Identification of a Novel EVC2 Variant in a Family with Non-Syndromic Tooth Agenesis and Its Potential Functional Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. WES and Bioinformatics Analysis
2.3. PCR Amplification and Sanger Sequencing
2.4. Prediction of Damaging Effects
2.5. Molecular Dynamics Simulations
2.5.1. System Construction
2.5.2. Energy Minimization and Production Dynamics
2.5.3. Root-Mean-Square Deviation (RMSD) Analysis
2.5.4. Molecular Mechanics-Generalized Born Surface Area (MM-GBSA) Binding Free Energy
2.6. Plasmid Construction and Cell Transfection
2.7. Subcellular Localization
2.8. Western Blot
2.9. RNA Extraction and RT-qPCR
2.10. Statistical Analysis
3. Results
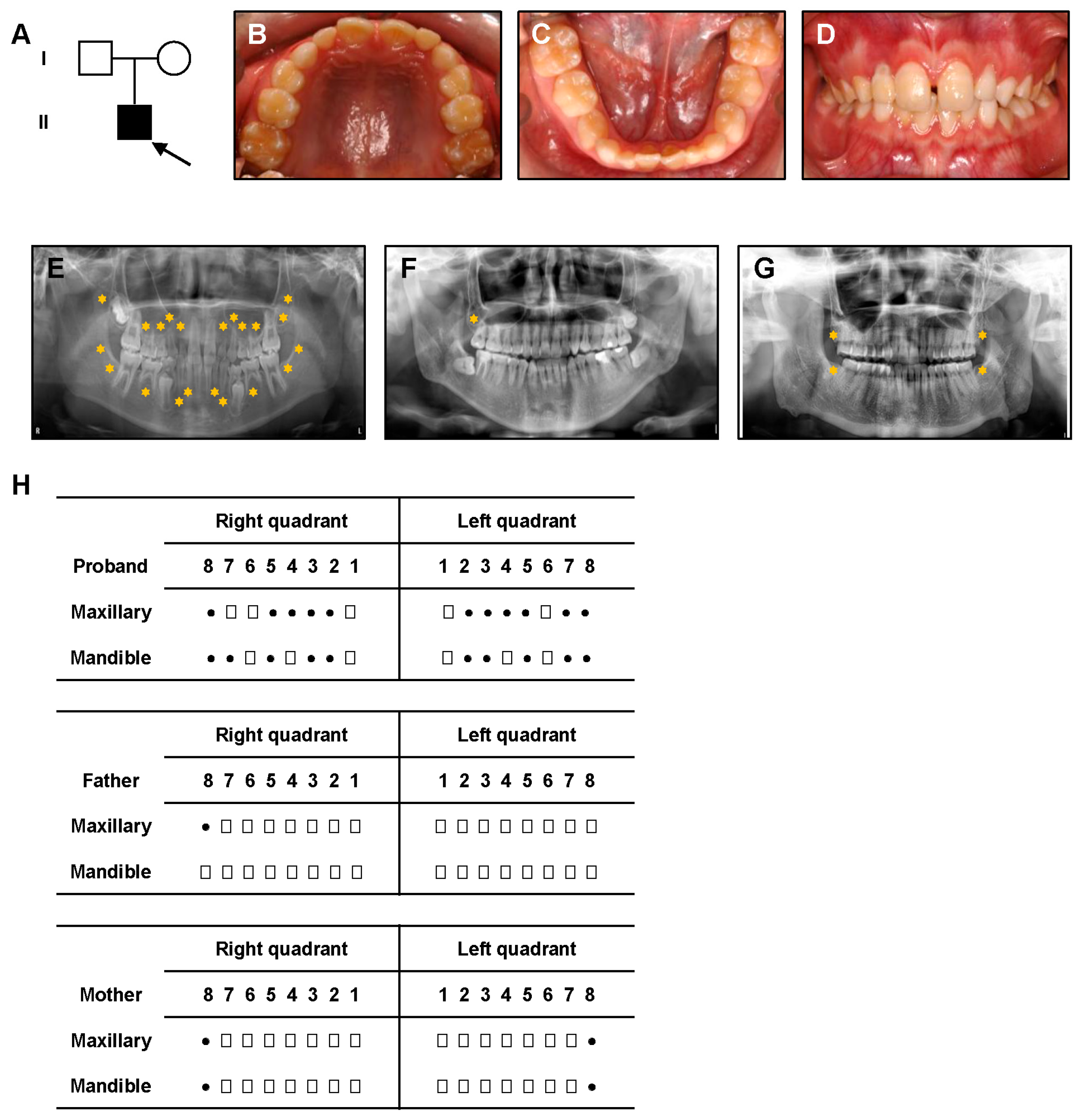
3.1. Clinical Manifestations
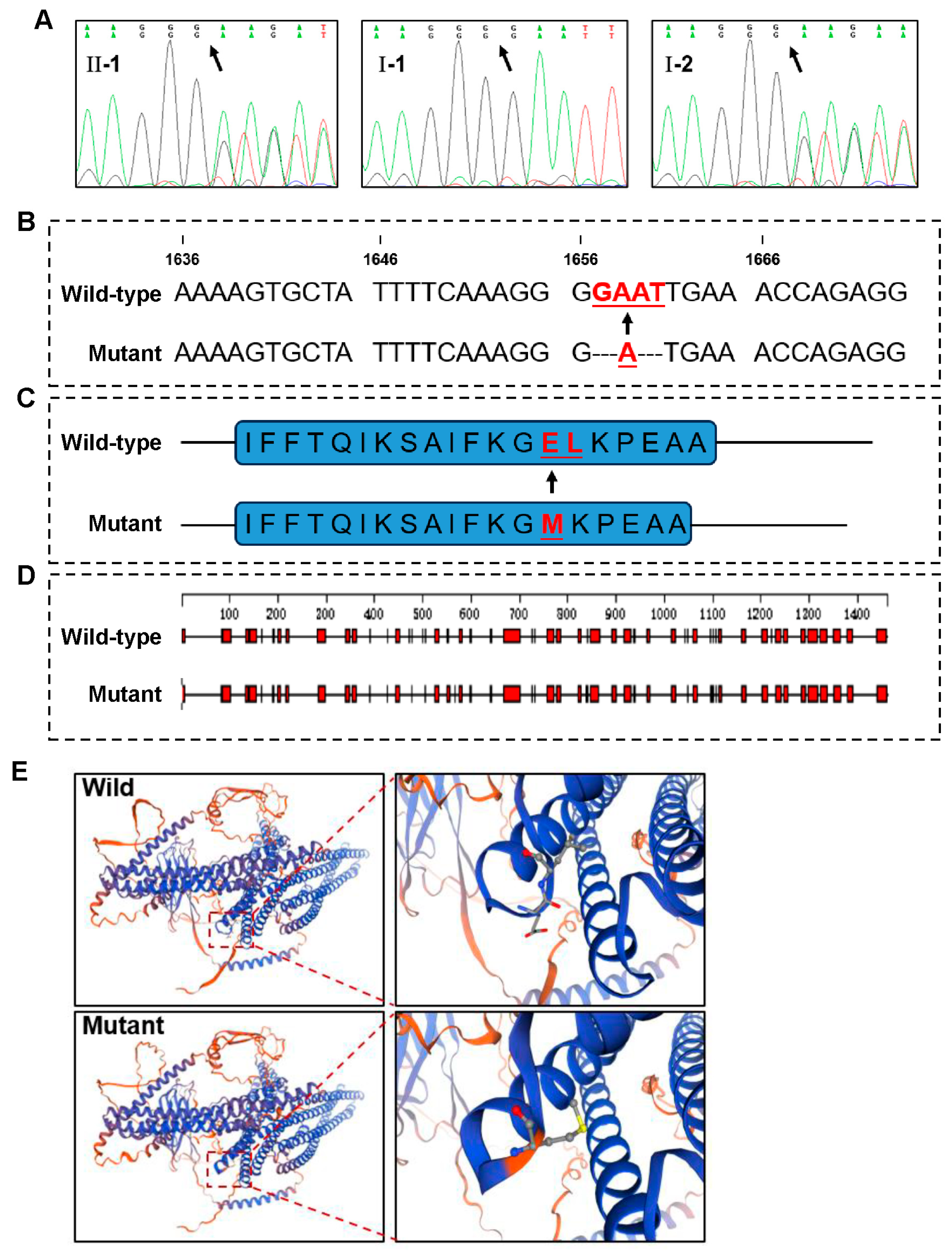
3.2. A Novel EVC2 Mutation Identified by WES and Sanger Sequencing
3.3. Potential Deleterious Effects of the EVC2 Mutation
3.4. Potential Impact of EVC2 Mutation on Protein Stability
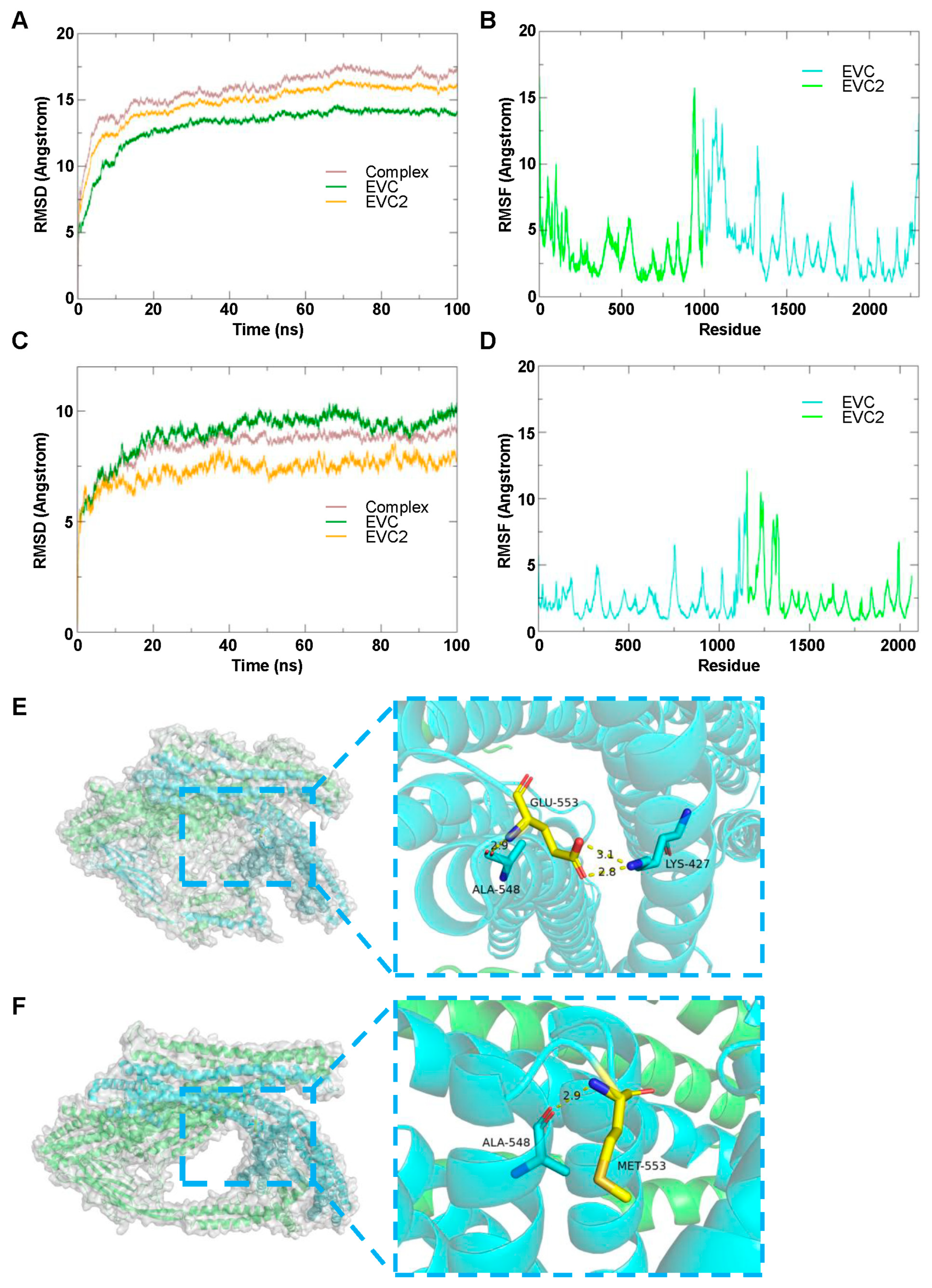
3.4.1. EVC2 and EVC Complex
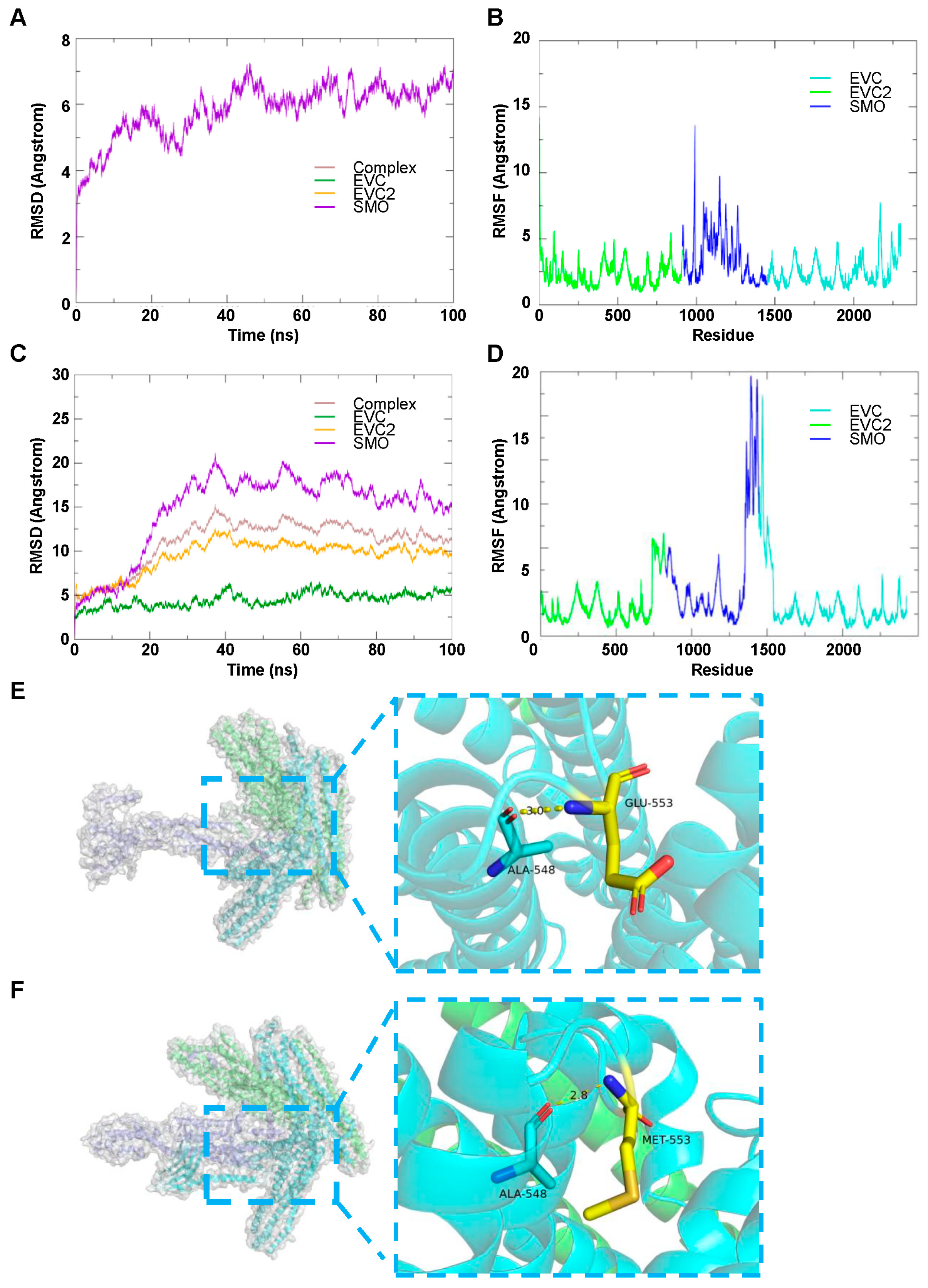
3.4.2. EVC2, EVC and SMO Complex
3.4.3. MM-GBSA Analysis
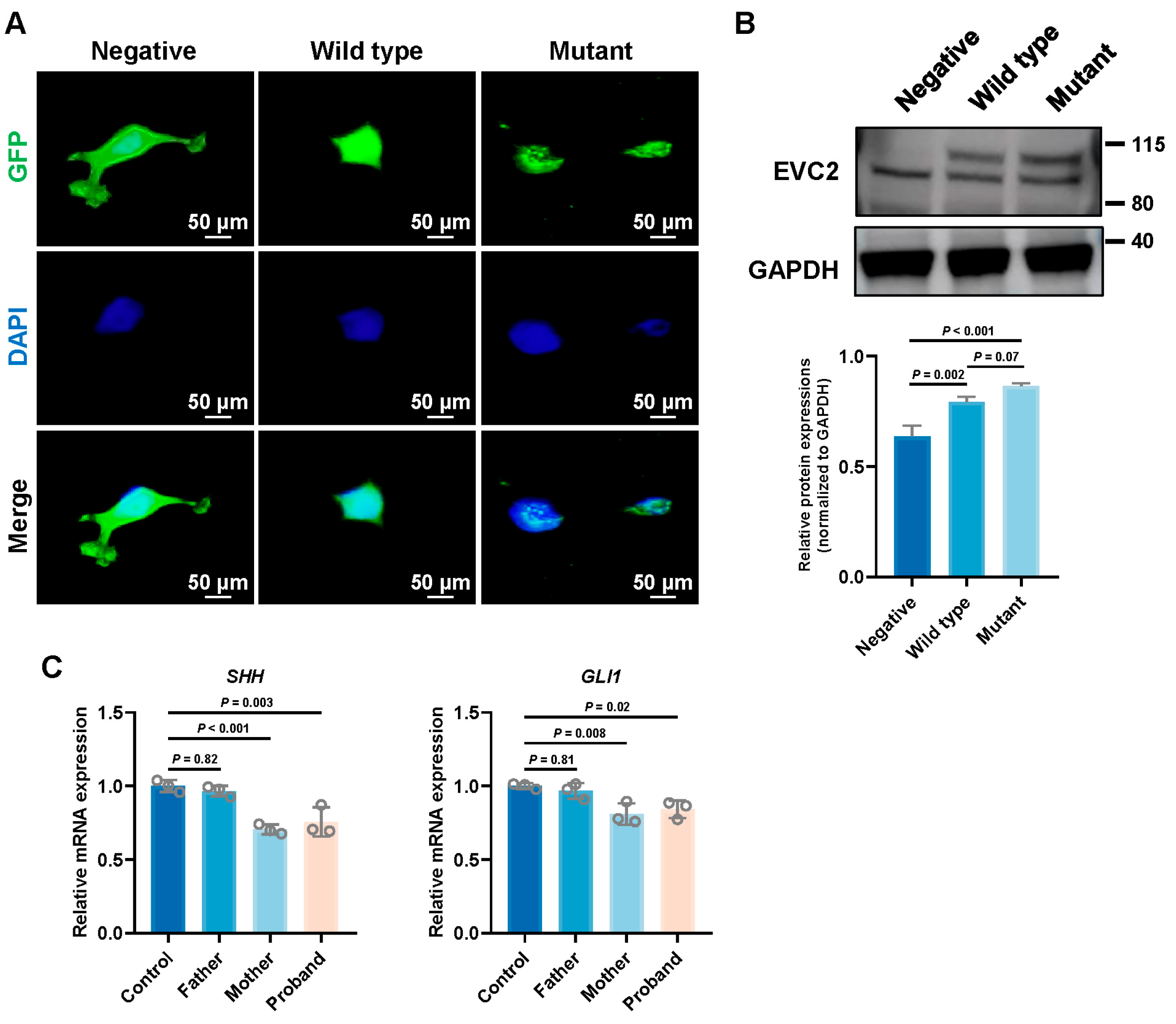
3.5. Impact of EVC2 Mutations on Subcellular Localization
3.6. Impact of EVC2 Mutation on Hedgehog Signaling Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NSTA | Non-syndromic tooth agenesis |
| WES | Whole-exome sequencing |
| SMO | Smoothened |
| RT-qPCR | Reverse transcription quantitative PCR |
| Hh | Hedgehog |
| RMSD | Root-mean-square deviation |
| MM-GBSA | Molecular Mechanics-Generalized Born Surface Area |
| SD | Standard deviation |
| RMSF | Root-mean-square fluctuation |
References
- Zhang, H.; Gong, X.; Xu, X.; Wang, X.; Sun, Y. Tooth number abnormality: From bench to bedside. Int. J. Oral Sci. 2023, 15, 5. [Google Scholar] [CrossRef]
