Non-Coding RNAs as Emerging Regulators in Kidney Pathophysiology: From Molecular Mechanisms to Therapeutic Potential
Abstract
1. Introduction
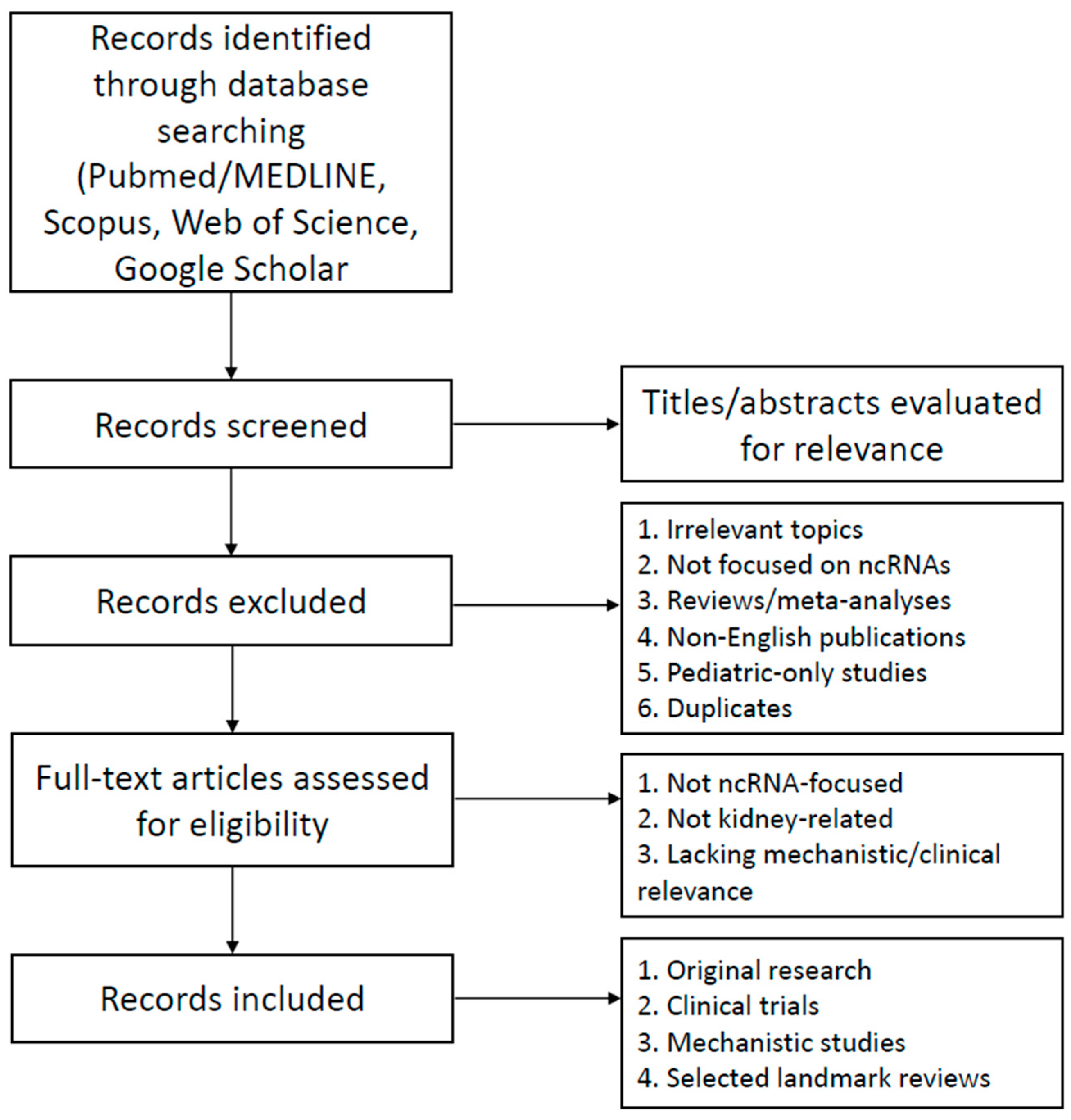
2. Literature Search Strategy
3. Molecular Classes of Non-Coding RNAs
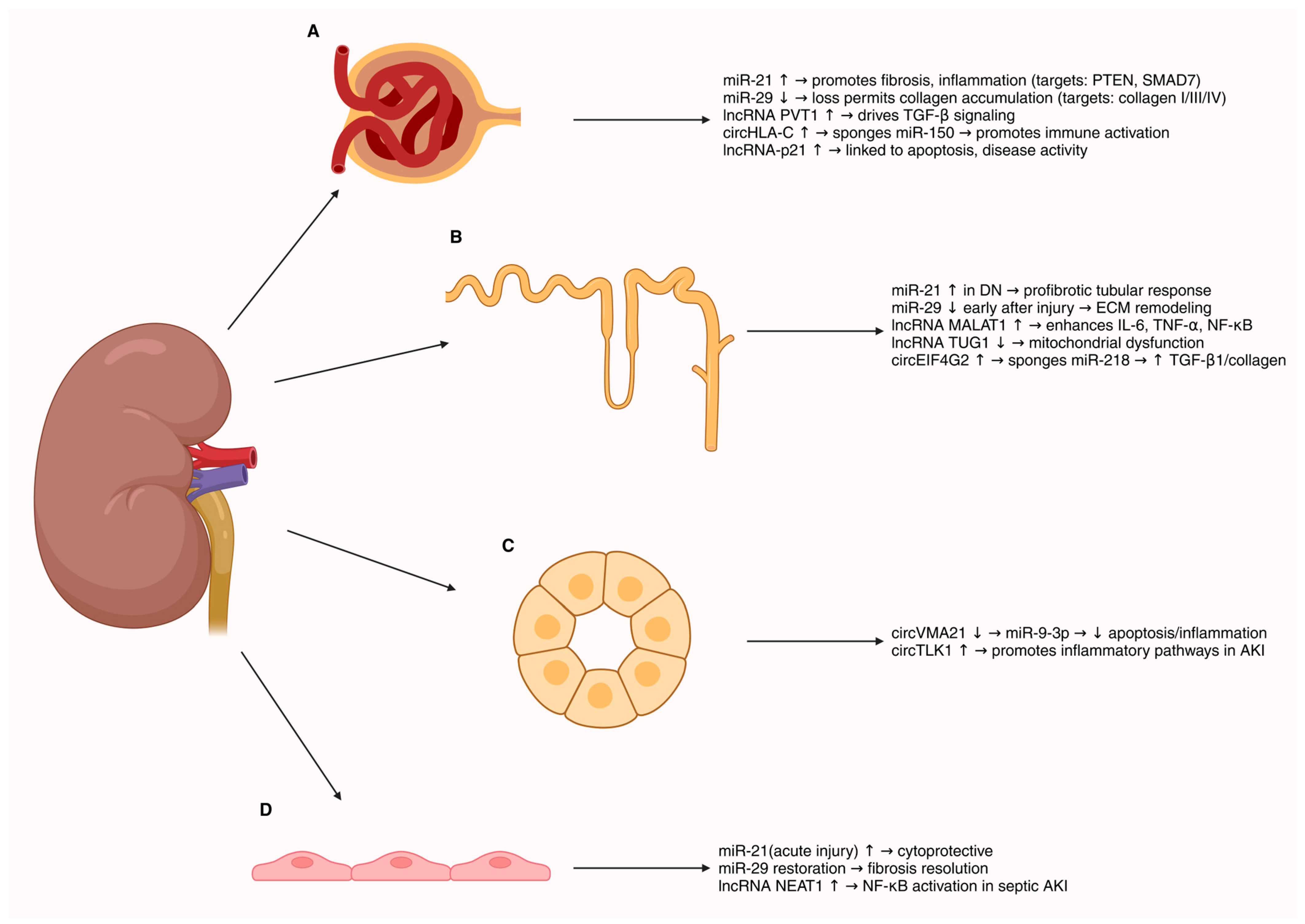
4. ncRNAs in Renal Cell Biology
5. ncRNAs in Metabolic and Acute Renal Injury
6. ncRNAs in Chronic and Immune-Mediated Kidney Diseases
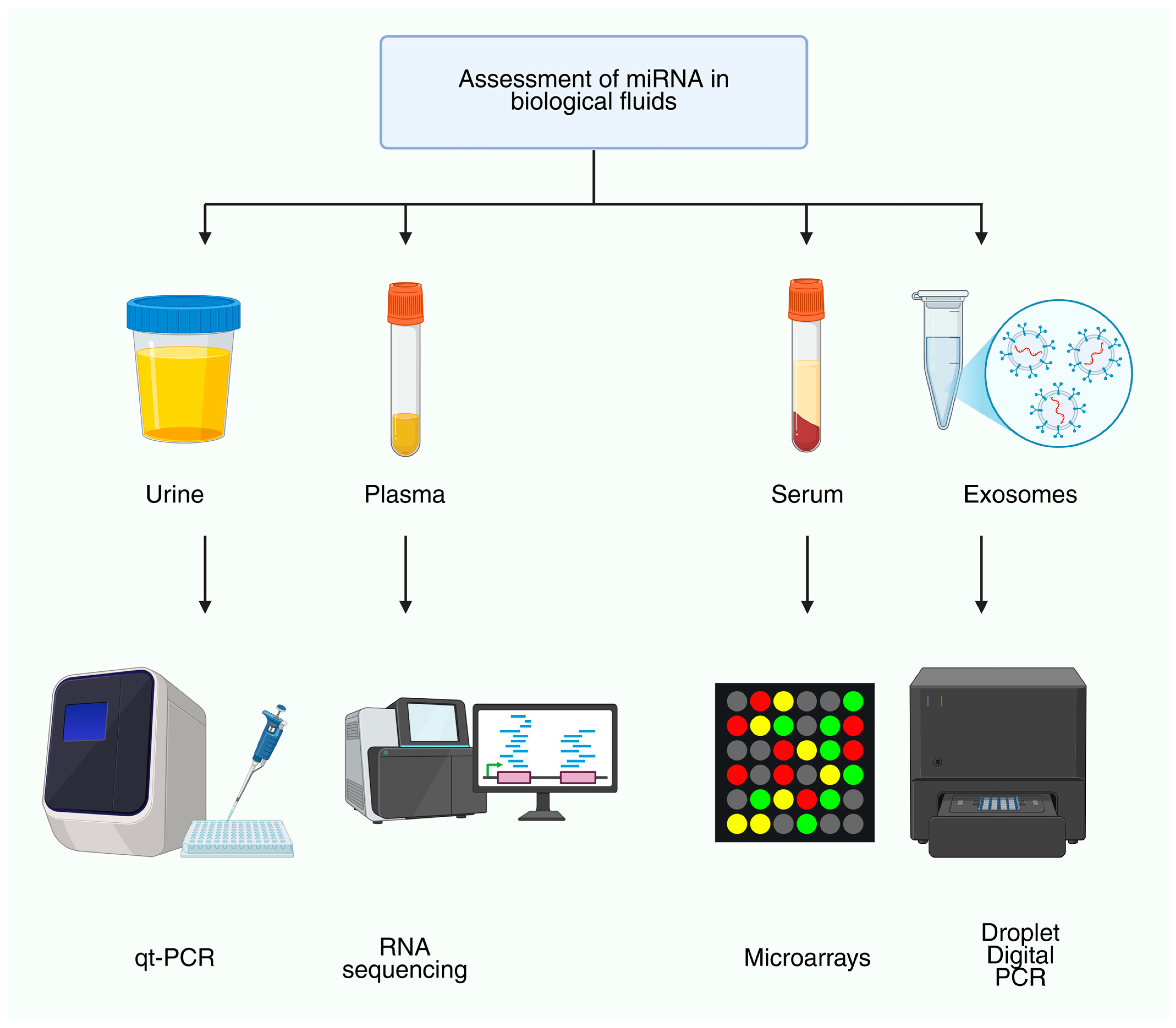
7. ncRNAs as Biomarkers in Nephrology
8. Therapeutic Targeting of ncRNAs
| Therapeutic Strategy | Mechanism/Rationale | Evidence/Key Findings | References |
|---|---|---|---|
| Anti-miR-21 (antagomirs/ASOs) | Inhibition of pro-fibrotic miR-21; derepression of PTEN/TIMP3 and anti-fibrotic pathways | Preclinical: reduced EMT, inflammation, fibrosis in DN/UUO; Clinical (Alport, lademirsen): safe but no eGFR benefit at 24–48 weeks | [79,119] |
| miR-29 mimics (remlarsen/MRG-201, MRG-229) | Restoration of miR-29 family to repress collagen/fibronectin genes | Preclinical: attenuates fibrosis in UUO and DN; Clinical (skin/lung fibrosis): proof-of-mechanism and safety established, anti-fibrotic PD relevant to CKD | [64,120] |
| Anti-miR-17 (RGLS4326, RGLS8429) | Blockade of miR-17 to relieve repression of PKD1/PKD2, metabolic/mTOR regulation | Preclinical: reduced cystogenesis in ADPKD models; Clinical: early-phase studies with kidney-preferential distribution, biomarker engagement, safety evaluation ongoing | [122,123,132,133] |
| lncRNA Erbb4-IR silencing (siRNA/ASO) | Interrupts Smad3-dependent pro-fibrotic signaling; restores Smad7/miR-29 axis | Preclinical: reduces matrix deposition in UUO and type 2 DN models | [124] |
| lncRNA Arid2-IR silencing (siRNA/ASO) | Inhibition of NF-κB-driven inflammatory pathways | Preclinical: diminished renal inflammation in vivo | [125] |
| Other lncRNA targets (KCNQ1OT1, IRAR, etc.) | Modulate tubular injury, chemokine induction, and fibrosis | Preclinical: silencing reduces injury/fibrosis in AKI and CKD models | [126,127] |
| Advanced delivery platforms (nanoparticles, peptide/ligand conjugates, EVs) | Broaden renal cell-type reach beyond proximal tubules | Preclinical: improved biodistribution and efficacy under development | [128,129] |
| Chemical modifications (2′-O-Me, 2′-F, LNA, novel backbones) | Reduce off-target and immune activation risks | Mitigated toxicity and improved stability demonstrated; translational safety still requires monitoring | [130] |
| Renal safety biomarkers (KIM-1, clusterin, β2-microglobulin) | Monitor nephrotoxicity and tubular injury during oligonucleotide therapy | Observed low-grade tubular effects; some ASOs (e.g., SPC5001) linked to nephrotoxicity → biomarkers integral for development | [131] |
9. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hombach, S.; Kretz, M. Non-Coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [CrossRef]
- Patil, V.S.; Zhou, R.; Rana, T.M. Gene Regulation by Non-Coding RNAs. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.P.; Goodwin, J.E.; Tripathi, P.; Kanasaki, K.; Koya, D. Interactions among Long Non-Coding RNAs and MicroRNAs Influence Disease Phenotype in Diabetes and Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 6027. [Google Scholar] [CrossRef]
- Jin, J.; Sun, H.; Shi, C.; Yang, H.; Wu, Y.; Li, W.; Dong, Y.-H.; Cai, L.; Meng, X.-M. Circular RNA in Renal Diseases. J. Cell Mol. Med. 2020, 24, 6523–6533. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-T.; Li, Z.-W.; Zhao, X.; Li, M.-L.; Hou, P.-F.; Chu, S.-F.; Zheng, J.-N.; Bai, J. Role of Circular RNA in Kidney-Related Diseases. Front. Pharmacol. 2021, 12, 615882. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Liu, C.L.; Kim, J.; Susztak, K. Understanding the Kidney One Cell at a Time. Kidney Int. 2019, 96, 862–870. [Google Scholar] [CrossRef]
- Liu, C.; Ma, K.; Zhang, Y.; He, X.; Song, L.; Chi, M.; Han, Z.; Li, G.; Zhang, Q.; Liu, C. Kidney Diseases and Long Non-Coding RNAs in the Limelight. Front. Physiol. 2022, 13, 932693. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Shu, S.; Cai, J.; Tang, C.; Dong, Z. Non-Coding RNAs in Kidney Injury and Repair. Am. J. Physiol. Cell Physiol. 2019, 317, C177–C188. [Google Scholar] [CrossRef]
- van Zonneveld, A.J.; Zhao, Q.; Rotmans, J.I.; Bijkerk, R. Circulating Non-Coding RNAs in Chronic Kidney Disease and Its Complications. Nat. Rev. Nephrol. 2023, 19, 573–586. [Google Scholar] [CrossRef]
