Acute Exposure to a Common Organic UV Filter Does Not Alter the mRNA of Gonadal Estrogen or Growth Hormone Receptors in Mozambique Tilapia (Oreochromis mossambicus) In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. RNA Extraction and qPCR
2.4. Data and Statistical Analyses
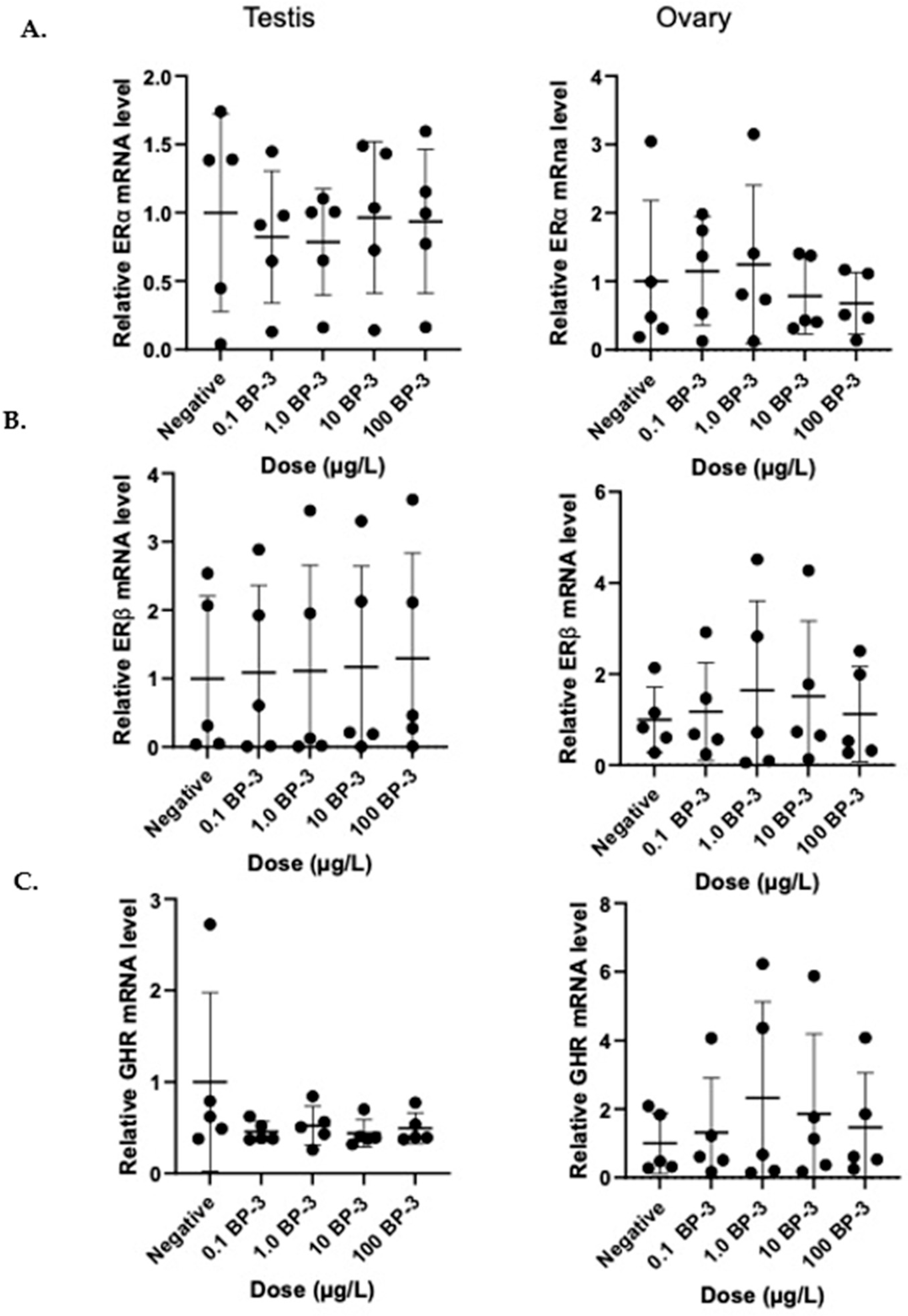
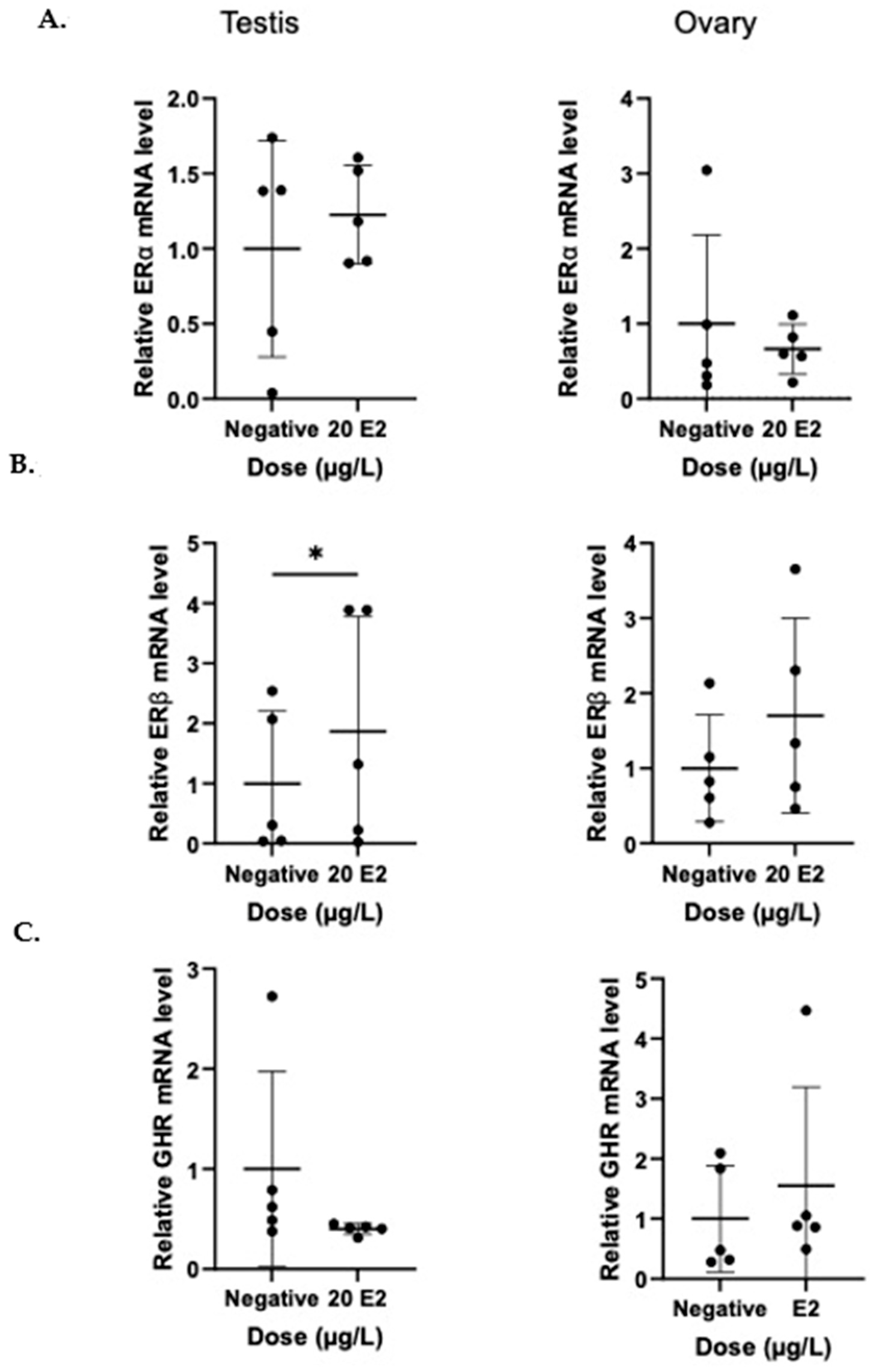
3. Results
ERα, ERβ, and GHR Regulation in Tilapia Gonads Following BP-3 Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP-3 | Hydroxy-4-methoxybenzophenone |
| 4MBC | 4-methylbenzylidene camphor |
| GHRs | Growth hormone receptors |
| ERs | Estrogen receptors |
| MS-222 | Methanesulfonate |
| LOEC | Lowest effective concentration |
| IACUC | Institutional Animal Care and Use Committee |
| PBS | Phosphate-buffered saline |
| L15 | Leibovitz’s 15 media |
| FBS | Fetal bovine serum |
| E2 | Estrogen |
| 4-OH-BP | 4-hydroxybenzophenone |
| EHMC | 2-ethyl-hexyl-4-trimethoxycinnamate |
| GnRH | Gonadotropin-releasing hormone |
| TH | Thyroid hormone |
| GTH | Gonadotropic hormone |
| HPG | Hypothalamic–Pituitary–Gonadal |
References
- Krause, M.; Klit, A.; Jensen, M.B.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.E.; Drzewiecki, K.T. Sunscreens: Are they Beneficial for Health? An Overview of Endocrine Disrupting Properties of UV-Filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.E.; Buser, H.R.; Müller, M.D.; Poiger, T. Occurrence of Some Organic UV Filters in Wastewater, in Surface Waters, and in Fish from Swiss Lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, Toxicities, and Ecological Risks of Benzophenone-3, a Common Component of Organic Sunscreen Products: A Mini-Review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Burnett, M.E.; Wang, S.Q. Current Sunscreen Controversies: A Critical Review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and Organic UV Filters: Their Role and Efficacy in Sunscreens and Suncare Products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Buser, H.R.; Müller, M.D.; Balmer, M.E.; Poiger, T.; Buerge, I.J. Stereoisomer Composition of the Chiral UV Filter 4-Methylbenzylidene Camphor in Environmental Samples. Environ. Sci. Technol. 2005, 39, 3013–3019. [Google Scholar] [CrossRef]
- Cuderman, P.; Heath, E. Determination of UV Filters and Antimicrobial Agents in Environmental Water Samples. Anal. Bioanal. Chem. 2007, 387, 1343–1350. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Fennix-Agudelo, M.; Miranda-Castro, W. Occurrence of Personal Care Products as Emerging Chemicals of Concern in Water Resources: A Review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef]
