharmacol. 2024, 17, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, D.; Dufu, K.; Patel, M.P.; Chuang, C.; Li, Z.; Xu, Q.; Silva-Garcia, A.; Zhou, C.; Hutchaleelaha, A.; Patskovska, L.; et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br. J. Haematol. 2016, 175, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Achebe, M.M.; Alkindi, S.; Brown, R.C.; et al. HOPE Trial Investigators. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Sickle Cell Disease Association of America, Inc. (SCDAA). Medical and Research Advisory Committee (MARAC) Statement: Pfizer’s Voxelotor (Oxbryta®) Withdrawal. 2024. Available online: https://www.sicklecelldisease.org/2024/09/28/marac-statement-oxbryta/ (accessed on 15 September 2025).
- Gupta, K.; Krishnamurti, L.; Jain, D. Sickle cell disease in India: The journey and hope for the future. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.; McGann, P.T. Hydroxyurea for children with sickle cell disease in sub-Saharan Africa: A summary of the evidence, opportunities, and challenges. Pharmacotherapy 2023, 43, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Keza, G.K.; Diallo, D.A.; Diagne, I.; de Montalembert, M.; Corbasson, A.; Bernaudin, F.; Ranque, B.; Coulibaly, B.; Frederic, G.; Diop, S.; et al. Availability and Cost of Basic Drugs for Sickle Cell Disease in 13 African Countries. Blood 2023, 142 (Suppl. 1). [Google Scholar] [CrossRef]
- Tshilolo, L.; Tomlinson, G.; Williams, T.N.; Santos, B.; Olupot-Olupot, P.; Lane, A.; Aygun, B.; Stuber, S.E.; Latham, T.S.; McGann, P.T.; et al. Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa. N. Engl. J. Med. 2019, 380, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Gray, D.; Lomova, A.; Kohn, D.B. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell 2017, 21, 574–590. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Thrasher, A.J.; Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2021, 22, 216–234. [Google Scholar] [CrossRef] [PubMed]
- American Society of Gene Plus Cell Therapy Patient Education. Gene Therapy Approaches. Available online: https://patienteducation.asgct.org/gene-therapy-101/gene-therapy-approaches (accessed on 28 August 2025).
- Kohn, D.B. Gene therapy for blood diseases. Curr. Opin. Biotechnol. 2019, 60, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Move over ZFNs. Nat. Biotechnol. 2011, 29, 681–684. [CrossRef]
- Mullard, A. Gene-editing pipeline takes off. Nat. Rev. Drug Discov. 2020, 19, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Kansal, R. The CRISPR-Cas System and Clinical Applications of CRISPR-Based Gene Editing in Hematology with a Focus on Inherited Germline Predisposition to Hematologic Malignancies. Genes 2024, 15, 863. [Google Scholar] [CrossRef] [PubMed]
- Shamshirgaran, Y.; Liu, J.; Sumer, H.; Verma, P.J.; Taheri-Ghahfarokhi, A. Tools for Efficient Genome Editing; ZFN, TALEN, and CRISPR. Methods Mol. Biol. 2022, 2495, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Doudna, J.A. CRISPR technology: A decade of genome editing is only the beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. Base editors hit the clinic. Nat. Rev. Drug Discov. 2022, 21, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. Lentiviral vector gene therapies come of age with two FDA approvals. Nat. Rev. Drug Discov. 2022, 21, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Thompson, A.A.; Kwiatkowski, J.L.; Porter, J.B.; Thrasher, A.J.; Hongeng, S.; Sauer, M.G.; Thuret, I.; Lal, A.; Algeri, M.; et al. Betibeglogene Autotemcel Gene Therapy for Non-β0/β0 Genotype β-Thalassemia. N. Engl. J. Med. 2022, 386, 415–427. [Google Scholar] [CrossRef] [PubMed]
- MHRA Authorises World-First Gene Therapy that Aims to Cure Sickle-Cell Disease and Transfusion-Dependent β-Thalassemia. The UK Medicines and Healthcare Products Regulatory Agency Press Release. 2023. Available online: https://www.gov.uk/government/news/mhra-authorises-world-first-gene-therapy-that-aims-to-cure-sickle-cell-disease-and-transfusion-dependent-thalassemia (accessed on 15 September 2025).
- FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease. US Food and Drug Administration News Release. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapies-treat-patients-sickle-cell-disease (accessed on 15 September 2025).
- Sureda, A.; Bader, P.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Dufour, C.; Falkenburg, J.H.; Farge-Bancel, D.; Gennery, A.; Kröger, N.; et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2015. Bone Marrow Transplant. 2015, 50, 1037–1056. [Google Scholar] [CrossRef] [PubMed]
- Balassa, K.; Danby, R.; Rocha, V. Haematopoietic stem cell transplants: Principles and indications. Br. J. Hosp. Med. 2019, 80, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.A.; Sánchez-Ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; de la Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Liem, R.I.; Bernaudin, F.; Bolaños-Meade, J.; Fitzhugh, C.D.; Hankins, J.S.; Murad, M.H.; Panepinto, J.A.; Rondelli, D.; Shenoy, S.; et al. American Society of Hematology 2021 guidelines for sickle cell disease: Stem cell transplantation. Blood Adv. 2021, 5, 3668–3689. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E.; Cappelli, B.; Bernaudin, F.; Labopin, M.; Volt, F.; Carreras, J.; Pinto Simões, B.; Ferster, A.; Dupont, S.; de la Fuente, J.; et al. Eurocord, the Pediatric Working Party of the European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research. Sickle cell disease: An international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood 2017, 129, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.M.; Fitzhugh, C.D.; Weitzel, R.P.; Link, M.E.; Coles, W.A.; Zhao, X.; Rodgers, G.P.; Powell, J.D.; Tisdale, J.F. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA 2014, 312, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.L.; Oh, A.L.; Patel, P.R.; Jalundhwala, Y.; Sweiss, K.; Koshy, M.; Campbell-Lee, S.; Gowhari, M.; Hassan, J.; Peace, D.; et al. Nonmyeloablative Stem Cell Transplantation with Alemtuzumab/Low-Dose Irradiation to Cure and Improve the Quality of Life of Adults with Sickle Cell Disease. Biol. Blood Marrow Transplant. 2016, 22, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Guilcher, G.M.T.; Truong, T.H.; Saraf, S.L.; Joseph, J.J.; Rondelli, D.; Hsieh, M.M. Curative therapies: Allogeneic hematopoietic cell transplantation from matched related donors using myeloablative, reduced intensity, and nonmyeloablative conditioning in sickle cell disease. Semin. Hematol. 2018, 55, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Inam, Z.; Jeffries, N.; Link, M.; Coles, W.; Pollack, P.; Luckett, C.; Phang, O.; Harvey, E.; Martin, T.; Farrey, T.; et al. Two Nonmyeloablative HLA-Matched Related Donor Allogeneic Hematopoietic Cell Transplantation Regimens in Patients with Severe Sickle Cell Disease. Transplant. Cell. Ther. 2025, 31, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, B.; Volt, F.; Tozatto-Maio, K.; Scigliuolo, G.M.; Ferster, A.; Dupont, S.; Simões, B.P.; Al-Seraihy, A.; Aljurf, M.D.; Almohareb, F.; et al. Risk factors and outcomes according to age at transplantation with an HLA-identical sibling for sickle cell disease. Haematologica 2019, 104, e543–e546. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.; Brazauskas, R.; Walters, M.C.; Bernaudin, F.; Bo-Subait, K.; Fitzhugh, C.D.; Hankins, J.S.; Kanter, J.; Meerpohl, J.J.; Bolaños-Meade, J.; et al. Effect of donor type and conditioning regimen intensity on allogeneic transplantation outcomes in patients with sickle cell disease: A retrospective multicentre, cohort study. Lancet Haematol. 2019, 6, e585–e596. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, W.C.; Heller, S.; Pearle, P.R.; Hackney, E.; Vichinsky, E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am. J. Pediatr. Hematol. Oncol. 1994, 16, 27–29. [Google Scholar] [PubMed]
- New York State Department of Health. The Need for Blood Stem Cell Donors. Available online: https://www.health.ny.gov/professionals/patients/donation/bone_marrow/ (accessed on 20 October 2025).