- Kota, S.K.; Kota, S.B. Noncoding RNA and Epigenetic Gene Regulation in Renal Diseases. Drug Discov. Today 2017, 22, 1112–1122. [Google Scholar] [CrossRef]
- Ignarski, M.; Islam, R.; Müller, R.-U. Long Non-Coding RNAs in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3276. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Shin, S.-I.; Jung, S.W.; An, H.; Choi, S.Y.; Eun, M.; Jun, C.-D.; Lee, S.; Park, J. Cell Type- and Age-Specific Expression of LncRNAs across Kidney Cell Types. J. Am. Soc. Nephrol. 2024, 35, 870–885. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Zhang, H.; Xing, Y.; Fang, Y.; Zheng, D.; Yang, H.; Qiao, Y.; Yang, B. The Potential Role of Non-Coding RNAs in Acute Kidney Injury: A Focus on Natural Medicine Treatment. Front. Mol. Biosci. 2025, 12, 1648526. [Google Scholar] [CrossRef]
- Metzinger-Le Meuth, V.; Fourdinier, O.; Charnaux, N.; Massy, Z.A.; Metzinger, L. The Expanding Roles of MicroRNAs in Kidney Pathophysiology. Nephrol. Dial. Transplant. 2019, 34, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, B.; Ma, L.; Fu, P. An Update of Long-Noncoding RNAs in Acute Kidney Injury. Front. Physiol. 2022, 13, 849403. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, S. Emerging Biomarkers and Advanced Diagnostics in Chronic Kidney Disease: Early Detection Through Multi-Omics and AI. Diagnostics 2025, 15, 1225. [Google Scholar] [CrossRef]
- Park, J.; Shrestha, R.; Qiu, C.; Kondo, A.; Huang, S.; Werth, M.; Li, M.; Barasch, J.; Suszták, K. Single-Cell Transcriptomics of the Mouse Kidney Reveals Potential Cellular Targets of Kidney Disease. Science 2018, 360, 758–763. [Google Scholar] [CrossRef]
- McEvoy, C.M.; Murphy, J.M.; Zhang, L.; Clotet-Freixas, S.; Mathews, J.A.; An, J.; Karimzadeh, M.; Pouyabahar, D.; Su, S.; Zaslaver, O.; et al. Single-Cell Profiling of Healthy Human Kidney Reveals Features of Sex-Based Transcriptional Programs and Tissue-Specific Immunity. Nat. Commun. 2022, 13, 7634. [Google Scholar] [CrossRef]
- Abedini, A.; Levinsohn, J.; Klötzer, K.A.; Dumoulin, B.; Ma, Z.; Frederick, J.; Dhillon, P.; Balzer, M.S.; Shrestha, R.; Liu, H.; et al. Single-Cell Multi-Omic and Spatial Profiling of Human Kidneys Implicates the Fibrotic Microenvironment in Kidney Disease Progression. Nat. Genet. 2024, 56, 1712–1724. [Google Scholar] [CrossRef]
- Polonsky, M.; Gerhardt, L.M.S.; Yun, J.; Koppitch, K.; Colón, K.L.; Amrhein, H.; Wold, B.; Zheng, S.; Yuan, G.-C.; Thomson, M.; et al. Spatial Transcriptomics Defines Injury Specific Microenvironments and Cellular Interactions in Kidney Regeneration and Disease. Nat. Commun. 2024, 15, 7010. [Google Scholar] [CrossRef]
- Raghubar, A.M.; Pham, D.T.; Tan, X.; Grice, L.F.; Crawford, J.; Lam, P.Y.; Andersen, S.B.; Yoon, S.; Teoh, S.M.; Matigian, N.A.; et al. Spatially Resolved Transcriptomes of Mammalian Kidneys Illustrate the Molecular Complexity and Interactions of Functional Nephron Segments. Front. Med. 2022, 9, 873923. [Google Scholar] [CrossRef]
- Wei, J.-W.; Huang, K.; Yang, C.; Kang, C.-S. Non-Coding RNAs as Regulators in Epigenetics. Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef]
- Hu, M.; Li, Z. A Mini Review of Noncoding RNAs in the Pathogenesis of Polycystic Kidney Disease. J. Transl. Genet. Genom. 2025, 9, 100–113. [Google Scholar] [CrossRef]
- Brandenburger, T.; Salgado Somoza, A.; Devaux, Y.; Lorenzen, J.M. Noncoding RNAs in Acute Kidney Injury. Kidney Int. 2018, 94, 870–881. [Google Scholar] [CrossRef]
- Bravo-Vázquez, L.A.; Paul, S.; Colín-Jurado, M.G.; Márquez-Gallardo, L.D.; Castañón-Cortés, L.G.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Exploring the Therapeutic Significance of MicroRNAs and LncRNAs in Kidney Diseases. Genes 2024, 15, 123. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a Molecular Understanding of MicroRNA-Mediated Gene Silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.W.; Guo, H.; McGeary, S.E.; Rodriguez-Mias, R.A.; Shin, C.; Baek, D.; Hsu, S.; Ghoshal, K.; Villén, J.; Bartel, D.P. MRNA Destabilization Is the Dominant Effect of Mammalian MicroRNAs by the Time Substantial Repression Ensues. Mol. Cell 2014, 56, 104–115. [Google Scholar] [CrossRef]