- Cammarata-Scalisi, F.; Willoughby, C.E.; El-Feghaly, J.R.; Tadich, A.C.; Castillo, M.A.; Alkhatib, S.; Elsherif, M.A.E.; El-Ghandour, R.K.; Coletta, R.; Morabito, A.; et al. Main genetic entities associated with tooth agenesis. Clin. Oral Investig. 2024, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Wu, Y.; Dai, Q.; Wang, F. Gene mutations and chromosomal abnormalities in syndromes with tooth agenesis. Oral Dis. 2022, 29, 2401–2408. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, H.; Camhi, H.; Seymen, F.; Koruyucu, M.; Kasimoglu, Y.; Kim, J.-W.; Kim-Berman, H.; Yuson, N.M.R.; Benke, P.J.; et al. Analyses of oligodontia phenotypes and genetic etiologies. Int. J. Oral Sci. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Baujat, G.; Le Merrer, M. Ellis-van Creveld syndrome. Orphanet. J. Rare Dis. 2007, 2, 27. [Google Scholar] [CrossRef]
- Ye, X.; Song, G.; Fan, M.; Shi, L.; Jabs, E.W.; Huang, S.; Guo, R.; Bian, Z. A novel heterozygous deletion in the EVC2 gene causes Weyers acrofacial dysostosis. Hum. Genet. 2006, 119, 199–205. [Google Scholar] [CrossRef]
- Altunoglu, U.; Palencia-Campos, A.; Güneş, N.; Turgut, G.T.; Nevado, J.; Lapunzina, P.; Valencia, M.; Iturrate, A.; Otaify, G.; Elhossini, R.; et al. Variant characterisation and clinical profile in a large cohort of patients with Ellis-van Creveld syndrome and a family with Weyers acrofacial dysostosis. J. Med. Genet. 2024, 61, 633–644. [Google Scholar] [CrossRef]
- Caparrós-Martín, J.A.; Valencia, M.; Reytor, E.; Pacheco, M.; Fernandez, M.; Perez-Aytes, A.; Gean, E.; Lapunzina, P.; Peters, H.; Goodship, J.A.; et al. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum. Mol. Genet. 2013, 22, 124–139. [Google Scholar] [CrossRef]
- Pusapati, G.V.; Hughes, C.E.; Dorn, K.V.; Zhang, D.; Sugianto, P.; Aravind, L.; Rohatgi, R. EFCAB7 and IQCE regulate hedgehog signaling by tethering the EVC-EVC2 complex to the base of primary cilia. Dev. Cell 2014, 28, 483–496. [Google Scholar] [CrossRef]