- O’Malley, E.; O’Brien, J.W.; Tscharke, B.; Thomas, K.V.; Mueller, J.F. Per Capita Loads of Organic UV Filters in Australian Wastewater Influent. Sci. Total Environ. 2019, 662, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in Analytical Methods and Occurrence of Organic UV-Filters in the Environment—A Review. Sci. Total Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef] [PubMed]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and Distribution of UV-Filters and Other Anthropogenic Contaminants in Coastal Surface Water, Sediment, and Coral Tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Zenker, A.; Rapp, M. Widespread Occurrence of Estrogenic UV-Filters in Aquatic Ecosystems in Switzerland. Environ. Pollut. 2010, 158, 1817–1824. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Lam, J.C.W.; Ng, T.; Ang, P.O.; Murphy, M.B.; Lam, P.K.S. Occurrence, Distribution, and Fate of Organic UV Filters in Coral Communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Lam, P.K.S.; Murphy, M.B. Seasonal Occurrence, Removal Efficiencies and Preliminary Risk Assessment of Multiple Classes of Organic UV Filters in Wastewater Treatment Plants. Water Res. 2014, 53, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV Filters Bioaccumulation in Fish from Iberian River Basins. Sci. Total Environ. 2015, 518–519, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Zenker, A.; Schmutz, H.; Fent, K. Simultaneous Trace Determination of Nine Organic UV-Absorbing Compounds (UV Filters) in Environmental Samples. J. Chromatogr. A 2008, 1202, 64–74. [Google Scholar] [CrossRef]
- Horricks, R.A.; Tabin, S.K.; Edwards, J.J.; Lumsden, J.S.; Marancik, D.P. Organic Ultraviolet Filters in Nearshore Waters and in the Invasive Lionfish (Pterois volitans) in Grenada, West Indies. PLoS ONE 2019, 14, e0220280. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter Oxybenzone (Benzophenone-3) on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Vione, D.; Caringella, R.; De Laurentiis, E.; Pazzi, M.; Minero, C. Phototransformation of the Sunlight Filter Benzophenone-3 (2-Hydroxy-4-Methoxybenzophenone) under Conditions Relevant to Surface Waters. Sci. Total Environ. 2013, 463–464, 243–251. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Zenker, A.; Rapp, M. A Tentative Environmental Risk Assessment of the UV-Filters 3-(4-Methylbenzylidene-Camphor), 2-Ethyl-Hexyl-4-Trimethoxycinnamate, Benzophenone-3, Benzophenone-4 and 3-Benzylidene Camphor. Mar. Environ. Res. 2010, 69, S4–S6. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic Effect of Active Ingredients in Sunscreen Products—A Contemporary Review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental Occurrence and Risk of Organic UV Filters and Stabilizers in Multiple Matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Alonso, M.B.; Bertozzi, C.P.; Marigo, J.; Barbosa, L.; Cremer, M.; Secchi, E.R.; Azevedo, A.; Lailson-Brito, J.; Torres, J.P.M.; et al. Erratum: First Determination of UV Filters in Marine Mammals. Octocrylene Levels in Franciscana Dolphins Environ. Sci. Technol. 2013, 47, 5619–5625. [Google Scholar] [CrossRef]
- Araújo, M.; Rocha, R.; Soares, A.; Benedé, J.; Chisvert, A.; Monteiro, M. Effects of UV Filter 4-Methylbenzylidene Camphor during Early Development of Solea senegalensis Kaup, 1858. Sci. Total Environ. 2018, 628–629, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Kinnberg, K.L.; Petersen, G.I.; Albrektsen, M.; Minghlani, M.; Awad, S.M.; Holbech, B.F.; Green, J.W.; Bjerregaard, P.; Holbech, H. Endocrine-Disrupting Effect of the Ultraviolet Filter Benzophenone-3 in Zebrafish (Danio rerio). Environ. Toxicol. Chem. 2015, 34, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.Y.; Gries, T.; Fent, K. The Ultraviolet Filter 3-Benzylidene Camphor Adversely Affects Reproduction in Fathead Minnow (Pimephales promelas). Toxicol. Sci. 2006, 93, 311–321. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of In Vitro and In Vivo Estrogenic Activity of UV Filters in Fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef]
- Weisbrod, C.J.; Kunz, P.Y.; Zenker, A.K.; Fent, K. Effects of the UV Filter Benzophenone-2 on Reproduction in Fish. Toxicol. Appl. Pharmacol. 2007, 225, 255–266. [Google Scholar] [CrossRef]
- Coronado, M.; De Haro, H.; Deng, X.; Rempel, M.A.; Lavado, R.; Schlenk, D. Estrogenic Activity and Reproductive Effects of the UV-Filter Oxybenzone (2-Hydroxy-4-Methoxyphenyl-Methanone) in Fish. Aquat. Toxicol. 2008, 90, 182–187. [Google Scholar] [CrossRef]
