Muscle Oxygen Saturation Responses During Maximal and Submaximal Exercise According to SLC16A1 (MCT1) Gene Polymorphism in Long-Distance Runners: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Experimental Procedures
2.3.1. Questionnaire
2.3.2. Genotyping
2.3.3. Body Composition Measurement
2.3.4. Graded Incremental Exercise Test (GXT)
2.3.5. SmO2 Measurement
2.4. Calculated Variables
2.5. Statistical Analysis
3. Results
3.1. Correlation Between the PBR and Physiological Parameters in the Participants
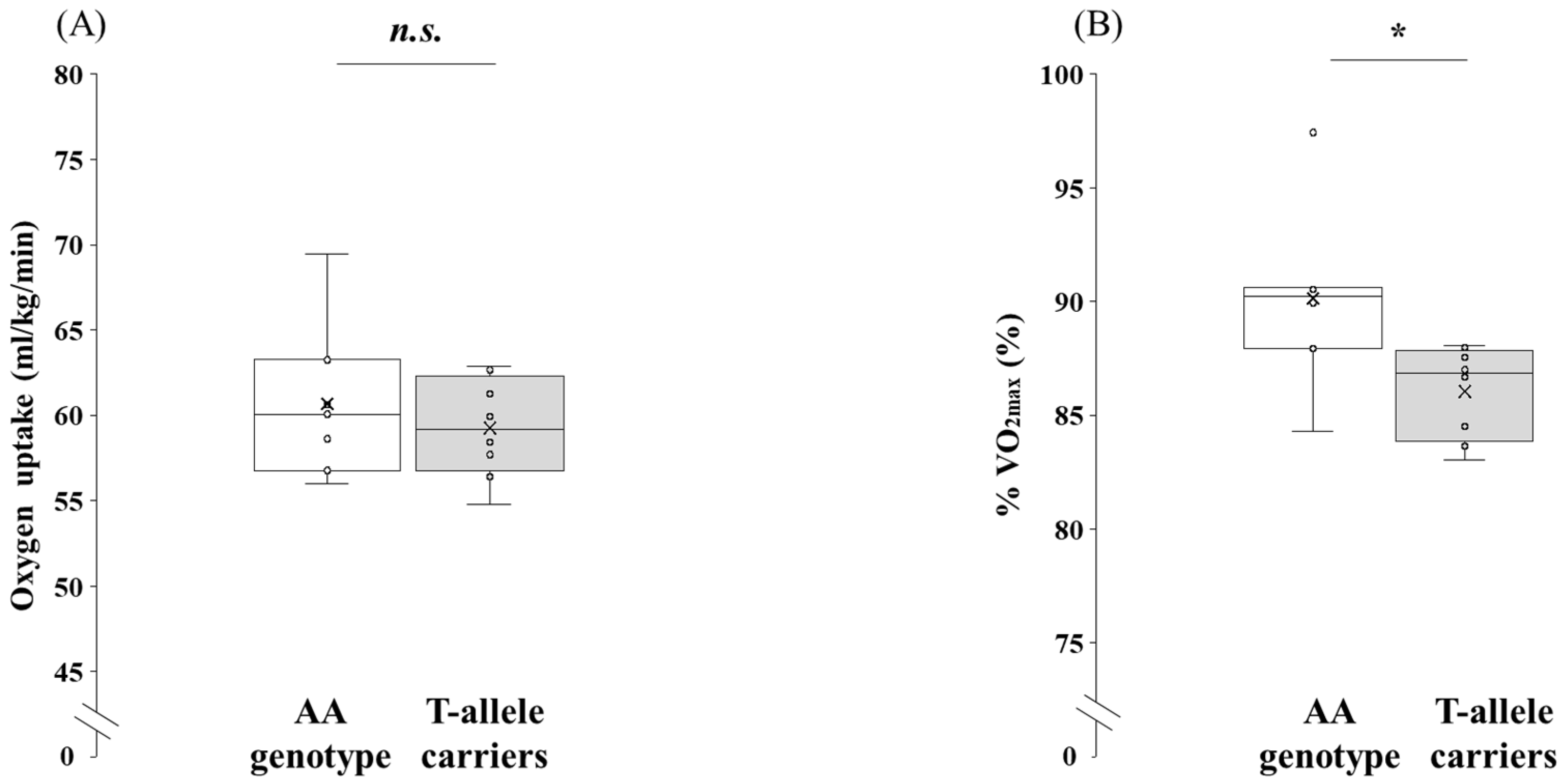
3.2. Comparison of Physiological Parameters at Maximal Exercise Between the AA Genotype and the T-Allele Carriers
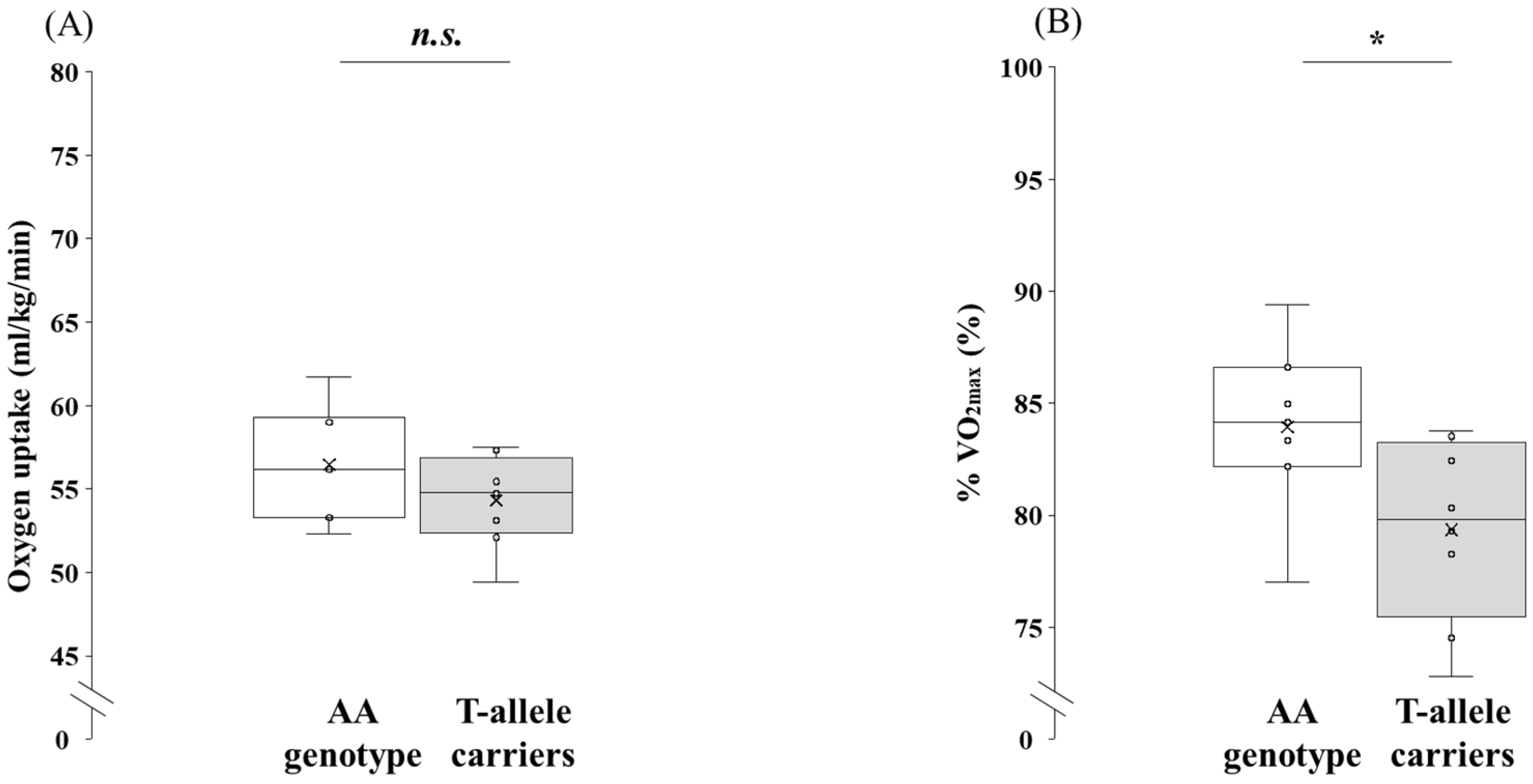
3.3. Comparison of Physiological Parameters at Submaximal Exercise Between the AA Genotype and the T-Allele Carriers
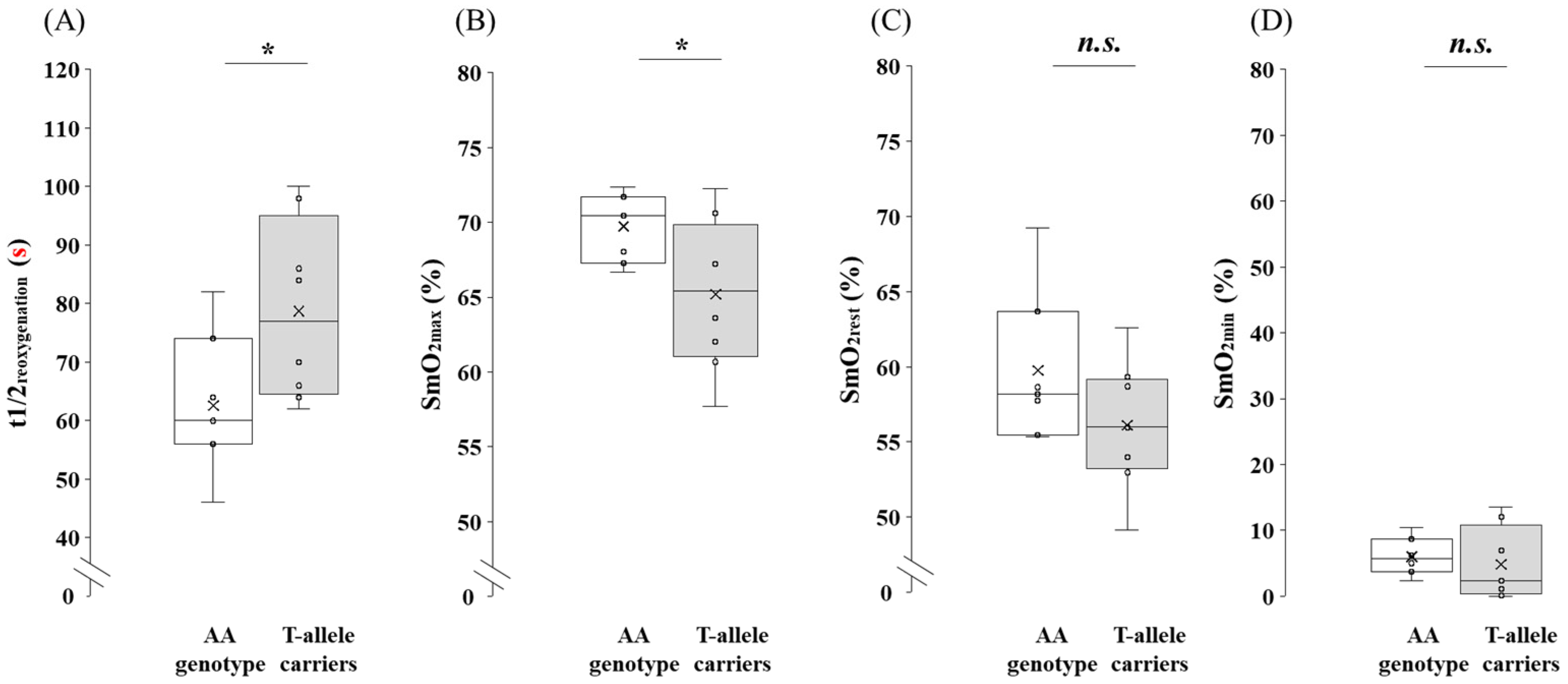
3.4. Comparison of SmO2 at Maximal Exercise Between the AA Genotype and the T-Allele Carriers
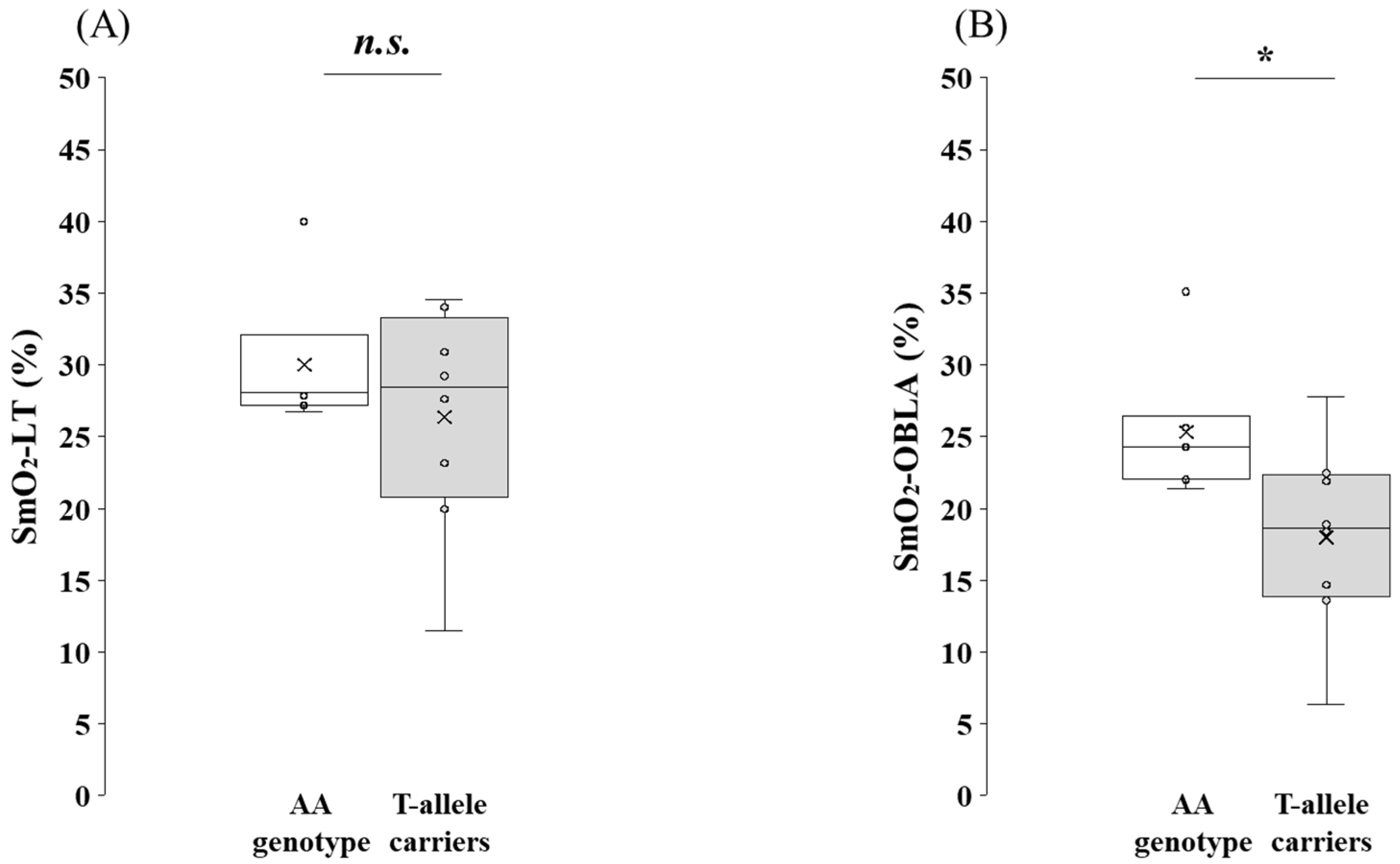
3.5. Comparison of SmO2 at Submaximal Exercise Between the AA Genotype and the T-Allele Carriers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, S.; Craig, I.; Wilson, J.; Aitchison, T. The relationship between 3 km running performance and selected physiological variables. J. Sports Sci. 1997, 15, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.W.; McNaughton, L.R.; Jones, A.M. Training to enhance the physiological determinants of long-distance running performance: Can valid recommendations be given to runners and coaches based on current scientific knowledge? Sports Med. 2007, 37, 857–880. [Google Scholar] [CrossRef] [PubMed]
- Llodio, I.; Gorostiaga, E.M.; Garcia-Tabar, I.; Granados, C.; Sánchez-Medina, L. Estimation of the maximal lactate steady state in endurance runners. Int. J. Sports Med. 2016, 37, 539–546. [Google Scholar] [CrossRef] [PubMed]