- Yang, C.; Chen, W.; Chen, Y.; Jiang, J. Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell Res. 2012, 22, 1593–1604. [Google Scholar] [CrossRef]
- Dorn, K.V.; Hughes, C.E.; Rohatgi, R. A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev. Cell 2012, 23, 823–835. [Google Scholar] [CrossRef]
- Cobourne, M.T.; Xavier, G.M.; Depew, M.; Hagan, L.; Sealby, J.; Webster, Z.; Sharpe, P.T. Sonic hedgehog signalling inhibits palatogenesis and arrests tooth development in a mouse model of the nevoid basal cell carcinoma syndrome. Dev. Biol. 2009, 331, 38–49. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, X.; Sun, X.; Zhang, C.; Zheng, S. Analysis of novel RUNX2 mutations in Chinese patients with cleidocranial dysplasia. PLoS ONE 2017, 12, e0181653. [Google Scholar] [CrossRef]
- Blair, H.J.; Tompson, S.; Liu, Y.-N.; Campbell, J.; MacArthur, K.; Ponting, C.P.; Ruiz-Perez, V.L.; A Goodship, J. Evc2 is a positive modulator of Hedgehog signalling that interacts with Evc at the cilia membrane and is also found in the nucleus. BMC Biol. 2011, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Perez, V.L.; Goodship, J.A. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am. J. Med. Genet. Part C Semin. Med. Genet. 2009, 151C, 341–351. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, J.; Zhang, C.; Ma, S.; Liu, Y.; Wu, X.; Gao, Q. The oligodontia phenotype in a X-linked hypohidrotic ectodermal dysplasia patient with a novel EVC2 variant. Heliyon 2024, 10, e23056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lian, M.; Zou, D.; Huang, W.; Zhou, W.; Shen, Y.; Wang, F.; Wu, Y. Novel mutations identified in patients with tooth agenesis by whole-exome sequencing. Oral Dis. 2019, 25, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Han, D.; Zhang, J.; Zhao, H.; Feng, H. Two novel heterozygous mutations of EVC2 cause a mild phenotype of Ellis-van Creveld syndrome in a Chinese family. Am. J. Med. Genet. Part A 2011, 155, 2131–2136. [Google Scholar] [CrossRef]