- National Marrow Donor Program. Available online: https://bethematch.org/transplant-basics/how-blood-stem-cell-transplants-work/how-does-a-patients-ethnic-background-affect-matching/ (accessed on 20 October 2025).
- Walters, M.C.; De Castro, L.M.; Sullivan, K.M.; Krishnamurti, L.; Kamani, N.; Bredeson, C.; Neuberg, D.; Hassell, K.L.; Farnia, S.; Campbell, A.; et al. Indications and Results of HLA-Identical Sibling Hematopoietic Cell Transplantation for Sickle Cell Disease. Biol. Blood Marrow Transplant. 2016, 22, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, E. Allogeneic transplantation strategies including haploidentical transplantation in sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Kassim, A.A.; Walters, M.C.; Eapen, M.; Smith, M.; Logan, B.R.; Solh, M.; McKinney, C.; Nieder, M.; Ross, M.; Kent, M.; et al. Haploidentical Bone Marrow Transplantation for Sickle Cell Disease. NEJM Evid. 2025, 4, EVIDoa2400192. [Google Scholar] [CrossRef] [PubMed]
- Eapen, M.; Brazauskas, R.; Williams, D.A.; Walters, M.C.; Martin, A.S.; Jacobs, B.L.; Antin, J.H.; Bona, K.; Chaudhury, S.; Coleman-Cowger, V.H.; et al. Secondary Neoplasms After Hematopoietic Cell Transplant for Sickle Cell Disease. J. Clin. Oncol. 2023, 41, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the intensity of conditioning regimens: Working definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Lawal, R.A.; Mukherjee, D.; Limerick, E.M.; Coles, W.; Hsieh, M.M.; Dillon, L.W.; Hourigan, C.S.; Fitzhugh, C.D. Increased incidence of hematologic malignancies in SCD after HCT in adults with graft failure and mixed chimerism. Blood 2022, 140, 2514–2518. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana-Calvo, M.; Payen, E.; Negre, O.; Wang, G.; Hehir, K.; Fusil, F.; Down, J.; Denaro, M.; Brady, T.; Westerman, K.; et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 2010, 467, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Ribeil, J.-A.; Hacein-Bey-Abina, S.; Payen, E.; Magnani, A.; Semeraro, M.; Magrin, E.; Caccavelli, L.; Neven, B.; Bourget, P.; El Nemer, W.; et al. Gene Therapy in a Patient with Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Walters, M.C.; Krishnamurti, L.; Mapara, M.Y.; Kwiatkowski, J.L.; Rifkin-Zenenberg, S.; Aygun, B.; Kasow, K.A.; Pierciey, F.J.; Bonner, M.; et al. Biologic and Clinical Efficacy of LentiGlobin for Sickle Cell Disease. N. Engl. J. Med. 2022, 386, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.M.; Bonner, M.; Pierciey, F.J.; Uchida, N.; Rottman, J.; Demopoulos, L.; Schmidt, M.; Kanter, J.; Walters, M.C.; Thompson, A.A.; et al. Myelodysplastic syndrome unrelated to lentiviral vector in a patient treated with gene therapy for sickle cell disease. Blood Adv. 2020, 4, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Tisdale, J.; Schmidt, M.; Kanter, J.; Jaroscak, J.; Whitney, D.; Bitter, H.; Gregory, P.D.; Parsons, G.; Foos, M.; et al. Acute Myeloid Leukemia Case after Gene Therapy for Sickle Cell Disease. N. Engl. J. Med. 2022, 386, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Thompson, A.A.; Pierciey, F.J.; Hsieh, M.; Uchida, N.; Leboulch, P.; Schmidt, M.; Bonner, M.; Guo, R.; Miller, A.; et al. Lovo-cel gene therapy for sickle cell disease: Treatment process evolution and outcomes in the initial groups of the HGB-206 study. Am. J. Hematol. 2023, 98, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Seminog, O.O.; Ogunlaja, O.I.; Yeates, D.; Goldacre, M.J. Risk of individual malignant neoplasms in patients with sickle cell disease: English national record linkage study. J. R. Soc. Med. 2016, 109, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Brunson, A.; Keegan, T.H.M.; Bang, H.; Mahajan, A.; Paulukonis, S.; Wun, T. Increased risk of leukemia among sickle cell disease patients in California. Blood 2017, 130, 1597–1599. [Google Scholar] [CrossRef] [PubMed]