- van Hall, G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol. 2010, 199, 499–508. [Google Scholar] [CrossRef]
- Bonen, A.; McCullagh, K.J.A.; Putman, C.T.; Hultman, E.; Jones, N.L.; Heigenhauser, G.J.F. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am. J. Physiol. 1998, 274, E102–E107. [Google Scholar] [CrossRef]
- Eydoux, N.; Py, G.; Lambert, K.; Dubouchaud, H.; Préfaut, C.; Mercier, J. Training does not protect against exhaustive exercise-induced lactate transport capacity alterations. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1045–E1052. [Google Scholar] [CrossRef]
- Juel, C. Current aspects of lactate exchange: Lactate/H+ transport in human skeletal muscle. Eur. J. Appl. Physiol. 2001, 86, 12–16. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Wu, H.; Liu, Y.; Yang, Y. Functional heterogeneity of MCT1 and MCT4 in metabolic reprogramming affects osteosarcoma growth and metastasis. J. Orthop. Surg. Res. 2023, 18, 131. [Google Scholar] [CrossRef]
- Pereira-Nunes, A.; Simões-Sousa, S.; Pinheiro, C.; Miranda-Gonçalves, V.; Granja, S.; Baltazar, F. Targeting lactate production and efflux in prostate cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165894. [Google Scholar] [CrossRef]
- Bonglack, E.N.; Messinger, J.E.; Cable, J.M.; Ch’ng, J.; Parnell, K.M.; Reinoso-Vizcaino, N.M.; Barry, A.P.; Russell, V.S.; Dave, S.S.; Christofk, H.R.; et al. Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proc. Natl. Acad. Sci. USA 2021, 118, e2022495118. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Fishbein, W.N.; Davis, J.I.; Foellmer, J.W. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 2000, 23, 90–97. [Google Scholar] [CrossRef]