- Denby, L.; Baker, A.H. Targeting Non-Coding RNA for the Therapy of Renal Disease. Curr. Opin. Pharmacol. 2016, 27, 70–77. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of MiRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Martínez Pabón, B.; Zaporozhchenko, I.; Konoshenko, M.; Murina, E.; Bryzgunova, O.; Laktionov, P. Influence of Pre-Analytical Conditions on Cell-Free MicroRNA Stability in Blood Plasma Samples. ExRNA 2025, 7, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Salehi, S.; Taheri, M.N.; Azarpira, N.; Zare, A.; Behzad--Behbahani, A. State of the Art Technologies to Explore Long Non--coding RNAs in Cancer. J. Cell Mol. Med. 2017, 21, 3120–3140. [Google Scholar] [CrossRef]
- Hu, M.; Ma, Q.; Liu, B.; Wang, Q.; Zhang, T.; Huang, T.; Lv, Z. Long Non-Coding RNAs in the Pathogenesis of Diabetic Kidney Disease. Front. Cell Dev. Biol. 2022, 10, 845371. [Google Scholar] [CrossRef]
- Shafaghat, Z.; Radmehr, S.; Saharkhiz, S.; Khosrozadeh, A.; Feiz, K.; Alkhathami, A.G.; Taheripak, G.; Ramezani Farani, M.; Rahmati, R.; Zarimeidani, F.; et al. Circular RNA, A Molecule with Potential Chemistry and Applications in RNA-Based Cancer Therapeutics: An Insight into Recent Advances. Top. Curr. Chem. 2025, 383, 21. [Google Scholar] [CrossRef]
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-Protein Interactions: Functions, Mechanisms, and Identification. Theranostics 2020, 10, 3503–3517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Hu, J.; Yu, H.; Shen, Y.; Lai, H.; Li, Q.; Zhang, H.; Li, Y.; Fang, Z.; et al. Circular RNA Landscape in Extracellular Vesicles from Human Biofluids. Genome Med. 2024, 16, 126. [Google Scholar] [CrossRef]
- Ross, R.J.; Weiner, M.M.; Lin, H. PIWI Proteins and PIWI-Interacting RNAs in the Soma. Nature 2014, 505, 353–359. [Google Scholar] [CrossRef]
- Ding, L.; Wang, R.; Xu, W.; Shen, D.; Cheng, S.; Wang, H.; Lu, Z.; Zheng, Q.; Wang, L.; Xia, L.; et al. PIWI-Interacting RNA 57125 Restrains Clear Cell Renal Cell Carcinoma Metastasis by Downregulating CCL3 Expression. Cell Death Discov. 2021, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- ’t Hart, L.M.; de Klerk, J.A.; Bouland, G.A.; Peerlings, J.H.D.; Blom, M.T.; Cramer, S.J.; Bijkerk, R.; Beulens, J.W.J.; Slieker, R.C. Small RNA Sequencing Reveals SnoRNAs and PiRNA-019825 as Novel Players in Diabetic Kidney Disease. Endocrine 2024, 86, 194–203. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, J.; Miao, J.; Shen, M.; Wang, H.; Huang, X.; Ni, A.; Wu, H.; Chen, J.; Xiao, L.; et al. SNORD3A Regulates STING Transcription to Promote Ferroptosis in Acute Kidney Injury. Adv. Sci. 2024, 11, e2400305. [Google Scholar] [CrossRef] [PubMed]
- Grützmann, K.; Salomo, K.; Krüger, A.; Lohse-Fischer, A.; Erdmann, K.; Seifert, M.; Baretton, G.; Aust, D.; William, D.; Schröck, E.; et al. Identification of Novel SnoRNA-Based Biomarkers for Clear Cell Renal Cell Carcinoma from Urine-Derived Extracellular Vesicles. Biol. Direct 2024, 19, 38. [Google Scholar] [CrossRef]
- Lu, C.-C.; Wang, G.-H.; Lu, J.; Chen, P.-P.; Zhang, Y.; Hu, Z.-B.; Ma, K.-L. Role of Podocyte Injury in Glomerulosclerosis. Adv. Exp. Med. Biol. 2019, 1165, 195–232. [Google Scholar] [PubMed]
- Qin, X.; Zhu, S.; Chen, Y.; Chen, D.; Tu, W.; Zou, H. Long Non-Coding RNA (LncRNA) CASC15 Is Upregulated in Diabetes-Induced Chronic Renal Failure and Regulates Podocyte Apoptosis. Med. Sci. Monit. 2020, 26, e919415. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Hadden, M.J.; Thieme, K.; Batchu, S.N.; Niveditha, D.; Chowdhury, S.; Yerra, V.G.; Advani, S.L.; Bowskill, B.B.; Liu, Y.; et al. Dysregulated Expression but Redundant Function of the Long Non-Coding RNA HOTAIR in Diabetic Kidney Disease. Diabetologia 2019, 62, 2129–2142. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Eddy, E.; Kiefer, J. Role of MicroRNA 1207-5P and Its Host Gene, the Long Non-Coding RNA Pvt1, as Mediators of Extracellular Matrix Accumulation in the Kidney: Implications for Diabetic Nephropathy. PLoS ONE 2013, 8, e77468. [Google Scholar] [CrossRef]
- Liu, D.-W.; Zhang, J.-H.; Liu, F.-X.; Wang, X.-T.; Pan, S.-K.; Jiang, D.-K.; Zhao, Z.-H.; Liu, Z.-S. Silencing of Long Noncoding RNA PVT1 Inhibits Podocyte Damage and Apoptosis in Diabetic Nephropathy by Upregulating FOXA1. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Hu, M.; Wang, R.; Li, X.; Fan, M.; Lin, J.; Zhen, J.; Chen, L.; Lv, Z. LncRNA MALAT 1 Is Dysregulated in Diabetic Nephropathy and Involved in High Glucose-induced Podocyte Injury via Its Interplay with Β-catenin. J. Cell Mol. Med. 2017, 21, 2732–2747. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Xu, J.; Zhu, Q.; Ye, X.; Ding, D.; Yao, W.; Lu, Y. Dysregulation of LncRNAs GM5524 and GM15645 Involved in High-glucose-induced Podocyte Apoptosis and Autophagy in Diabetic Nephropathy. Mol. Med. Rep. 2018, 18, 3657–3664. [Google Scholar] [CrossRef]
