The Chemoreceptive Molecular Mechanism Underlying CSP-Mediated Recognition of Seed Elaiosome from Stemona tuberosa by Hornets
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Tissue Collection
2.2. RNA Extraction and cDNA Synthesis
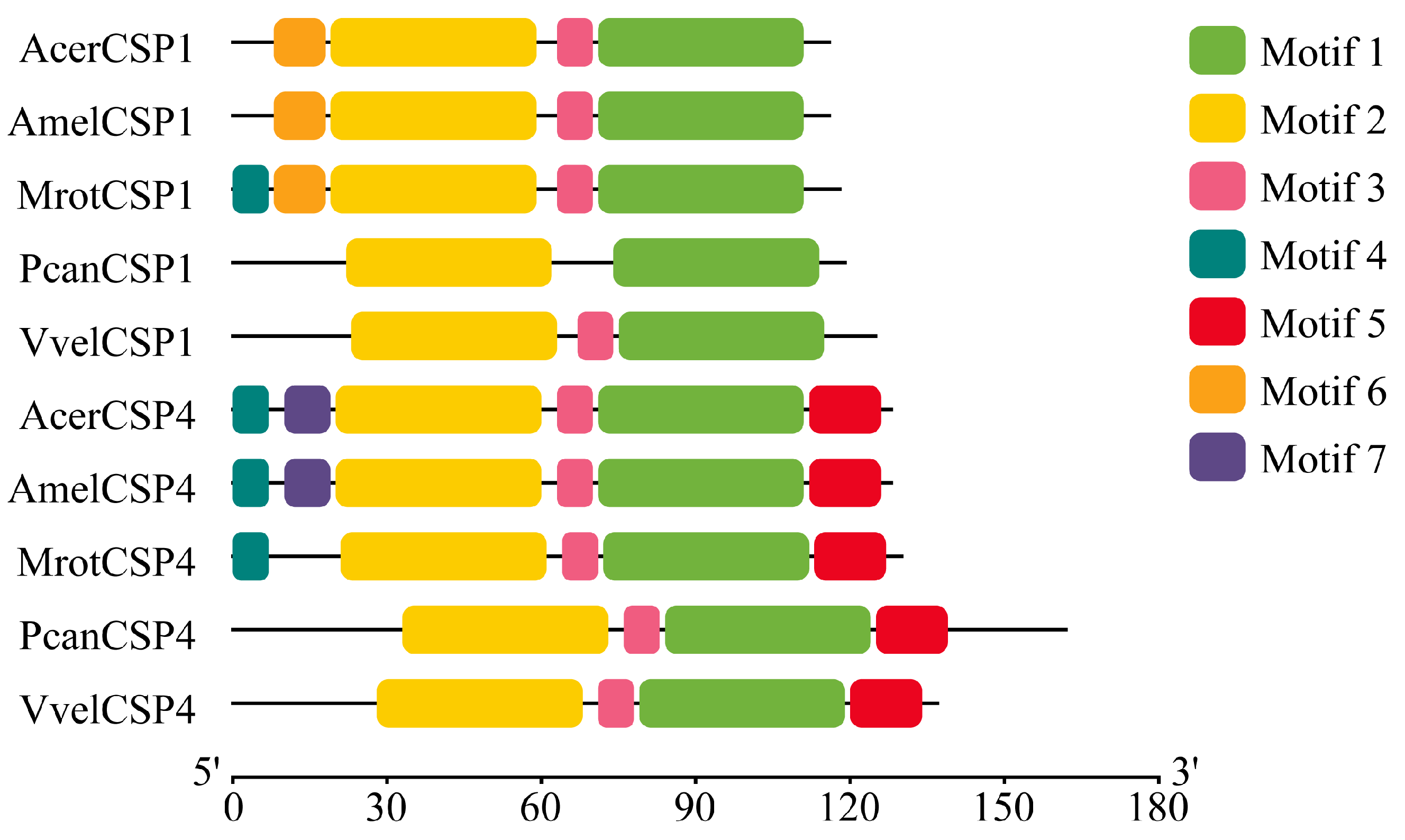
2.3. Sequence Alignment and Motif Analysis
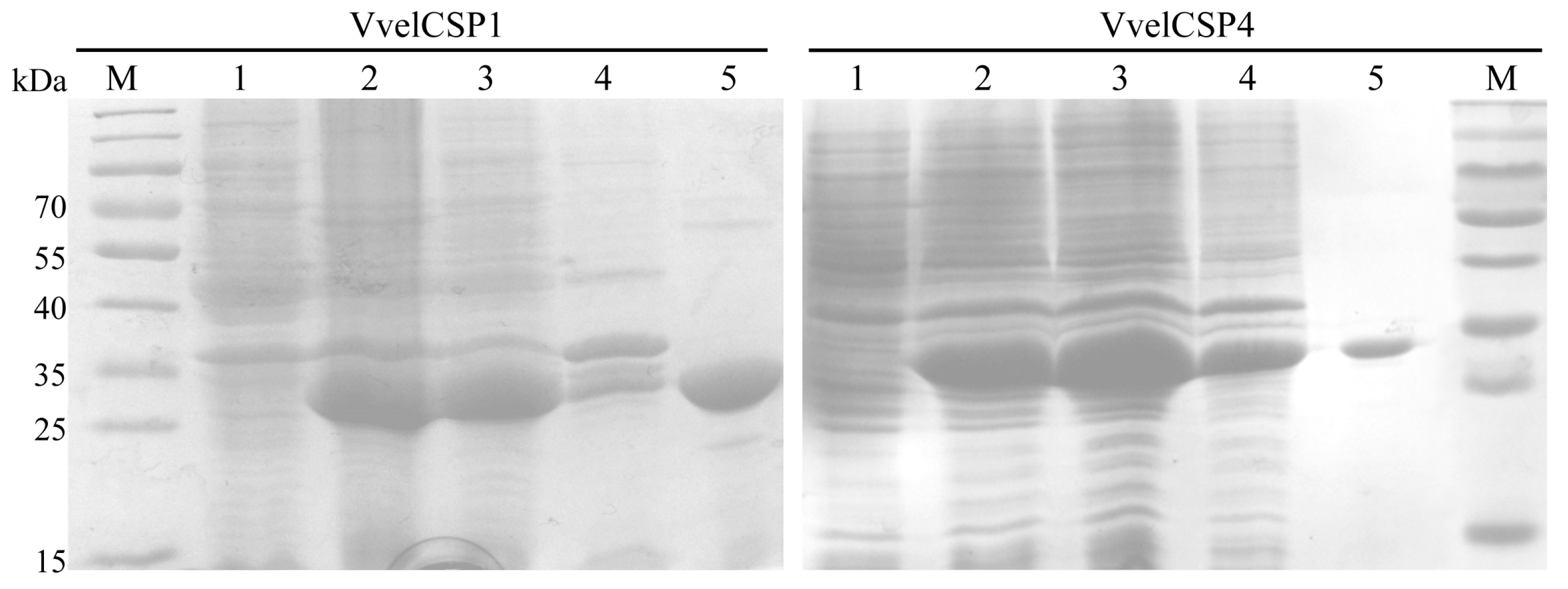
2.4. Prokaryotic Expression and Purification of VvelCSPs
2.5. Fluorescence Competitive Binding Assay
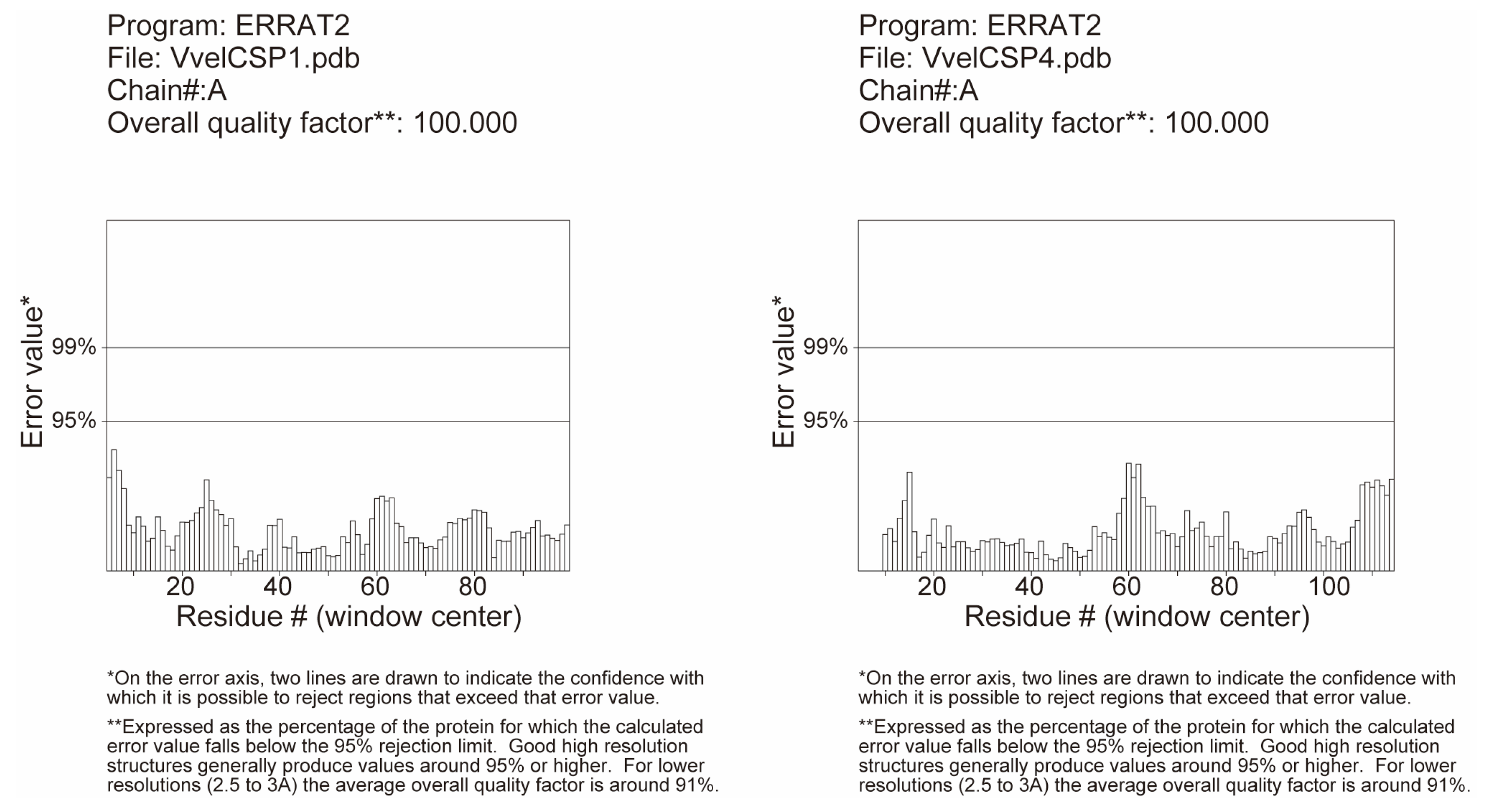
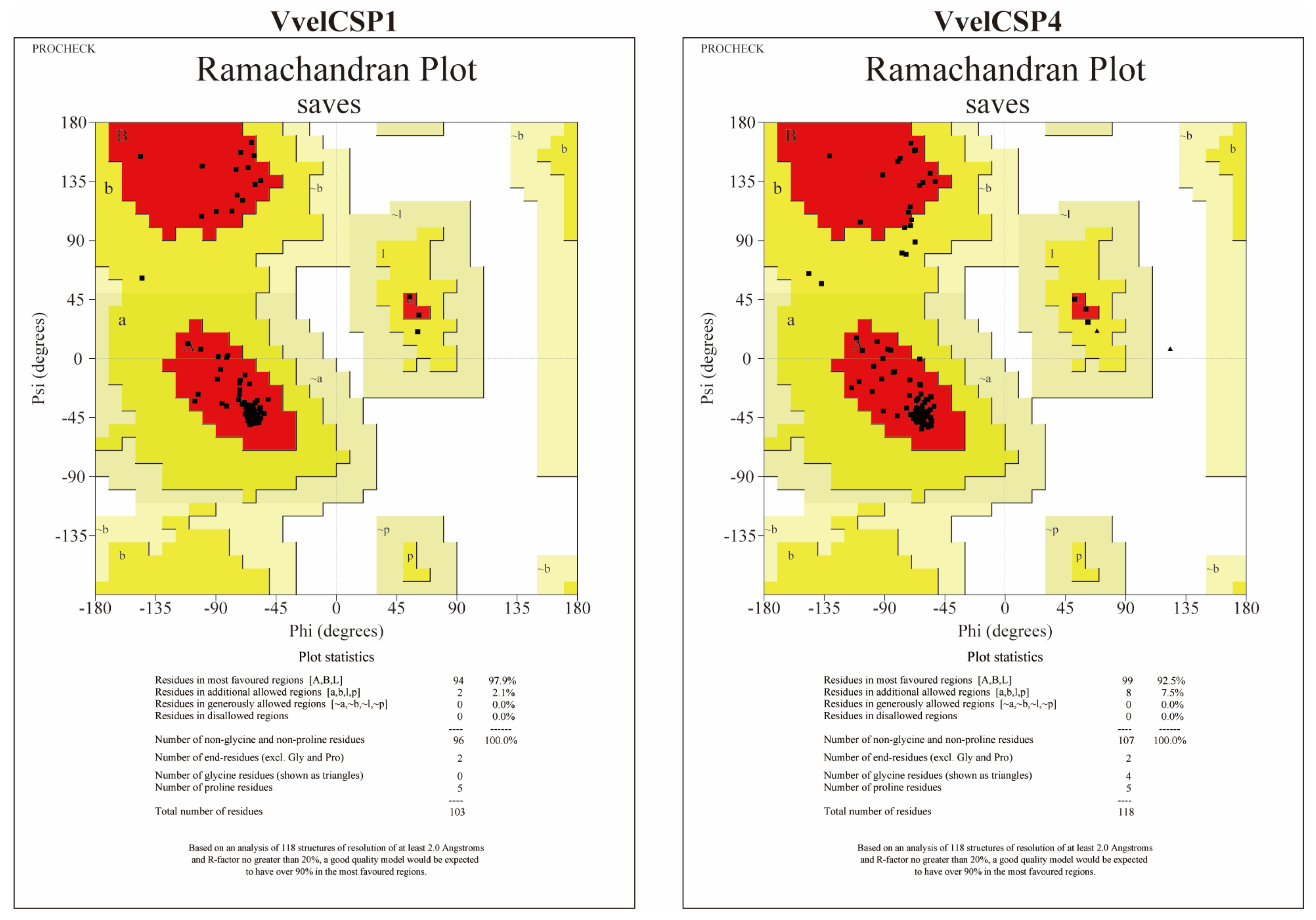
2.6. Structural Modeling and Molecular Docking
3. Results
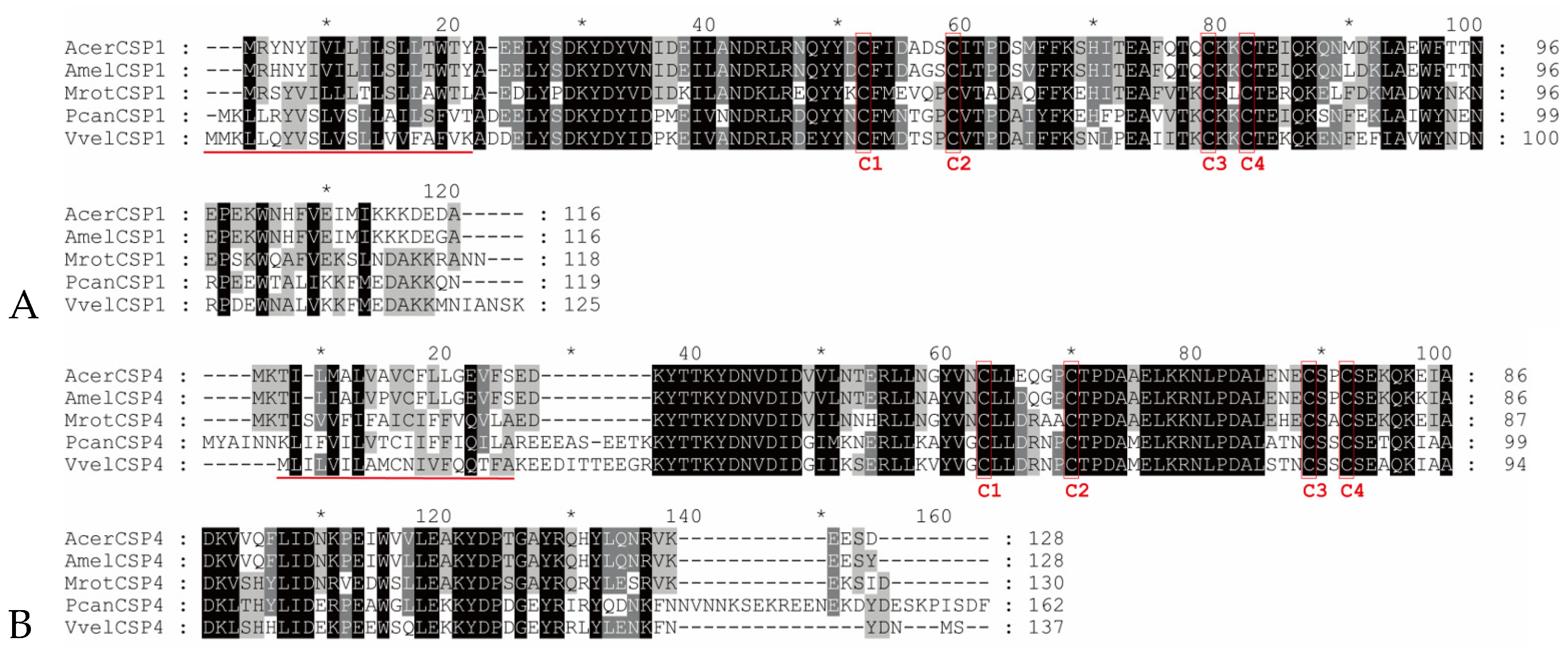
3.1. Sequence Conservation and Key Structural Motifs
3.2. Expression and Purification of VvelCSPs
3.3. Fluorescence Competitive Binding Analysis of VvelCSPs
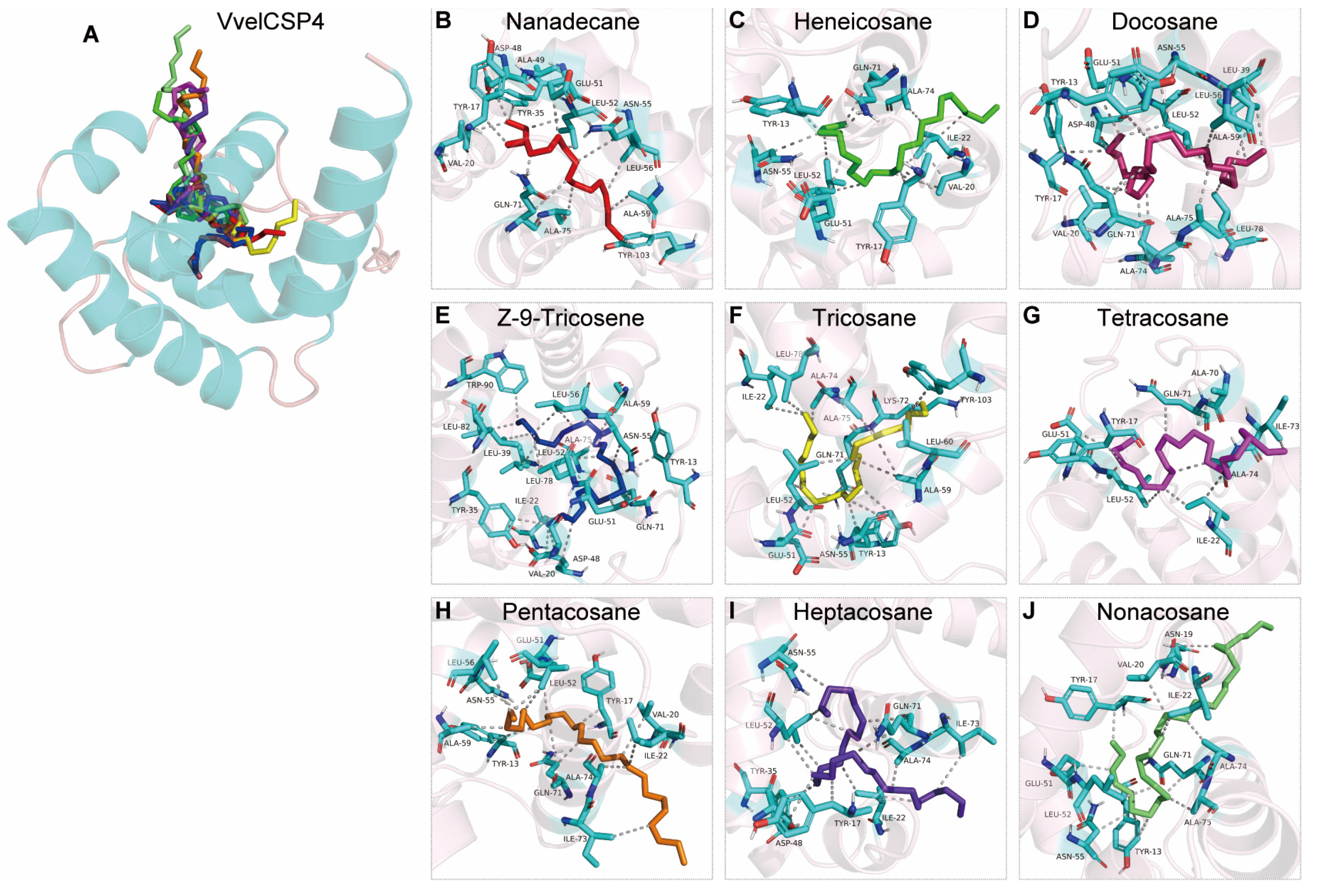
3.4. Structural Modeling and Molecular Docking of VvelCSP1 and VvelCSP4 with Different Ligands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, P.J.; Ford, S.M.; Poidatz, J.; Thiéry, D.; Osborne, J.L. Searching for Nests of the Invasive Asian Hornet (Vespa velutina) Using Radio-Telemetry. Commun. Biol. 2018, 1, 88. [Google Scholar] [CrossRef]