- Valencia, M.; Lapunzina, P.; Lim, D.; Zannolli, R.; Bartholdi, D.; Wollnik, B.; Al-Ajlouni, O.; Eid, S.S.; Cox, H.; Buoni, S.; et al. Widening the mutation spectrum of EVC and EVC2: Ectopic expression of Weyer variants in NIH 3T3 fibroblasts disrupts Hedgehog signaling. Hum. Mutat. 2009, 30, 1667–1675. [Google Scholar] [CrossRef]
- D’Asdia, M.C.; Torrente, I.; Consoli, F.; Ferese, R.; Magliozzi, M.; Bernardini, L.; Guida, V.; Digilio, M.C.; Marino, B.; Dallapiccola, B.; et al. Novel and recurrent EVC and EVC2 mutations in Ellis-van Creveld syndrome and Weyers acrofacial dyostosis. Eur. J. Med. Genet. 2013, 56, 80–87. [Google Scholar] [CrossRef]
- Ruiz-Perez, V.L.; Tompson, S.W.J.; Blair, H.J.; Espinoza-Valdez, C.; Lapunzina, P.; O Silva, E.; Hamel, B.; Gibbs, J.L.; Young, I.D.; Wright, M.J.; et al. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am. J. Hum. Genet. 2003, 72, 728–732. [Google Scholar] [CrossRef]
- Barbeito, P.; Martin-Morales, R.; Palencia-Campos, A.; Cerrolaza, J.; Rivas-Santos, C.; Gallego-Colastra, L.; Caparros-Martin, J.A.; Martin-Bravo, C.; Martin-Hurtado, A.; Sánchez-Bellver, L.; et al. EVC-EVC2 complex stability and ciliary targeting are regulated by modification with ubiquitin and SUMO. Front. Cell Dev. Biol. 2023, 11, 1190258. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of β-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef]
- Sharp, K.A.; O’BRien, E.; Kasinath, V.; Wand, A.J. On the relationship between NMR-derived amide order parameters and protein backbone entropy changes. Proteins Struct. Funct. Bioinform. 2015, 83, 922–930. [Google Scholar] [CrossRef]