- Hu, S.; Han, R.; Shi, J.; Zhu, X.; Qin, W.; Zeng, C.; Bao, H.; Liu, Z. The Long Noncoding RNA LOC105374325 Causes Podocyte Injury in Individuals with Focal Segmental Glomerulosclerosis. J. Biol. Chem. 2018, 293, 20227–20239. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, X.; Ru, Y.; Xu, J. Circ_0060077 Knockdown Alleviates High-Glucose-Induced Cell Apoptosis, Oxidative Stress, Inflammation and Fibrosis in HK-2 Cells via MiR-145-5p/VASN Pathway. Inflammation 2022, 45, 1911–1923. [Google Scholar] [CrossRef]
- Feng, F.; Yang, J.; Wang, G.; Huang, P.; Li, Y.; Zhou, B. Circ_0068087 Promotes High Glucose-Induced Human Renal Tubular Cell Injury through Regulating MiR-106a-5p/ROCK2 Pathway. Nephron 2023, 147, 212–222. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Yang, B.; Hou, B.; Sun, L.; Pang, H.; Wang, H.; Fan, Y. CircTAOK1 regulates high glucose induced inflammation, oxidative stress, ECM accumulation, and apoptosis in diabetic nephropathy via targeting miR-142-3p/SOX6 axis. Environ. Toxicol. 2024, 39, 2197–2207. [Google Scholar] [CrossRef]
- Wang, X.; Jing, R.; Yang, T.; Shao, R.; Yang, F.; Shi, Y.; Yang, X.; An, D.; Liang, Y. Research Progress on Non-Coding RNA Regulatory Networks and Targeted Therapy in Diabetic Nephropathy. Front. Endocrinol. 2025, 16, 1625307. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Benitez, M.B.; Navarro, Y.; Azuara-Liceaga, E.; Cruz, A.; Flores, J.; Lopez-Canovas, L. Circular RNAs and the Regulation of Gene Expression in Diabetic Nephropathy (Review). Int. J. Mol. Med. 2024, 53, 44. [Google Scholar] [CrossRef] [PubMed]
- van Zonneveld, A.J.; Kölling, M.; Bijkerk, R.; Lorenzen, J.M. Circular RNAs in Kidney Disease and Cancer. Nat. Rev. Nephrol. 2021, 17, 814–826. [Google Scholar] [CrossRef]
- Lv, J.; Wu, Y.; Mai, Y.; Bu, S. Noncoding RNAs in Diabetic Nephropathy: Pathogenesis, Biomarkers, and Therapy. J. Diabetes Res. 2020, 2020, 3960857. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, W.; Qian, L.; Sun, D. Insights into Non-Coding RNAS: Biogenesis, Function and Their Potential Regulatory Roles in Acute Kidney Disease and Chronic Kidney Disease. Mol. Cell Biochem. 2025, 480, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Zhang, Z.; Liu, J.; Chen, P.; Yang, C.; Wu, Y.; Wu, D.; Cao, Y.; Chu, Y.; Li, L. Circular RNAs: An Emerging Precise Weapon for Diabetic Nephropathy Diagnosis and Therapy. Biomed. Pharmacother. 2023, 168, 115818. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Liao, J.; Tang, F.; Gao, G.; Peng, J.; Fu, X.; Zhan, Y.; Chen, Z.; Xu, W.; et al. MicroRNA-21: A Critical Pathogenic Factor of Diabetic Nephropathy. Front. Endocrinol. 2022, 13, 895010. [Google Scholar] [CrossRef]
- Song, N.; Zhang, T.; Xu, X.; Lu, Z.; Yu, X.; Fang, Y.; Hu, J.; Jia, P.; Teng, J.; Ding, X. MiR-21 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Preventing Epithelial Cell Apoptosis and Inhibiting Dendritic Cell Maturation. Front. Physiol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Fan, P.-C.; Chen, C.-C.; Chen, Y.-C.; Chang, Y.-S.; Chu, P.-H. MicroRNAs in Acute Kidney Injury. Hum. Genom. 2016, 10, 29. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Luo, S.; Zhang, H.; Hu, F.; Chen, R.; Huang, C.; Su, Z. The MicroRNA MiR-29c Alleviates Renal Fibrosis via TPM1-Mediated Suppression of the Wnt/β-Catenin Pathway. Front. Physiol. 2020, 11, 331. [Google Scholar] [CrossRef]
- Moreno, J.A.; Hamza, E.; Guerrero-Hue, M.; Rayego-Mateos, S.; García-Caballero, C.; Vallejo-Mudarra, M.; Metzinger, L.; Metzinger-Le Meuth, V. Non-Coding RNAs in Kidney Diseases: The Long and Short of Them. Int. J. Mol. Sci. 2021, 22, 6077. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, Y.; Yang, X.; Long, Y.; Chen, S.; Lin, X.; Dong, R.; Yuan, J. Silencing of Long Noncoding RNA XIST Protects against Renal Interstitial Fibrosis in Diabetic Nephropathy via MicroRNA-93-5p-Mediated Inhibition of CDKN1A. Am. J. Physiol. Ren. Physiol. 2019, 317, F1350–F1358. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Q.; Li, W.; Xia, L.; Ge, X.; Shen, L.; Cang, Z.; Peng, W.; Shao, K.; Huang, S. Circular RNA CircEIF4G2 Aggravates Renal Fibrosis in Diabetic Nephropathy by Sponging MiR-218. J. Cell Mol. Med. 2022, 26, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-F.; Chenier, I.; Lavoie, J.L.; Chan, J.S.D.; Hamet, P.; Tremblay, J.; Chen, X.M.; Wang, D.H.; Inagami, T. Development of Hypertension and Kidney Hypertrophy in Transgenic Mice Overexpressing ARAP1 Gene in the Kidney. Hypertension 2006, 48, 453–459. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, T.; Ding, D.; Zhang, W.; Wang, X.; Sun, Z.; Hu, L.; Qin, S.; Shen, L.; He, B. Circulating MicroRNA-188, -30a, and -30e as Early Biomarkers for Contrast--Induced Acute Kidney Injury. J. Am. Heart Assoc. 2016, 5, e004138. [Google Scholar] [CrossRef]
- Huo, R.; Dai, M.; Fan, Y.; Zhou, J.-Z.; Li, L.; Zu, J. Predictive value of miRNA-29a and miRNA-10a-5p for 28-day mortality in patients with sepsis-induced acute kidney injury. Nan Fang. Yi Ke Da Xue Xue Bao 2017, 37, 646. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, H.; Liu, W.; Liu, S.; Wang, X.Y.; Diao, Z.L.; Zhang, A.H.; Guo, W.; Han, X.; Dong, X.; et al. Endothelial Progenitor Cells-Derived Exosomal MicroRNA-21-5p Alleviates Sepsis-Induced Acute Kidney Injury by Inhibiting RUNX1 Expression. Cell Death Dis. 2021, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fadeal, N.M.; Sultan, B.O.; AbdelMaogood, A.K.K.; Al Ageeli, E.; Mekhamer, F.T.; Rohayem, S.; Shahidy, A.; Hosny, N.; Fawzy, M.S.; Ismail, M.M.; et al. The Association of Cell-Free LncH19 and MiR-29b Expression with the PI3K/AKT/HIF-1/VEGF Pathway in Patients with Diabetic Nephropathy: In Silico Prediction and Clinical Validation. Curr. Issues Mol. Biol. 2024, 47, 20. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Wang, G.-Y.; Zhao, J.-W.; Jiang, H.-T. Knockdown of LncRNA MALAT1 Ameliorates Acute Kidney Injury by Mediating the MiR-204/APOL1 Pathway. J. Clin. Lab. Anal. 2021, 35, e23881. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, F.; Zhu, T.; Li, J.; Gu, D.; Jiang, W.; Lu, Y.; Zhou, D. Mechanism of Long Non-Coding RNA MALAT1 in Lipopolysaccharide-Induced Acute Kidney Injury Is Mediated by the MiR-146a/NF-ΚB Signaling Pathway. Int. J. Mol. Med. 2017, 41, 446–454. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Li, Q.; Dong, K.; Chen, C.; Mao, E.; Jiang, W. Downregulation of LncRNA NEAT1 Alleviates Sepsis-Induced Acute Kidney Injury. Cent. Eur. J. Immunol. 2022, 47, 8–19. [Google Scholar] [CrossRef]
- Susianti, H.; Sutrisnani, C.S.; Santosa, I.P.A.; Febrianto, W.; Kusdjianto, A.Y.; Kuwoyo, K.P.; Riyu, E. Evaluation of MicroRNA-10a and MicroRNA-210 as Biomarkers in Sepsis Patients with Acute Kidney Injury. Int. J. Nephrol. 2024, 2024, 1555811. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, C.; Ge, W.; Du, Y.; Hu, N. Circular RNA VMA21 Ameliorates Sepsis--associated Acute Kidney Injury by Regulating MiR--9--3p/SMG1/Inflammation Axis and Oxidative Stress. J. Cell Mol. Med. 2020, 24, 11397–11408. [Google Scholar] [CrossRef] [PubMed]
- Chioccioli, M.; Roy, S.; Newell, R.; Pestano, L.; Dickinson, B.; Rigby, K.; Herazo-Maya, J.; Jenkins, G.; Ian, S.; Saini, G.; et al. A Lung Targeted MiR-29 Mimic as a Therapy for Pulmonary Fibrosis. EBioMedicine 2022, 85, 104304. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.P.; Gross, O.; Wang, F.; Esteban de la Rosa, R.J.; Hall, M.; Sayer, J.A.; Appel, G.; Hariri, A.; Liu, S.; Maski, M.; et al. A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 995–1004. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Tang, P.M.-K.; Zhang, Y.-Y.; Lan, H.-Y. LncRNAs in TGF-β-Driven Tissue Fibrosis. Noncoding RNA 2018, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yu, C.; Yu, J.; Li, Z.; Lan, H.; Zhou, Q. Arid2-IR Promotes NF-ΚB-Mediated Renal Inflammation by Targeting NLRC5 Transcription. Cell. Mol. Life Sci. 2021, 78, 2387–2404. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Z.; Hu, J.; Su, H.; Liu, B.; Lang, Y.; Yu, Q.; Liu, Y.; Fan, X.; Yang, M.; et al. LncRNA PVT1 Induces Mitochondrial Dysfunction of Podocytes via TRIM56 in Diabetic Kidney Disease. Cell Death Dis. 2024, 15, 697. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.; Yu, X.; Lan, H.-Y. Smad3 Signatures in Renal Inflammation and Fibrosis. Int. J. Biol. Sci. 2022, 18, 2795–2806. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, Y.; Yang, Y.; Wu, Z.; Peng, N.; Xiao, J.; Dou, F.; Xu, J.; Pei, W.; Fu, C.; et al. Urinary Exosomes Derived CircRNAs as Biomarkers for Chronic Renal Fibrosis. Ann. Med. 2022, 54, 1966–1976. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Liu, X.-S.; Lan, H.-Y. Therapeutic Potential for Renal Fibrosis by Targeting Smad3-Dependent Noncoding RNAs. Mol. Ther. 2024, 32, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ye, Z.; Xue, Z.; Wu, L.; Ouyang, Y.; Yao, C.; Cui, C.; Xu, N.; Ma, J.; Hou, G.; et al. Identification of Renal Long Non-Coding RNA RP11-2B6.2 as a Positive Regulator of Type I Interferon Signaling Pathway in Lupus Nephritis. Front. Immunol. 2019, 10, 975. [Google Scholar] [CrossRef]