- Requier, F.; Fournier, A.; Pointeau, S.; Rome, Q.; Courchamp, F. Economic Costs of the Invasive Yellow-Legged Hornet on Honey Bees. Sci. Total Environ. 2023, 898, 165576. [Google Scholar] [CrossRef]
- Southon, R.J.; Fernandes, O.A.; Nascimento, F.S.; Sumner, S. Social Wasps Are Effective Biocontrol Agents of Key Lepidopteran Crop Pests. Proc. Biol. Sci. 2019, 286, 20191676. [Google Scholar] [CrossRef]
- Pedersen, S.; Kennedy, P.J.; O’Shea-Wheller, T.A.; Poidatz, J.; Christie, A.; Osborne, J.L.; Tyler, C.R. Broad Ecological Threats of an Invasive Hornet Revealed through a Deep Sequencing Approach. Sci. Total Environ. 2025, 970, 178978. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Diéguez-Antón, A.; Seijo-Coello, M.C.; Escuredo, O. Flora Volatile Profiles of Plants Visited by Vespa velutina: A Preliminary Assessment in the Interaction of Plant-Insect. J. Plant Res. 2025, 138, 807–823. [Google Scholar] [CrossRef]
- Gong, Z.W.; Tan, K.; Nieh, J.C. Hornets Possess Long-Lasting Olfactory Memories. J. Exp. Biol. 2019, 222, jeb200881. [Google Scholar] [CrossRef]