- Liggett, L.A.; Cato, L.D.; Weinstock, J.S.; Zhang, Y.; Nouraie, S.M.; Gladwin, M.T.; Garrett, M.E.; Ashley-Koch, A.; Telen, M.J.; Custer, B.; et al. Clonal hematopoiesis in sickle cell disease. J. Clin. Investig. 2022, 132, e156060. [Google Scholar] [CrossRef] [PubMed]
- Pincez, T.; Lee, S.S.K.; Ilboudo, Y.; Preuss, M.; Pham Hung d’Alexandry d’Orengiani, A.L.; Bartolucci, P.; Galactéros, F.; Joly, P.; Bauer, D.E.; Loos, R.J.F.; et al. Clonal hematopoiesis in sickle cell disease. Blood 2021, 138, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Rotin, L.E.; Viswabandya, A.; Kumar, R.; Patriquin, C.J.; Kuo, K.H.M. A systematic review comparing allogeneic hematopoietic stem cell transplant to gene therapy in sickle cell disease. Hematology 2023, 28, 2163357. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Long Term Follow-Up After Administration of Human Gene Therapy Products: Guidance for Industry. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-administration-human-gene-therapy-products (accessed on 15 September 2025).
- Frangoul, H.; Locatelli, F.; Sharma, A.; Bhatia, M.; Mapara, M.; Molinari, L.; Wall, D.; Liem, R.I.; Telfer, P.; Shah, A.J.; et al. Exagamglogene Autotemcel for Severe Sickle Cell Disease. N. Engl. J. Med. 2024, 390, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Stein, R. Sickle Cell Patient’s Success with Gene Editing Raises Hopes and Questions. Available online: https://www.npr.org/sections/health-shots/2023/03/16/1163104822/crispr-gene-editing-sickle-cell-success-cost-ethics (accessed on 20 October 2025).
- Menzel, S.; Garner, C.; Gut, I.; Matsuda, F.; Yamaguchi, M.; Heath, S.; Foglio, M.; Zelenika, D.; Boland, A.; Rooks, H.; et al. A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007, 39, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Keller, J.R.; Ortiz, M.; Tessarollo, L.; Rachel, R.A.; Nakamura, T.; Jenkins, N.A.; Copeland, N.G. Bcl11a is essential for normal lymphoid development. Nat. Immunol. 2003, 4, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, R.; Teichert, K.; Montbleau, K.E.; Verboon, J.M.; Voit, R.A.; Sankaran, V.G. Pathogenic BCL11A variants provide insights into the mechanisms of human fetal hemoglobin silencing. PLoS Genet. 2021, 17, e1009835. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Menne, T.F.; Xu, J.; Akie, T.E.; Lettre, G.; Van Handel, B.; Mikkola, H.K.A.; Hirschhorn, J.N.; Cantor, A.B.; Orkin, S.H. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008, 322, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, C.; Sankaran, V.G.; Shao, Z.; Esrick, E.B.; Chong, B.G.; Ippolito, G.C.; Fujiwara, Y.; Ebert, B.L.; Tucker, P.W.; et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 2011, 334, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell 2018, 173, 430–442.e17. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.E.; Kamran, S.C.; Lessard, S.; Xu, J.; Fujiwara, Y.; Lin, C.; Shao, Z.; Canver, M.C.; Smith, E.C.; Pinello, L.; et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 2013, 342, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Canver, M.C.; Smith, E.C.; Sher, F.; Pinello, L.; Sanjana, N.E.; Shalem, O.; Chen, D.D.; Schupp, P.G.; Vinjamur, D.S.; Garcia, S.P.; et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 2015, 527, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Luc, S.; Croney, D.M.; Woodworth, M.B.; Greig, L.C.; Fujiwara, Y.; Nguyen, M.; Sher, F.; Macklis, J.D.; Bauer, D.E.; et al. Strict in vivo specificity of the Bcl11a erythroid enhancer. Blood 2016, 128, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Smith, S.E.; Sullivan, T.; Chen, K.; Zhou, Q.; West, J.A.; Liu, M.; Liu, Y.; Vieira, B.F.; Sun, C.; et al. Long-Term Engraftment and Fetal Globin Induction upon BCL11A Gene Editing in Bone-Marrow-Derived CD34+ Hematopoietic Stem and Progenitor Cells. Mol. Ther. Methods Clin. Dev. 2017, 4, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, J.; Roscoe, B.P.; Liu, P.; Yao, Q.; Lazzarotto, C.R.; Clement, K.; Cole, M.A.; Luk, K.; Baricordi, C.; et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat. Med. 2019, 25, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.R. “Packaging” of fetal hemoglobin in sickle cell anemia. Blood 2014, 123, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Biopharma Dive. Pricey New Gene Therapies for Sickle Cell Pose Access Test. 2023. Available online: https://www.biopharmadive.com/news/crispr-sickle-cell-price-millions-gene-therapy-vertex-bluebird/702066/ (accessed on 15 September 2025).
- Cohen, J. A cut above: Pair that developed CRISPR earns historic award. Science 2020, 370, 271–272. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Summary Basis for Regulatory Action. 2024. Available online: https://www.fda.gov/media/175842/download?attachment (accessed on 15 September 2025).
- The National Human Genome Research Institute. Sickle Cell Disease Gene Therapy FAQ. Available online: https://www.genome.gov/sites/default/files/media/files/2022-09/Sickle_cell_disease_gene_therapy_FAQ_0.pdf (accessed on 18 October 2025).
- Gonzalez Sepulveda, J.M.; Yang, J.C.; Reed, S.D.; Lee, T.H.; Ng, X.; Stothers, S.; Irony, T.; Ho, M.; Rothman, J.A.; Badawy, S.; et al. Preferences for potential benefits and risks for gene therapy in the treatment of sickle cell disease. Blood Adv. 2023, 7, 7371–7381. [Google Scholar] [CrossRef] [PubMed]
- Individuals with Severe Sickle Cell Disease Express High Risk Tolerance for Gene Therapies. American Society of Hematology Press Release. 2023. Available online: https://www.hematology.org/newsroom/press-releases/2023/individuals-with-severe-sickle-cell-disease-express-high-risk-tolerance-for-gene-therapies (accessed on 18 October 2025).
- Johnson, L.M.; Sharma, A.; Carroll, Y.; Goodson, D.; Mandrell, B.N.; Gattuso, J.; Young, A.; Boggs, J.; Wilfond, B.S.; Unguru, Y. Listening to patients and parents with sickle cell disease: The totality of gene therapy risks may outweigh the perceived benefits. Blood Adv. 2024, 8, 5723–5724. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.; Zappala, Z.; Fine, R.S.; Majarian, T.D.; Sripakdeevong, P.; Altshuler, D. Specificity of CRISPR-Cas9 Editing in Exagamglogene Autotemcel. N. Engl. J. Med. 2024, 390, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Herring, W.L.; Gallagher, M.E.; Shah, N.; Morse, K.C.; Mladsi, D.; Dong, O.M.; Chawla, A.; Leiding, J.W.; Zhang, L.; Paramore, C.; et al. Cost-Effectiveness of Lovotibeglogene Autotemcel (Lovo-Cel) Gene Therapy for Patients with Sickle Cell Disease and Recurrent Vaso-Occlusive Events in the United States. Pharmacoeconomics 2024, 42, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Winn, A.N.; Johnson, K.M.; Jiao, B.; Devine, B.; Hankins, J.S.; Arnold, S.D.; Bender, M.A.; Ramsey, S.D. Gene Therapy Versus Common Care for