- Luan, J.; Jiao, C.; Kong, W.; Fu, J.; Qu, W.; Chen, Y.; Zhu, X.; Zeng, Y.; Guo, G.; Qi, H.; et al. CircHLA-C Plays an Important Role in Lupus Nephritis by Sponging MiR-150. Mol. Ther. Nucleic Acids 2018, 10, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-C.; Li, J.; Leng, R.-X.; Li, X.-P.; Li, X.-M.; Wang, D.-G.; Pan, H.-F.; Ye, D.-Q. Identification of Long Non-Coding RNAs GAS5, Linc0597 and Lnc-DC in Plasma as Novel Biomarkers for Systemic Lupus Erythematosus. Oncotarget 2017, 8, 23650–23663. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, R.; Tang, Z.; Wang, J.; Wang, C.; Song, Z.; Ni, B.; Zhang, L.; He, X.; You, Y. Circular RNA CircLOC101928570 Suppresses Systemic Lupus Erythematosus Progression by Targeting the MiR-150-5p/c-Myb Axis. J. Transl. Med. 2022, 20, 547. [Google Scholar] [CrossRef]
- Khoshmirsafa, M.; Kianmehr, N.; Falak, R.; Mowla, S.J.; Seif, F.; Mirzaei, B.; Valizadeh, M.; Shekarabi, M. Elevated Expression of MiR--21 and MiR--155 in Peripheral Blood Mononuclear Cells as Potential Biomarkers for Lupus Nephritis. Int. J. Rheum. Dis. 2019, 22, 458–467. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Etemadi, J.; Pourlak, T.; Mirhosaini, Z.; Vahed, S.Z.; Abediazar, S. Plasma levels of miR-21, miR-150, miR-423 in patients with lupus nephritis. Iran. J. Kidney Dis. 2019, 13, 198–206. [Google Scholar]
- Przybyciński, J.; Czerewaty, M.; Kwiatkowska, E.; Dziedziejko, V.; Safranow, K.; Domański, L.; Pawlik, A. MicroRNAs MiR-148a-3p, MiR-425-3p, and MiR-20a-5p in Patients with IgA Nephropathy. Genes 2025, 16, 125. [Google Scholar] [CrossRef]
- Serino, G.; Sallustio, F.; Cox, S.N.; Pesce, F.; Schena, F.P. Abnormal MiR-148b Expression Promotes Aberrant Glycosylation of IgA1 in IgA Nephropathy. J. Am. Soc. Nephrol. 2012, 23, 814–824. [Google Scholar] [CrossRef]
- Han, R.; Hu, S.; Qin, W.; Shi, J.; Zeng, C.; Bao, H.; Liu, Z. Upregulated Long Noncoding RNA LOC105375913 Induces Tubulointerstitial Fibrosis in Focal Segmental Glomerulosclerosis. Sci. Rep. 2019, 9, 716. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Lu, F.-H.; Huang, X.-R.; Zhang, L.; Mao, W.; Yu, X.-Q.; Liu, X.-S.; Lan, H.-Y. Non-Coding RNAs as Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Front. Pharmacol. 2020, 11, 583528. [Google Scholar] [CrossRef]
- Zheng, Q.; Reid, G.; Eccles, M.R.; Stayner, C. Non-Coding RNAs as Potential Biomarkers and Therapeutic Targets in Polycystic Kidney Disease. Front. Physiol. 2022, 13, 1006427. [Google Scholar] [CrossRef] [PubMed]
- Hüttenhofer, A.; Mayer, G. Circulating MiRNAs as Biomarkers of Kidney Disease. Clin. Kidney J. 2017, 10, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Clementi, A.; Virzì, G.M.; Ronco, C.; Monciino, P.; Zanella, M. Urinary and Plasma MiRNAs in the Early Detection of Acute Kidney Injury and Their Possible Role as Therapeutic Targets. J. Clin. Med. 2025, 14, 2306. [Google Scholar] [CrossRef] [PubMed]
- Elkahwagy, D.M.; Kiriacos, C.J.; Sobeih, M.E.; Khorshid, O.M.R.; Mansour, M. The LncRNAs Gas5, MALAT1 and SNHG8 as Diagnostic Biomarkers for Epithelial Malignant Pleural Mesothelioma in Egyptian Patients. Sci. Rep. 2024, 14, 4823. [Google Scholar] [CrossRef]
- Yu, Y.; Niu, Y.-Y.; Zhang, Y.-Y.; Yu, C. Urinary Long Non-Coding RNA GAS5 as a Noninvasive Diagnostic Biomarker for Renal Fibrosis. Ren. Fail. 2025, 47, 2534493. [Google Scholar] [CrossRef]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Gastebois, C.; Meugnier, E.; Mohan, V.; Balasubramanyam, M. MicroRNAs from Urinary Extracellular Vesicles Are Non-Invasive Early Biomarkers of Diabetic Nephropathy in Type 2 Diabetes Patients with the “Asian Indian Phenotype”. Diabetes Metab. 2019, 45, 276–285. [Google Scholar] [CrossRef]