- Richter, M.R. Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu. Rev. Entomol. 2000, 45, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, K.W.; Choe, J.C. Temporal Polyethism in Korean Yellowjacket Foragers, Vespula koreensis (Hymenoptera, Vespidae). Insectes Sociaux 2012, 59, 263–268. [Google Scholar] [CrossRef]
- O’Shea-Wheller, T.A.; Curtis, R.J.; Kennedy, P.J.; Groom, E.K.J.; Poidatz, J.; Raffle, D.S.; Rojas-Nossa, S.V.; Bartolomé, C.; Dasilva-Martins, D.; Maside, X.; et al. Quantifying the Impact of an Invasive Hornet on Bombus terrestris Colonies. Commun. Biol. 2023, 6, 990. [Google Scholar] [CrossRef]
- Xia, X.H. Phylogeographic Analysis for Understanding Origin, Speciation, and Biogeographic Expansion of Invasive Asian Hornet, Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae). Life 2024, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Nie, P.X.; Hu, X.K.; Feng, J.M. Future Range Expansions of Invasive Wasps Suggest Their Increasing Impacts on Global Apiculture. Insects 2024, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; Crescenzi, O.; Sanfelice, D.; Ab, E.; Wechselberger, R.; Angeli, S.; Scaloni, A.; Boelens, R.; Tancredi, T.; Pelosi, P.; et