- Sasaki, S.; Futagi, Y.; Kobayashi, M.; Ogura, J.; Iseki, K. Functional characterization of 5-oxoproline transport via SLC16A1/MCT1. J. Biol. Chem. 2015, 290, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Sawczuk, M.; Banting, L.K.; Cięszczyk, P.; Maciejewska-Karłowska, A.; Zarębska, A.; Leońska-Duniec, A.; Jastrzębski, Z.; Bishop, D.J.; Eynon, N. MCT1 A1470T: A novel polymorphism for sprint performance? J. Sci. Med. Sport 2015, 18, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Saito, M.; Mochizuki, Y.; Shinogi, M.; Kobatake, N.; Okamoto, T.; Nishiyama, T.; Nakazato, K.; Kikuchi, N. Association between MCT1 T1470A polymorphism and athlete status in Japanese power-oriented athletes. Gazz. Med. Ital.-Arch. Sci. Med. 2023, 182, 43–48. [Google Scholar] [CrossRef]
- Fedotovskaya, O.N.; Mustafina, L.J.; Popov, D.V.; Vinogradova, O.L.; Ahmetov, I.I. A common polymorphism of the MCT1 gene and athletic performance. Int. J. Sports Physiol. Perform. 2014, 9, 173–180. [Google Scholar] [CrossRef]
- Kikuchi, N.; Fuku, N.; Matsumoto, R.; Matsumoto, S.; Murakami, H.; Miyachi, M.; Nakazato, K. The association between MCT1 T1470A polymorphism and power-oriented athletic performance. Int. J. Sports Med. 2017, 38, 76–80. [Google Scholar] [CrossRef]
- Cupeiro, R.; Pérez-Prieto, R.; Amigo, T.; Gortázar, P.; Redondo, C.; González-Lamuño, D. Role of the monocarboxylate transporter MCT1 in the uptake of lactate during active recovery. Eur. J. Appl. Physiol. 2016, 116, 1005–1010. [Google Scholar] [CrossRef]
- Guilherme, J.P.L.F.; Bosnyák, E.; Semenova, E.A.; Szmodis, M.; Griff, A.; Móra, Á.; Almási, G.; Trájer, E.; Udvardy, A.; Kostryukova, E.S.; et al. The MCT1 gene Glu490Asp polymorphism (rs1049434) is associated with endurance athlete status, lower blood lactate accumulation and higher maximum oxygen uptake. Biol. Sport 2021, 38, 465–474. [Google Scholar] [CrossRef]