- Małachowska, B.; Tkaczyk, M.; Chrul, S.; Zwiech, R.; Młynarski, W.; Fendler, W. Serum MicroRNA Profiles in Patients with Autosomal Dominant Polycystic Kidney Disease Show Systematic Dysregulation Partially Reversible by Hemodialysis. Arch. Med. Sci. 2021, 17, 1730–1741. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Hsu, C.-T.; Tsai, S.-F.; Chen, C.-H. Association between Circulating MicroRNAs (MiR-21-5p, MiR-20a-5p, MiR-29b-3p, MiR-126-3p and MiR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients. Int. J. Mol. Sci. 2022, 23, 2253. [Google Scholar] [CrossRef]
- Hu, M.; Shen, X.; Zhou, L. Role of Extracellular Vesicle-Derived Noncoding RNAs in Diabetic Kidney Disease. Kidney Dis. 2024, 10, 303–312. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, Q.; Fang, L.; Li, G.; Liu, Y.; Li, J.; Pan, S.; Zhou, S.; Duan, J.; Liu, D.; et al. Identification of Urinary Extracellular Vesicles Differentially Expressed RNAs in Diabetic Nephropathy via Whole-Transcriptome Integrated Analysis. Comput. Biol. Med. 2023, 166, 107480. [Google Scholar] [CrossRef]
- Luo, C.; Liu, H.; Shao, L.; Tang, J.; He, Q.; Jin, J. The Role of Small Extracellular Vesicle Non-Coding RNAs in Kidney Diseases. Front. Genet. 2022, 13, 1013637. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cui, Y.; Yin, M.; Liu, F. Screening Potential Prognostic Biomarkers of Long Non-Coding RNAs for Predicting the Risk of Chronic Kidney Disease. Braz. J. Med. Biol. Res. 2019, 52, e8333. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Gu, L.; Li, X.; Gao, Y.; Lyu, X.; Chen, L.; Luo, G.; Wang, L.; Xie, Y.; et al. LncRNAs Act as Prognostic and Diagnostic Biomarkers in Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Oncotarget 2016, 7, 74325–74336. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, D.; Huang, N.; Zhou, Q. Circular RNAs as Novel Diagnostic Biomarkers and Therapeutic Targets in Kidney Disease. Front. Med. 2021, 8, 714958. [Google Scholar] [CrossRef]
- Franco-Acevedo, A.; Melo, Z.; Echavarria, R. Diagnostic, Prognostic, and Therapeutic Value of Non-Coding RNA Expression Profiles in Renal Transplantation. Diagnostics 2020, 10, 60. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (MiRNAs) and Long Non-Coding RNAs (LncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
- Cordier, A.-G.; Zerbib, E.; Favier, A.; Dabi, Y.; Daraï, E. Value of Non-Coding RNA Expression in Biofluids to Identify Patients at Low Risk of Pathologies Associated with Pregnancy. Diagnostics 2024, 14, 729. [Google Scholar] [CrossRef]
- Cheong, J.K.; Rajgor, D.; Lv, Y.; Chung, K.Y.; Tang, Y.C.; Cheng, H. Noncoding RNome as Enabling Biomarkers for Precision Health. Int. J. Mol. Sci. 2022, 23, 390. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Olszewski, R.; Parolczyk, M.; Rysz-Górzyńska, M.; Rysz, J. MiRNA Biomarkers in Renal Disease. Int. Urol. Nephrol. 2022, 54, 575–588. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Tsotakos, N. Editorial: Non-Coding RNA and Renal Disease. Front. Mol. Biosci. 2024, 11, 1404930. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ni, W.; Wu, Y.; Zhai, B.; Zhao, Q.; Zheng, T.; Liu, Q.; Ding, D. Application of Biomarkers in the Diagnosis of Kidney Disease. Front. Med. 2025, 12, 1560222. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ming, X.; Carver, K.; Laing, B. Cellular Uptake and Intracellular Trafficking of Oligonucleotides: Implications for Oligonucleotide Pharmacology. Nucleic Acid. Ther. 2014, 24, 101–113. [Google Scholar] [CrossRef]
- Szostak, J.; Gorący, A.; Durys, D.; Dec, P.; Modrzejewski, A.; Pawlik, A. The Role of MicroRNA in the Pathogenesis of Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 6214. [Google Scholar] [CrossRef]
- Lv, W.; Fan, F.; Wang, Y.; Gonzalez-Fernandez, E.; Wang, C.; Yang, L.; Booz, G.W.; Roman, R.J. Therapeutic Potential of MicroRNAs for the Treatment of Renal Fibrosis and CKD. Physiol. Genom. 2018, 50, 20–34. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudehen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. MicroRNA-17 Family Promotes Polycystic Kidney Disease Progression through Modulation of Mitochondrial Metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef]
- Yheskel, M.; Lakhia, R.; Cobo-Stark, P.; Flaten, A.; Patel, V. Anti-MicroRNA Screen Uncovers MiR-17 Family within MiR-17~92 Cluster as the Primary Driver of Kidney Cyst Growth. Sci. Rep. 2019, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Tang, P.M.-K.; Huang, X.-R.; Sun, S.-F.; You, Y.-K.; Xiao, J.; Lv, L.-L.; Xu, A.-P.; Lan, H.-Y. TGF-β Mediates Renal Fibrosis via the Smad3-Erbb4-IR Long Noncoding RNA Axis. Mol. Ther. 2018, 26, 148–161. [Google Scholar] [CrossRef]