- Pei, F.; Ma, L.; Guo, T.; Zhang, M.; Jing, J.; Wen, Q.; Feng, J.; Lei, J.; He, J.; Janečková, E.; et al. Sensory nerve regulates progenitor cells via FGF-SHH axis in tooth root morphogenesis. Development 2024, 151, dev202043. [Google Scholar] [CrossRef]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Shimo, T.; Irie, K. Sonic Hedgehog Signaling and Tooth Development. Int. J. Mol. Sci. 2020, 21, 1587. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Li, J.; Zhao, H.; Ho, T.-V.; Chai, Y. An Nfic-hedgehog signaling cascade regulates tooth root development. Development 2015, 142, 3374–3382. [Google Scholar] [CrossRef]
- van der Burght, S.N.; Rademakers, S.; Johnson, J.L.; Li, C.; Kremers, G.J.; Houtsmuller, A.B.; Leroux, M.R.; Jansen, G. Ciliary Tip Signaling Compartment Is Formed and Maintained by Intraflagellar Transport. Curr. Biol. 2020, 30, 4299–4306.e5. [Google Scholar] [CrossRef]
- Louie, K.W.; Mishina, Y.; Zhang, H. Molecular and Cellular Pathogenesis of Ellis-van Creveld Syndrome: Lessons from Targeted and Natural Mutations in Animal Models. J. Dev. Biol. 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.H.; Brugmann, S.A. Sending mixed signals: Cilia-dependent signaling during development and disease. Dev. Biol. 2019, 447, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Lloyd, I.C.; Clayton-Smith, J.; Churchill, A.; Van Heyningen, V.; Hanson, I.; Taylor, D.; McKeown, C.; Super, M.; Kerr, B.; et al. Phenotypic variability and asymmetry of Rieger syndrome associated with PITX2 mutations. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2456–2460. [Google Scholar]
- Li, M.; Popovic, N.; Wang, Y.; Chen, C.; Polychronakos, C. Incomplete penetrance and variable expressivity in monogenic diabetes; a challenge but also an opportunity. Rev. Endocr. Metab. Disord. 2023, 24, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Delgado, M.; Cámara-Checa, A.; Rubio-Alarcón, M.; Heredero-Jung, D.; de la Fuente-Blanco, L.; Rapún, J.; Plata-Izquierdo, B.; Pérez-Martín, S.; Cebrián, J.; de Redrojo, L.M.; et al. Variable Penetrance and Expressivity of a Rare Pore Loss-of-Function Mutation (p.L889V) of Nav1.5 Channels in Three Spanish Families. Int. J. Mol. Sci. 2024, 25, 4686. [Google Scholar] [CrossRef]