- Weber, L.P.; Hill, R.L.; Janz, D.M. Developmental Estrogenic Exposure in Zebrafish (Danio rerio): II. Histological Evaluation of Gametogenesis and Organ Toxicity. Aquat. Toxicol. 2003, 63, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Petro-Sakuma, C.; Celino-Brady, F.T.; Breves, J.P.; Seale, A.P. Growth Hormone Regulates Intestinal Gene Expression of Nutrient Transporters in Tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2020, 292, 113464. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Hiramatsu, N.; Hiramatsu, K.; Reading, B.J.; Matsubara, T.; Hara, A.; Sullivan, C.V.; Pierce, A.L.; Hirano, T.; Grau, E.G. Induction of Three Vitellogenins by 17β-Estradiol with Concurrent Inhibition of the Growth Hormone–Insulin-Like Growth Factor 1 Axis in a Euryhaline Teleost, the Tilapia (Oreochromis mossambicus). Biol. Reprod. 2007, 77, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.K.; Pierce, A.L.; Hiramatsu, N.; Sullivan, C.V.; Hirano, T.; Grau, E.G. Gender-Specific Expression of Multiple Estrogen Receptors, Growth Hormone Receptors, Insulin-Like Growth Factors and Vitellogenins, and Effects of 17β-Estradiol in the Male Tilapia (Oreochromis mossambicus). Gen. Comp. Endocrinol. 2008, 156, 544–551. [Google Scholar] [CrossRef]
- Inokuchi, M.; Hiroi, J.; Kaneko, T. Why Can Mozambique Tilapia Acclimate to Both Freshwater and Seawater? Insights from the Plasticity of Ionocyte Functions in the Euryhaline Teleost. Front. Physiol. 2022, 13, 914277. [Google Scholar] [CrossRef]
- Osman, G.A.; El-Khateeb, M.A. Impact of water contamination on tilapia (Oreochromis niloticus) fish yield. Int. J. Chem. Technol. Res. 2016, 9, 166–181. [Google Scholar]
- Nivelle, R.; Gennotte, V.; Kalala, E.J.K.; Ngoc, N.B.; Muller, M.; Mélard, C.; Rougeot, C. Temperature Preference of Nile Tilapia (Oreochromis niloticus) Juveniles Induces Spontaneous Sex Reversal. PLoS ONE 2019, 14, e0212504. [Google Scholar]
- Chen, L.; Jiang, X.; Feng, H.; Shi, H.; Sun, L.; Tao, W.; Xie, Q.; Wang, D. Simultaneous Exposure to Estrogen and Androgen Resulted in Feminization and Endocrine Disruption. J. Endocrinol. 2016, 228, 205–218. [Google Scholar] [CrossRef]
- Ribeiro, C.; Urbatzka, R.; Castro, L.F.C.; Carrola, J.; Fontainhas-Fernandes, A.; Monteiro, R.A.; Rocha, E.; Rocha, M.J. In Vitro Exposure of Nile Tilapia (Oreochromis niloticus) Testis to Estrogenic Endocrine Disrupting Chemicals: mRNA Expression of Genes Encoding Steroidogenic Enzymes. Toxicol. Mech. Methods 2012, 22, 47–53. [Google Scholar] [CrossRef]