- Ben-Zaken, S.; Meckel, Y.; Nemet, D.; Kassem, E.; Eliakim, A. Genetic basis for the dominance of Israeli long-distance runners of Ethiopian origin. J. Strength Cond. Res. 2021, 35, 1885–1896. [Google Scholar] [CrossRef]
- Seki, S.; Kobayashi, T.; Beppu, K.; Nojo, M.; Hoshina, K.; Kikuchi, N.; Okamoto, T.; Nakazato, K.; Hwang, I. Association among MCT1 rs1049434 polymorphism, athlete status, and physiological parameters in Japanese long-distance runners. Genes 2024, 15, 1627. [Google Scholar] [CrossRef]
- Feldmann, A.; Schmitz, R.; Erlacher, D. Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: Reliability and validity of the Moxy Monitor. J. Biomed. Opt. 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.S.; Clark, D.A.; Bradley, E.M.; Wilkins, B.W. The balance of muscle oxygen supply and demand reveals critical metabolic rate and predicts time to exhaustion. J. Appl. Physiol. (1985) 2021, 130, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Batterson, P.M.; Kirby, B.S.; Hasselmann, G.; Feldmann, A. Muscle oxygen saturation rates coincide with lactate-based exercise thresholds. Eur. J. Appl. Physiol. 2023, 123, 2249–2258. [Google Scholar] [CrossRef]
- Yogev, A.; Arnold, J.; Nelson, H.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M.S. Comparing the reliability of muscle oxygen saturation with common performance and physiological markers across cycling exercise intensity. Front. Sports Act. Living 2023, 5, 1143393. [Google Scholar] [CrossRef]
- Flück, M.; Protte, C.; Giraud, M.-N.; Gsponer, T.; Dössegger, A. Genotypic influences on actuators of aerobic performance in tactical athletes. Genes 2024, 15, 1535. [Google Scholar] [CrossRef]
- Porter, M.; Langley, J. The relationship between muscle oxygen saturation kinetics and maximal blood lactate accumulation rate across varying sprint cycle durations. Eur. J. Sport Sci. 2025, 25, e12242. [Google Scholar] [CrossRef]