- Ellingford, J.M.; Hufnagel, R.B.; Arno, G. Phenotype and Genotype Correlations in Inherited Retinal Diseases: Population-Guided Variant Interpretation, Variable Expressivity and Incomplete Penetrance. Genes 2020, 11, 1274. [Google Scholar] [CrossRef]
- McKeown, H.F.; Robinson, D.L.; Elcock, C.; Al-Sharood, M.; Brook, A.H. Tooth dimensions in hypodontia patients, their unaffected relatives and a control group measured by a new image analysis system. Eur. J. Orthod. 2002, 24, 131–141. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Singer-Berk, M.; Son, R.; Sveden, A.; Wood, J.; England, E.; Cole, J.B.; Weisburd, B.; Watts, N.; Caulkins, L.; et al. Determinants of penetrance and variable expressivity in monogenic metabolic conditions across 77,184 exomes. Nat. Commun. 2021, 12, 3505. [Google Scholar] [CrossRef]
- Khan, A.; Shang, N.; Nestor, J.G.; Weng, C.; Hripcsak, G.; Harris, P.C.; Gharavi, A.G.; Kiryluk, K. Polygenic risk alters the penetrance of monogenic kidney disease. Nat. Commun. 2023, 14, 8318. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Barbieri, S.; Bruni, A.; Sinesi, A.; Ricci, M.; Trombini, J.; et al. Assessment of Genetical, Pre, Peri and Post Natal Risk Factors of Deciduous Molar Hypomineralization (DMH), Hypomineralized Second Primary Molar (HSPM) and Molar Incisor Hypomineralization (MIH): A Narrative Review. Children 2021, 8, 432. [Google Scholar] [CrossRef]
- Stewart, O.; Gruber, C.; Randolph, H.E.; Patel, R.; Ramba, M.; Calzoni, E.; Huang, L.H.; Levy, J.; Buta, S.; Lee, A.; et al. Monoallelic expression can govern penetrance of inborn errors of immunity. Nature 2025, 637, 1186–1197. [Google Scholar] [CrossRef]