Eligible Individuals With Sickle Cell Disease in the United States: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2024, 177, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Esrick, E.B.; Lehmann, L.E.; Biffi, A.; Achebe, M.; Brendel, C.; Ciuculescu, M.F.; Daley, H.; MacKinnon, B.; Morris, E.; Federico, A.; et al. Post-Transcriptional Genetic Silencing of BCL11A to Treat Sickle Cell Disease. N. Engl. J. Med. 2021, 384, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Psatha, N.; Reik, A.; Phelps, S.; Zhou, Y.; Dalas, D.; Yannaki, E.; Levasseur, D.N.; Urnov, F.D.; Holmes, M.C.; Papayannopoulou, T. Disruption of the BCL11A Erythroid Enhancer Reactivates Fetal Hemoglobin in Erythroid Cells of Patients with β-Thalassemia Major. Mol. Ther. Methods Clin. Dev. 2018, 10, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lessard, S.; Rimmelé, P.; Ling, H.; Moran, K.; Vieira, B.; Lin, Y.D.; Rajani, G.M.; Hong, V.; Reik, A.; Boismenu, R.; et al. Zinc finger nuclease-mediated gene editing in hematopoietic stem cells results in reactivation of fetal hemoglobin in sickle cell disease. Sci. Rep. 2024, 14, 24298. [Google Scholar] [CrossRef] [PubMed]
- Grimley, M.; Davies, S.M.; Shrestha, A.; Shova, A.; Asnani, M.; Kent, M.; Sayani, F.; Quinn, C.T.; Niss, O.; Lutzko, C.; et al. Lentiviral gene therapy with reduced-intensity conditioning for sickle cell disease: A phase 1/2 trial. Nat. Med. 2025, 31, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Forget, B.G. Molecular basis of hereditary persistence of fetal hemoglobin. Ann. N. Y. Acad. Sci. 1998, 850, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Akinsheye, I.; Alsultan, A.; Solovieff, N.; Ngo, D.; Baldwin, C.T.; Sebastiani, P.; Chui, D.H.; Steinberg, M.H. Fetal hemoglobin in sickle cell anemia. Blood 2011, 118, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H. Fetal Hemoglobin in Sickle Hemoglobinopathies: High HbF Genotypes and Phenotypes. J. Clin. Med. 2020, 9, 3782. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, J.; Tan, Y.; Beyer, A.I.; Xie, F.; Muench, M.O.; Kan, Y.W. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc. Natl. Acad. Sci. USA 2016, 113, 10661–10665. [Google Scholar] [CrossRef] [PubMed]
- Traxler, E.A.; Yao, Y.; Wang, Y.D.; Woodard, K.J.; Kurita, R.; Nakamura, Y.; Hughes, J.R.; Hardison, R.C.; Blobel, G.A.; Li, C.; et al. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016, 22, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.S.; Wienert, B.; Wyman, S.K.; Bell, H.W.; George, A.; Mahalingam, G.; Vu, J.T.; Prasad, K.; Bandlamudi, B.P.; Devaraju, N.; et al. Identification of novel HPFH-like mutations by CRISPR base editing that elevate the expression of fetal hemoglobin. Elife 2022, 11, e65421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Boelens, J.-J.; Cancio, M.; Hankins, J.S.; Bhad, P.; Azizy, M.; Lewandowski, A.; Zhao, X.; Chitnis, S.; Peddinti, R.; et al. CRISPR-Cas9 Editing of the HBG1 and HBG2 Promoters to Treat Sickle Cell Disease. N. Engl. J. Med. 2023, 389, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Newby, G.A.; Yen, J.S.; Woodard, K.J.; Mayuranathan, T.; Lazzarotto, C.R.; Li, Y.; Sheppard-Tillman, H.; Porter, S.N.; Yao, Y.; Mayberry, K.; et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature 2021, 595, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Krigel, M. Novel Gene Therapy Trial for Sickle Cell Disease Launches. 2024. Available online: https://www.ucsf.edu/news/2024/11/428941/novel-gene-therapy-trial-sickle-cell-disease-launches (accessed on 15 September 2025).