- Austin, K.G.; Daigle, K.A.; Patterson, P.; Cowman, J.; Chelland, S.; Haymes, E.M. Reliability of near-infrared spectroscopy for determining muscle oxygen saturation during exercise. Res. Q. Exerc. Sport 2005, 76, 440–449. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. Improved detection of lactate threshold during exercise using a log-log transformation. J. Appl. Physiol. (1985) 1985, 59, 1936–1940. [Google Scholar] [CrossRef]
- Gasser, B.; Franchi, M.V.; Ruoss, S.; Frei, A.; Popp, W.L.; Niederseer, D.; Catuogno, S.; Frey, W.O.; Flück, M. Accelerated muscle deoxygenation in aerobically fit subjects during exhaustive exercise is associated with the ACE insertion allele. Front. Sports Act. Living 2022, 4, 814975. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Science; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Legaz-Arrese, A.; Munguia-Izquierdo, D.; Nuviala, A.N.; Serveto-Galindo, O.; Urdiales, D.M.; Masia, J.R. Average VO2max as a function of running performances on different distances. Sci. Sport 2007, 22, 43–49. [Google Scholar] [CrossRef]
- Gasser, B.; Dössegger, A.; Giraud, M.-N.; Flück, M. T-Allele carriers of mono carboxylate transporter one gene polymorphism rs1049434 demonstrate altered substrate metabolization during exhaustive exercise. Genes 2024, 15, 918. [Google Scholar] [CrossRef]
- Clifford, P.S.; Hellsten, Y. Vasodilatory mechanisms in contracting skeletal muscle. J. Appl. Physiol. (1985) 2004, 97, 393–403. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Iotti, S.; Kendrick, K.; Wang, Z.; Posner, J.D.; Leigh, J.; Chance, B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J. Appl. Physiol. (1985) 1994, 77, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Yogev, A.; Arnold, J.I.; Nelson, H.; Rosenblat, M.A.; Clarke, D.C.; Guenette, J.A.; Sporer, B.C.; Koehle, M.S. The effects of endurance training on muscle oxygen desaturation during incremental exercise tests: A systematic review and meta-analysis. Front. Sports Act. Living 2024, 6, 1406987. [Google Scholar] [CrossRef] [PubMed]
- Dalamitros, A.A.; Semaltianou, E.; Toubekis, A.G.; Kabasakalis, A. Muscle oxygenation, heart rate, and blood lactate concentration during submaximal and maximal interval swimming. Front. Sports Act. Living 2021, 3, 759925. [Google Scholar] [CrossRef] [PubMed]
- Grassi, B.; Quaresima, V.; Marconi, C.; Ferrari, M.; Cerretelli, P. Blood lactate accumulation and muscle deoxygenation during incremental exercise. J. Appl. Physiol. 1999, 87, 348–355. [Google Scholar] [CrossRef]
- Chuang, M.L.; Ting, H.; Otsuka, T.; Sun, X.G.; Chiu, F.Y.; Hansen, J.E.; Wasserman, K. Muscle deoxygenation as related to work rate. Med. Sci. Sports Exerc. 2002, 34, 1614–1623. [Google Scholar] [CrossRef]
- Jones, B.; Parry, D.; Cooper, C.E. Underwater near-infrared spectroscopy can measure training adaptations in adolescent swimmers. PeerJ 2018, 6, e4393. [Google Scholar] [CrossRef]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef]
- Hashimoto, T.; Brooks, G.A. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med. Sci. Sport. Exerc. 2008, 40, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Leija, R.G.; Arevalo, J.A.; Xing, D.; Vázquez-Medina, J.P.; Brooks, G.A. The mitochondrial lactate oxidation complex: Endpoint for carbohydrate carbon disposal. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E126–E136. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life 2012, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, I.; Kimura, Y.; Kobayashi, M.; Narumi, K.; Furugen, A.; Miyoshi, H.; Nakamura, A.; Yamada, T.; Atsumi, T.; Iseki, K. Relationships between plasma lactate, plasma alanine, genetic variations in lactate transporters and type 2 diabetes in the Japanese population. Drug Metab. Pharmacokinet. 2020, 35, 131–138. [Google Scholar] [CrossRef]
- Bıçakçı, B.; Cięszczyk, P.; Humińska-Lisowska, K. Genetic determinants of endurance: A narrative review on elite athlete status and performance. Int. J. Mol. Sci. 2024, 25, 13041. [Google Scholar] [CrossRef]

| All Participants (n = 15) | AA Genotype (n = 7) | T-Allele Carriers (n = 8) | |||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |
| Age (years) | 20.6 | ± | 0.9 | 20.6 | ± | 0.8 | 20.6 | ± | 1.2 |
| Career (years) | 6.9 | ± | 1.9 | 7.3 | ± | 1.6 | 6.5 | ± | 2.4 |
| Height (cm) | 172.8 | ± | 6.1 | 174.9 | ± | 6.2 | 170.9 | ± | 5.8 |
| Weight (kg) | 58.3 | ± | 3.4 | 58.6 | ± | 3.6 | 58 | ± | 3.5 |
| FM (kg) | 5.5 | ± | 1.8 | 5.4 | ± | 2.2 | 5.5 | ± | 1.6 |
| LBM (kg) | 52.8 | ± | 3.2 | 53.2 | ± | 4.2 | 52.5 | ± | 2.3 |
| %BF (%) | 9.1 | ± | 2.4 | 8.8 | ± | 2.9 | 9.4 | ± | 2.1 |
| BMI (kg/m2) | 19.6 | ± | 1.1 | 19.2 | ± | 1.0 | 19.9 | ± | 1.2 |
| Skinfold thickness of the vastus lateralis (mm) | 6.4 | ± | 1.2 | 6.3 | ± | 1.1 | 6.5 | ± | 1.3 |
| 5000-m PBRs (s) | 889.6 | ± | 22.0 | 883.1 | ± | 18.8 | 895.3 | ± | 24.3 |
| Variables (Unit) | Genotypes (n) | p-Value | 95% CI | Effect Size | 95% CI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA Genotype (n = 7) | T-Allele Carriers (n = 8) | |||||||||||||
| O2max (mL/kg/min) | 67.3 | ± | 3.3 | 68.6 | ± | 4.4 | 0.543 | −5.64 | to | 3.11 | d = 0.32 | −1.34 | to | 0.71 |
| CO2max (mL/kg/min) | 76.3 | ± | 5.5 | 81.4 | ± | 5.8 | 0.109 | −11.37 | to | 1.30 | d = 0.89 | −1.94 | to | 0.20 |