- Letra, A. Rethinking the Genetic Etiology of Nonsyndromic Tooth Agenesis. Curr. Osteoporos. Rep. 2022, 20, 389–397. [Google Scholar] [CrossRef]
| Primer | Primer Sequence (5′-3′) | Location | Size of Amplified Fragment (bp) |
|---|---|---|---|
| Forward | CAACCTTCTGCGGACCCT | Exon 11 | 380 |
| Reverse | TCCCTGCTTACTGGAAACTCAC | Exon 11 | 380 |
| Genes | Primer | Primer Sequence (5′-3′) |
|---|---|---|
| GLI1 | Forward | AGCGTGAGCCTGAATCTGTG |
| Reverse | CAGCATGTACTGGGCTTTGAA | |
| SHH | Forward | CTCGCTGCTGGTATGCTCG |
| Reverse | ATCGCTCGGAGTTTCTGGAGA | |
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG |
| Reverse | ACCACCCTGTTGCTGTAGCCAA |
| Subject | Genotype | Phenotype |
|---|---|---|
| Proband | c.1657_1660delinsA | NSTA; absence of 17 permanent teeth |
| Father | Wild-type | Absence of 1 third molar (within normal variation) |
| Mother | c.1657_1660delinsA | No clinical anomalies; absence of third molars (within normal variation) |
| Energy (kcal/mol) | Wild-Type EVC2/EVC (Mean ± SD) | Mutant EVC2/EVC (Mean ± SD) | p Value |
|---|---|---|---|
| ΔGvdw | 46,467.8 ± 7.0 | 41,592.0 ± 26.8 | <0.01 |
| ΔGele | −81,154.6 ± 31.5 | −71,398.6 ± 36.1 | <0.01 |
| ΔGpolar | −26,876.0 ± 43.6 | −27,205.3 ± 56.1 | <0.01 |
| ΔGnonpolar | 674.8 ± 0.7 | 600.5 ± 0.7 | <0.01 |
| ΔGtotal | −60,888.0 ± 14.5 | −56,411.4 ± 19.8 | <0.01 |
| Variants | Location | Variant Type | Clinical Features | References |
|---|---|---|---|---|
| c.198_199insGGCGG | Exon1 | Homozygous | atrial septal defect, short limbs, genu valgum, postaxial polydactyly, multiple oral frenulae, oligodontia, teeth dysplasia | [21] |
| c.1472C>T | Exon11 | Heterozygous | oligodontia | [17] |
| c.1772C>T | Exon12 | Homozygous | sparse hair, dry skin, prominent ears, oligodontia | [16] |
| c.1855C>T | Exon12 | Heterozygous | short stature, short limbs, short ribs, postaxial polydactyly, hypoplastic nails, multiple oral frenulae, oligodontia, teeth dysplasia, fusion of the hamate and capitate | [21] |