- Magis, W.; DeWitt, M.A.; Wyman, S.K.; Vu, J.T.; Heo, S.J.; Shao, S.J.; Hennig, F.; Romero, Z.G.; Campo-Fernandez, B.; Said, S.; et al. High-level correction of the sickle mutation is amplified in vivo during erythroid differentiation. iScience 2022, 25, 104374. [Google Scholar] [CrossRef] [PubMed]
- Dever, D.P.; Bak, R.O.; Reinisch, A.; Camarena, J.; Washington, G.; Nicolas, C.E.; Pavel-Dinu, M.; Saxena, N.; Wilkens, A.B.; Mantri, S.; et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 2016, 539, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Romero, Z.; Lomova, A.; Said, S.; Miggelbrink, A.; Kuo, C.Y.; Campo-Fernandez, B.; Hoban, M.D.; Masiuk, K.E.; Clark, D.N.; Long, J.; et al. Editing the Sickle Cell Disease Mutation in Human Hematopoietic Stem Cells: Comparison of Endonucleases and Homologous Donor Templates. Mol. Ther. 2019, 27, 1389–1406. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, A.; Camarena, J.; Lahiri, P.; Segal, H.; Srifa, W.; Vakulskas, C.A.; Frock, R.L.; Kenrick, J.; Lee, C.; Talbott, N.; et al. Development of β-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci. Transl. Med. 2021, 13, eabf2444. [Google Scholar] [CrossRef] [PubMed]
- Everette, K.A.; Newby, G.A.; Levine, R.M.; Mayberry, K.; Jang, Y.; Mayuranathan, T.; Nimmagadda, N.; Dempsey, E.; Li, Y.; Bhoopalan, S.V.; et al. Ex vivo prime editing of patient haematopoietic stem cells rescues sickle-cell disease phenotypes after engraftment in mice. Nat. Biomed. Eng. 2023, 7, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Frangoul, H.; Hobbs, W.E.; Locatelli, F. Studies of Exagamglogene Autotemcel—Age and Place. N. Engl. J. Med. 2024, 391, 572. [Google Scholar] [CrossRef] [PubMed]
- Rulu, P.; Tabassum, H. A Delphi study to identify and prioritize research areas in sickle cell disease in India. Sci. Rep. 2025, 15, 18319. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Psatha, N.; Wang, H.; Singh, M.; Samal, H.B.; Zhang, W.; Ehrhardt, A.; Izsvák, Z.; Papayannopoulou, T.; Lieber, A. Integrating HDAd5/35++ Vectors as a New Platform for HSC Gene Therapy of Hemoglobinopathies. Mol. Ther. Methods Clin. Dev. 2018, 9, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Georgakopoulou, A.; A Newby, G.; Chen, P.J.; A Everette, K.; Paschoudi, K.; Vlachaki, E.; Gil, S.; Anderson, A.K.; Koob, T.; et al. In vivo HSC prime editing rescues sickle cell disease in a mouse model. Blood 2023, 141, 2085–2099. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mishra, A.S.; Gil, S.; Wang, M.; Georgakopoulou, A.; Papayannopoulou, T.; Hawkins, R.D.; Lieber, A. Targeted Integration and High-Level Transgene Expression in AAVS1 Transgenic Mice after In Vivo HSC Transduction with HDAd5/35++ Vectors. Mol. Ther. 2019, 27, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, H.; Gil, S.; Germond, A.; Fountain, C.; Baldessari, A.; Kim, J.; Liu, Z.; Georgakopoulou, A.; Radtke, S.; et al. Safe and efficient in vivo hematopoietic stem cell transduction in nonhuman primates using HDAd5/35++ vectors. Mol. Ther. Methods Clin. Dev. 2021, 24, 127–141, Erratum in Mol. Ther. Methods Clin. Dev. 2022, 25, 533. https://doi.org/10.1016/j.omtm.2022.05.003. PMID: 35662812. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Anderson, A.K.; Ruminski, P.; Rettig, M.; Karpova, D.; Kiem, H.P.; DiPersio, J.F.; Lieber, A. A simplified G-CSF-free procedure allows for in vivo HSC gene therapy of sickle cell disease in a mouse model. Blood Adv. 2024, 8, 4089–4101. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Georgakopoulou, A.; Paschoudi, K.; Anderson, A.K.; Huang, L.; Gil, S.; Giannaki, M.; Vlachaki, E.; Newby, G.A.; Liu, D.R.; et al. Introducing a hemoglobin G-Makassar variant in HSCs by in vivo base editing treats sickle cell disease in mice. Mol. Ther. 2024, 32, 4353–4371. [Google Scholar] [CrossRef] [PubMed]
- Flomenberg, N.; Devine, S.M.; Dipersio, J.F.; Liesveld, J.L.; McCarty, J.M.; Rowley, S.D.; Vesole, D.H.; Badel, K.; Calandra, G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005, 106, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Ball, C.R.; Pilz, I.H.; Schmidt, M.; Fessler, S.; Williams, D.A.; von Kalle, C.; Glimm, H. Stable differentiation and clonality of murine long-term hematopoiesis after extended reduced-intensity selection for MGMT P140K transgene expression. Blood 2007, 110, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, C.; Paunovska, K.; Hatit, M.Z.C.; Lokugamage, M.P.; Dahlman, J.E. Therapeutic RNA Delivery for COVID and Other Diseases. Adv. Healthc. Mater. 2021, 10, e2002022. [Google Scholar] [CrossRef] [PubMed]
- Schindeler, A.; Chu, J.; Au-Yeung, C.; Kao, H.Y.; Ginn, S.L.; O’Donohue, A.K. In vivo precision base editing to rescue mouse models of disease. Mol. Ther. Nucleic Acids 2025, 36, 102622. [Google Scholar] [CrossRef] [PubMed]
- Breda, L.; Papp, T.E.; Triebwasser, M.P.; Yadegari, A.; Fedorky, M.T.; Tanaka, N.; Abdulmalik, O.; Pavani, G.; Wang, Y.; Grupp, S.A.; et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 2023, 381, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, L.; Schiroli, G.; Heshmati, Y.; Cao, Y.; Monte, M.T.; Wangweerawong, A.; Rottman, J.B.; Palchaudhuri, R.; Ribeil, J.-A.; Manis, J.; et al. In Vivo HSC Gene Editing for Correction of the Sickle Cell Mutation Using RNA Gene Writers. Blood 2024, 144 (Suppl. 1). [Google Scholar] [CrossRef]
- Musunuru, K.; Grandinette, S.A.; Wang, X.; Hudson, T.R.; Briseno, K.; Berry, A.M.; Hacker, J.L.; Hsu, A.; Silverstein, R.A.; Hille, L.T.; et al. Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease. N. Engl. J. Med. 2025, 392, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansal, R. Curing Sickle Cell Disease by Allogeneic Hematopoietic Stem Cell (HSC) Transplantation Toward In Vivo HSC Gene Therapy. Genes 2025, 16, 1367. https://doi.org/10.3390/genes16111367
Kansal R. Curing Sickle Cell Disease by Allogeneic Hematopoietic Stem Cell (HSC) Transplantation Toward In Vivo HSC Gene Therapy. Genes. 2025; 16(11):1367. https://doi.org/10.3390/genes16111367
Chicago/Turabian StyleKansal, Rina. 2025. "Curing Sickle Cell Disease by Allogeneic Hematopoietic Stem Cell (HSC) Transplantation Toward In Vivo HSC Gene Therapy" Genes 16, no. 11: 1367. https://doi.org/10.3390/genes16111367
APA StyleKansal, R. (2025). Curing Sickle Cell Disease by Allogeneic Hematopoietic Stem Cell (HSC) Transplantation Toward In Vivo HSC Gene Therapy. Genes, 16(11), 1367. https://doi.org/10.3390/genes16111367