- Sun, S.F.; Tang, P.M.K.; Feng, M.; Xiao, J.; Huang, X.R.; Li, P.; Ma, R.C.W.; Lan, H.Y. Novel LncRNA Erbb4-IR Promotes Diabetic Kidney Injury in Db/Db Mice by Targeting MiR-29b. Diabetes 2018, 67, 731–744. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, Y.; Yu, W.; Li, H.; He, D. Silencing of LncRNA KCNQ1OT1 Confers an Inhibitory Effect on Renal Fibrosis through Repressing MiR-124-3p Activity. Bioengineered 2022, 13, 10399–10411. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xu, S.; Ren, T.; Pan, T.; Wang, X.; Zhang, Y.; Zou, Z.; Guo, M.; Zeng, Q.; Shen, B.; et al. LncRNA IRAR Regulates Chemokines Production in Tubular Epithelial Cells Thus Promoting Kidney Ischemia-Reperfusion Injury. Cell Death Dis. 2022, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wahane, A.; Tham, M.S.; Somlo, S.; Gupta, A.; Bahal, R. Efficient and Selective Kidney Targeting by Chemically Modified Carbohydrate Conjugates. Mol. Ther. 2024, 32, 4383–4400. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Ma, J.-X.; Lu, B.; Criswell, T.; Zhang, Y. Exosome-Mediated Renal Protection: Halting the Progression of Fibrosis. Genes. Dis. 2024, 11, 101117. [Google Scholar] [CrossRef]
- Dumoulin, B.; Yamada, K.; Susztak, K. SNA-Modified Antisense Oligonucleotides: A New Pathway for Renal Targeting? Mol. Ther. Nucleic Acids 2025, 36, 102476. [Google Scholar] [CrossRef]
- Nieskens, T.T.G.; Magnusson, O.; Andersson, P.; Söderberg, M.; Persson, M.; Sjögren, A.-K. Nephrotoxic Antisense Oligonucleotide SPC5001 Induces Kidney Injury Biomarkers in a Proximal Tubule-on-a-Chip. Arch. Toxicol. 2021, 95, 2123–2136. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Valencia, T.; Allerson, C.; Schairer, A.; Flaten, A.; Yheskel, M.; Kersjes, K.; Li, J.; Gatto, S.; Takhar, M.; et al. Discovery and Preclinical Evaluation of Anti-MiR-17 Oligonucleotide RGLS4326 for the Treatment of Polycystic Kidney Disease. Nat. Commun. 2019, 10, 4148. [Google Scholar] [CrossRef]
- Kamel, A.; Owen, T.; Cole, I.; Valencia, T.; Lee, E.C. Pharmacokinetics and Absorption, Distribution, Metabolism and Excretion of RGLS4326 in Mouse and Monkey, an Anti–MiR-17 Oligonucleotide for the Treatment of Polycystic Kidney Disease. Drug Metab. Dispos. 2023, 51, 1536–1546. [Google Scholar] [CrossRef]
- Plawgo, K.; Raczynska, K.D. Context-Dependent Regulation of Gene Expression by Non-Canonical Small RNAs. Noncoding RNA 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Hueso, M.; Mallén, A.; Suñé-Pou, M.; Aran, J.M.; Suñé-Negre, J.M.; Navarro, E. NcRNAs in Therapeutics: Challenges and Limitations in Nucleic Acid-Based Drug Delivery. Int. J. Mol. Sci. 2021, 22, 11596. [Google Scholar] [CrossRef]
- Saliba, A.; Du, Y.; Feng, T.; Garmire, L. Multi-Omics Integration in Nephrology: Advances, Challenges, and Future Directions. Semin. Nephrol. 2024, 44, 151584. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Decoding Kidney Pathophysiology: Omics-Driven Approaches in Precision Medicine. J. Pers. Med. 2024, 14, 1157. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorović, P.; Pavlović, N.; Maglica, M.; Bajt, P.; Kelam, N.; Raguž, F.; Vukojević, K. Non-Coding RNAs as Emerging Regulators in Kidney Pathophysiology: From Molecular Mechanisms to Therapeutic Potential. Genes 2025, 16, 1328. https://doi.org/10.3390/genes16111328
Todorović P, Pavlović N, Maglica M, Bajt P, Kelam N, Raguž F, Vukojević K. Non-Coding RNAs as Emerging Regulators in Kidney Pathophysiology: From Molecular Mechanisms to Therapeutic Potential. Genes. 2025; 16(11):1328. https://doi.org/10.3390/genes16111328
Chicago/Turabian StyleTodorović, Petar, Nikola Pavlović, Mirko Maglica, Patricija Bajt, Nela Kelam, Fila Raguž, and Katarina Vukojević. 2025. "Non-Coding RNAs as Emerging Regulators in Kidney Pathophysiology: From Molecular Mechanisms to Therapeutic Potential" Genes 16, no. 11: 1328. https://doi.org/10.3390/genes16111328
APA StyleTodorović, P., Pavlović, N., Maglica, M., Bajt, P., Kelam, N., Raguž, F., & Vukojević, K. (2025). Non-Coding RNAs as Emerging Regulators in Kidney Pathophysiology: From Molecular Mechanisms to Therapeutic Potential. Genes, 16(11), 1328. https://doi.org/10.3390/genes16111328