| c.2653C>T | Exon15 | Compound heterozygous | short stature, short limbs, genu valgum, postaxial polydactyly, hypoplastic nails, teeth dysplasia, oligodontia | [18] |
| c.3793del | Exon22 | Homozygous | polydactyly, hypoplastic nails, multiple oral frenulae, delayed teeth eruption, oligodontia | [6] |
| c.3797T>A | Exon22 | Heterozygous | postaxial polydactyly, hypoplastic nails, multiple oral frenulae, teeth dysplasia, oligodontia | [19] |
| c.3797T>G | Exon22 | Heterozygous | postaxial polydactyly, hypoplastic nails, teeth dysplasia, oligodontia, osteopenia, mental delay | [19] |
| c.3805G>T | Exon22 | Heterozygous | postaxial polydactyly, hypoplastic nails, enamel hypoplasia, oligodontia | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Li, J.; Zhang, C.; Liu, Y.; Wang, X.; Zheng, S. Identification of a Novel EVC2 Variant in a Family with Non-Syndromic Tooth Agenesis and Its Potential Functional Implications. Genes 2025, 16, 1288. https://doi.org/10.3390/genes16111288
Yan C, Li J, Zhang C, Liu Y, Wang X, Zheng S. Identification of a Novel EVC2 Variant in a Family with Non-Syndromic Tooth Agenesis and Its Potential Functional Implications. Genes. 2025; 16(11):1288. https://doi.org/10.3390/genes16111288
Chicago/Turabian StyleYan, Changqing, Jie Li, Chenying Zhang, Yang Liu, Xiaozhe Wang, and Shuguo Zheng. 2025. "Identification of a Novel EVC2 Variant in a Family with Non-Syndromic Tooth Agenesis and Its Potential Functional Implications" Genes 16, no. 11: 1288. https://doi.org/10.3390/genes16111288
APA StyleYan, C., Li, J., Zhang, C., Liu, Y., Wang, X., & Zheng, S. (2025). Identification of a Novel EVC2 Variant in a Family with Non-Syndromic Tooth Agenesis and Its Potential Functional Implications. Genes, 16(11), 1288. https://doi.org/10.3390/genes16111288