| HRmax (beats/min) | 191.0 | ± | 9.1 | 188.8 | ± | 6.0 | 0.578 | −6.27 | to | 10.77 | d = 0.30 | −0.73 | to | 1.31 |
| Respiratory Quotient | 1.3 | ± | 0.1 | 1.2 | ± | 0.1 | 0.512 | −0.07 | to | 0.13 | d = 0.35 | −0.68 | to | 1.37 |
| VEmax (mL/kg/min) | 2.9 (2.5 to 3.5) | 2.7 (2.3 to 4.4) | 0.536 | −0.26 | to | 0.54 | r = 0.18 | |||||||
| RPE | 19.0 (18.0 to 20.0) | 19.5 (17.0 to 20.0) | 0.779 | −2.00 | to | 1.00 | r = 0.08 | |||||||
| BLamax (mmol/L) | 11.4 | ± | 1.1 | 11.6 | ± | 1.4 | 0.711 | −1.69 | to | 1.18 | d = 0.20 | −1.21 | to | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seki, S.; Kobayashi, T.; Kikuchi, N.; Hoshina, K.; Hwang, I. Muscle Oxygen Saturation Responses During Maximal and Submaximal Exercise According to SLC16A1 (MCT1) Gene Polymorphism in Long-Distance Runners: A Cross-Sectional Pilot Study. Genes 2025, 16, 1324. https://doi.org/10.3390/genes16111324
Seki S, Kobayashi T, Kikuchi N, Hoshina K, Hwang I. Muscle Oxygen Saturation Responses During Maximal and Submaximal Exercise According to SLC16A1 (MCT1) Gene Polymorphism in Long-Distance Runners: A Cross-Sectional Pilot Study. Genes. 2025; 16(11):1324. https://doi.org/10.3390/genes16111324
Chicago/Turabian StyleSeki, Shotaro, Tetsuro Kobayashi, Naoki Kikuchi, Kosaku Hoshina, and Inkwan Hwang. 2025. "Muscle Oxygen Saturation Responses During Maximal and Submaximal Exercise According to SLC16A1 (MCT1) Gene Polymorphism in Long-Distance Runners: A Cross-Sectional Pilot Study" Genes 16, no. 11: 1324. https://doi.org/10.3390/genes16111324
APA StyleSeki, S., Kobayashi, T., Kikuchi, N., Hoshina, K., & Hwang, I. (2025). Muscle Oxygen Saturation Responses During Maximal and Submaximal Exercise According to SLC16A1 (MCT1) Gene Polymorphism in Long-Distance Runners: A Cross-Sectional Pilot Study. Genes, 16(11), 1324. https://doi.org/10.3390/genes16111324