- Esterhuyse, M.M.; Helbing, C.C.; van Wyk, J.H. Temporal Expression of Two Cytochrome P450 Aromatase Isoforms during Development in Oreochromis mossambicus, in Association with Histological Development. Comp. Biochem. Physiol. D Genomics Proteomics 2008, 3, 297–306. [Google Scholar] [CrossRef]
- Park, C.B.; Takemura, A.; Aluru, N.; Park, Y.-J.; Kim, B.-H.; Lee, C.-H.; Lee, Y.-D.; Moon, T.W.; Vijayan, M.M. Tissue-Specific Suppression of Estrogen, Androgen and Glucocorticoid Receptor Gene Expression in Feral Vitellogenic Male Mozambique Tilapia. Chemosphere 2007, 69, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Tacon, P.; Baroiller, J.F.; Le Bail, P.Y.; Prunet, P.; Jalabert, B. Effect of Egg Deprivation on Sex Steroids, Gonadotropin, Prolactin, and Growth Hormone Profiles during the Reproductive Cycle of the Mouthbrooding Cichlid Fish Oreochromis niloticus. Gen. Comp. Endocrinol. 2000, 117, 54–65. [Google Scholar] [CrossRef]
- Chen, T.H.; Wu, Y.T.; Ding, W.H. UV-Filter Benzophenone-3 Inhibits Agonistic Behavior in Male Siamese Fighting Fish (Betta splendens). Ecotoxicology 2016, 25, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Mijangos, L.; Montes, R.; Rodil, R.; Anakabe, E.; Izagirre, U.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Study of Bioconcentration of Oxybenzone in Gilt-Head Bream and Characterization of Its By-Products. Chemosphere 2018, 208, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Farbrot, A.; Larkö, O.; Wennberg, A.M. Percutaneous Absorption of the Sunscreen Benzophenone-3 after Repeated Whole-Body Applications, with and without Ultraviolet Irradiation. Br. J. Dermatol. 2006, 154, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Kumar, A.; Trang, P.N. The use of fish cell lines as in vitro ecotoxicological tools: A cellular solution to aquaculture sustainability. Aquaculture 2024, 593, 741302. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and Antiandrogenic Activities of 17 Benzophenone Derivatives Used as UV Stabilizers and Sunscreens. Toxicol. Appl. Pharmacol. 2005, 203, 9–17. [Google Scholar] [CrossRef]
- Zucchi, S.; Oggier, D.M.; Fent, K. Global Gene Expression Profile Induced by the UV-Filter 2-Ethyl-Hexyl-4-Trimethoxycinnamate (EHMC) in Zebrafish (Danio rerio). Environ. Pollut. 2011, 159, 3086–3096. [Google Scholar] [CrossRef]
- Du, Y.; Wang, W.-Q.; Pei, Z.-T.; Ahmad, F.; Xu, R.-R.; Zhang, Y.-M.; Sun, L.-W. Acute Toxicity and Ecological Risk Assessment of Benzophenone-3 (BP-3) and Benzophenone-4 (BP-4) in Ultraviolet (UV) Filters. Int. J. Environ. Res. Public Health 2017, 14, 1414. [Google Scholar] [CrossRef]
- Nelson, E.R.; Habibi, H.R. Estrogen Receptor Function and Regulation in Fish and Other Vertebrates. Gen. Comp. Endocrinol. 2013, 192, 15–24. [Google Scholar] [CrossRef]
- Tovo-Neto, A.; da Silva Rodrigues, M.; Habibi, H.R.; Nóbrega, R.H. Thyroid Hormone Actions on the Male Reproductive System of Teleost Fish. Gen. Comp. Endocrinol. 2018, 265, 230–236. [Google Scholar] [CrossRef]
- Zohar, Y.; Muñoz-Cueto, J.A.; Elizur, A.; Kah, O. Neuroendocrinology of Reproduction in Teleost Fish. Gen. Comp. Endocrinol. 2010, 165, 438–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, G.; Liu, J.; Yan, Z.; Wang, Y. Toxicological Responses of Carassius auratus Induced by Benzophenone-3 Exposure and the Association with Alteration of Gut Microbiota. Sci. Total Environ. 2020, 747, 141255. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yeung, K.; Kwok, M.L.; Chung, C.T.; Hu, X.L.; Chan, K.M. Toxic Effects and Transcriptome Analyses of Zebrafish (Danio rerio) Larvae Exposed to Benzophenones. Environ. Pollut. 2020, 265, 114857. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maur, G.; Silva-Picazo, K.; Dores, C.; Marancik, D.; Allan, E.R.O. Acute Exposure to a Common Organic UV Filter Does Not Alter the mRNA of Gonadal Estrogen or Growth Hormone Receptors in Mozambique Tilapia (Oreochromis mossambicus) In Vitro. Genes 2025, 16, 1357. https://doi.org/10.3390/genes16111357
Maur G, Silva-Picazo K, Dores C, Marancik D, Allan ERO. Acute Exposure to a Common Organic UV Filter Does Not Alter the mRNA of Gonadal Estrogen or Growth Hormone Receptors in Mozambique Tilapia (Oreochromis mossambicus) In Vitro. Genes. 2025; 16(11):1357. https://doi.org/10.3390/genes16111357
Chicago/Turabian StyleMaur, Glenna, Kelly Silva-Picazo, Camila Dores, David Marancik, and Euan R. O. Allan. 2025. "Acute Exposure to a Common Organic UV Filter Does Not Alter the mRNA of Gonadal Estrogen or Growth Hormone Receptors in Mozambique Tilapia (Oreochromis mossambicus) In Vitro" Genes 16, no. 11: 1357. https://doi.org/10.3390/genes16111357
APA StyleMaur, G., Silva-Picazo, K., Dores, C., Marancik, D., & Allan, E. R. O. (2025). Acute Exposure to a Common Organic UV Filter Does Not Alter the mRNA of Gonadal Estrogen or Growth Hormone Receptors in Mozambique Tilapia (Oreochromis mossambicus) In Vitro. Genes, 16(11), 1357. https://doi.org/10.3390/genes16111357