al. Solution Structure of a Chemosensory Protein from the Desert Locust Schistocerca gregaria. Biochemistry 2006, 45, 10606–10613. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zeng, H.; Zhang, J.B.; Gao, S.F.; Xiao, N.; Tang, J.; Dong, X.L.; Xie, W. The Crystal Structure of the Spodoptera litura Chemosensory Protein CSP8. Insects 2021, 12, 602. [Google Scholar] [CrossRef]
- Liu, G.X.; Xuan, N.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Comprehensive History of CSP Genes: Evolution, Phylogenetic Distribution and Functions. Genes 2020, 11, 413. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, L.Y.; Ni, C.X.; Shang, H.W.; Zhuang, S.L.; Li, J. Molecular Recognition of Floral Volatile with Two Olfactory Related Proteins in the Eastern Honeybee (Apis cerana). Int. J. Biol. Macromol. 2013, 56, 114–121. [Google Scholar] [CrossRef]
- Li, H.L.; Ni, C.X.; Tan, J.; Zhang, L.Y.; Hu, F.L. Chemosensory Proteins of the Eastern Honeybee, Apis cerana: Identification, Tissue Distribution and Olfactory Related Functional Characterization. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 194–195, 11–19. [Google Scholar] [CrossRef]
- Li, H.L.; Tan, J.; Song, X.M.; Wu, F.; Tang, M.Z.; Hua, Q.Y.; Zheng, H.Q.; Hu, F.L. Sublethal Doses of Neonicotinoid Imidacloprid Can Interact with Honey Bee Chemosensory Protein 1 (CSP1) and Inhibit Its Function. Biochem. Biophys. Res. Commun. 2017, 486, 391–397. [Google Scholar] [CrossRef]
- Forêt, S.; Wanner, K.W.; Maleszka, R. Chemosensory Proteins in the Honey Bee: Insights from the Annotated Genome, Comparative Analyses and Expressional Profiling. Insect Biochem. Mol. Biol. 2007, 37, 19–28. [Google Scholar] [CrossRef]
- Maleszka, J.; Forêt, S.; Saint, R.; Maleszka, R. RNAi-Induced Phenotypes Suggest a Novel Role for a Chemosensory Protein CSP5 in the Development of Embryonic Integument in the Honeybee (Apis mellifera). Dev. Genes Evol. 2007, 217, 189–196. [Google Scholar] [CrossRef]
- Briand, L.; Swasdipan, N.; Nespoulous, C.; Bézirard, V.; Blon, F.; Huet, J.-C.; Ebert, P.; Penollet, J.-C. Characterization of a Chemosensory Protein (ASP3c) from Honeybee (Apis mellifera L.) as a Brood Pheromone Carrier. Eur. J. Biochem. 2002, 269, 4586–4596. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Z.W.; Qin, Y.; Sun, W.B. Seed Dispersal by Hornets: An Unusual Insect-Plant Mutualism. J. Integr. Plant Biol. 2017, 59, 792–796. [Google Scholar] [CrossRef]
- Chen, G.; Liu, C.Q.; Sun, W.B. Pollination and Seed Dispersal of Aquilaria sinensis (Lour.) Gilg (Thymelaeaceae): An Economic Plant Species with Extremely Small Populations in China. Plant Divers. 2016, 38, 227–232. [Google Scholar] [CrossRef]
- Qin, R.M.; Wen, P.; Corlett, R.T.; Zhang, Y.Y.; Wang, G.; Chen, J. Plant-Defense Mimicry Facilitates Rapid Dispersal of Short-Lived Seeds by Hornets. Curr. Biol. 2022, 32, 3429–3435.e5. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Z.W.; Wen, P.; Wei, W.; Chen, Y.; Ai, H.; Sun, W.B. Hydrocarbons Mediate Seed Dispersal: A New Mechanism of Vespicochory. New Phytol. 2018, 220, 714–725. [Google Scholar] [CrossRef]
- Ai, H.; Liu, Y.Y.; Long, G.Y.; Yuan, Y.; Huang, S.P.; Chen, Y. Functional Characteristics of a Novel Odorant Binding Protein in the Legume Pod Borer, Maruca vitrata. Sci. Rep. 2021, 11, 14027. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.; Okamura, J.Y. Foraging Navigation of Hornets Studied in Natural Habitats and Laboratory Experiments. Zoolog. Sci. 2003, 20, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Chen, G.; Tan, K. Both Olfactory and Visual Cues Promote the Hornet Vespa velutina to Locate Its Honeybee Prey Apis cerana. Insectes Sociaux 2014, 61, 67–70. [Google Scholar] [CrossRef]
- Forstner, M.; Breer, H.; Krieger, J. A Receptor and Binding Protein Interplay in the Detection of a Distinct Pheromone Component in the Silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 2009, 5, 745–757. [Google Scholar] [CrossRef]
- Wang, B.; Dong, W.Y.; Li, H.M.; D’Onofrio, C.; Bai, P.; Chen, R.P.; Yang, L.L.; Wu, J.N.; Wang, X.Q.; Wang, B.; et al. Molecular Basis of (E)-β-Farnesene-Mediated Aphid Location in the Predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962.e7. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Cao, S.; Jacquin-Joly, E.; Zhou, Q.; Liu, Y.; Wang, G.R. An Odorant Receptor Mediates the Avoidance of Plutella xylostella against Parasitoid. BMC Biol. 2024, 22, 61. [Google Scholar] [CrossRef]
- Guo, X.G.; Chen, Y.; Xing, Q.T.; Han, Y.C.; Qiao, H.L. Expression and Binding Characterization of Chemosensory Protein CSP16 in the Silkworm, Bombyx mori. Acta Entomol. Sin. 2016, 59, 613–621. [Google Scholar] [CrossRef]
- Dani, F.R.; Iovinella, I.; Felicioli, A.; Niccolini, A.; Calvello, M.A.; Carucci, M.G.; Qiao, H.; Pieraccini, G.; Turillazzi, S.; Moneti, G.; et al. Mapping the Expression of Soluble Olfactory Proteins in the Honeybee. J. Proteome Res. 2010, 9, 1822–1833. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, S.N.; Li, K.M.; Liu, J.T.; Zheng, Y.; Shan, S.; Yang, Y.Q.; Li, R.J.; Zhang, Y.J.; Guo, Y.Y. Identification of Odorant Binding Proteins and Chemosensory Proteins in Microplitis mediator as Well as Functional Characterization of Chemosensory Protein 3. PLoS ONE 2017, 12, e0180775. [Google Scholar] [CrossRef] [PubMed]
- Han, K.R.; Wang, W.W.; Li, X.; Liu, T.X.; Zhang, S.Z. Involvement of Chemosensory Protein CrufCSP3 in Perception of the Host Location in a Parasitic Wasp Cotesia ruficrus. J. Agric. Food Chem. 2024, 72, 10828–10841. [Google Scholar] [CrossRef]
- Yang, W.; Ye, C.; Wang, L.; Nie, J.; Liu, X.; Zhang, T.; Zhang, W.; Saba, N.U.; Yin, L.; Xing, L.; et al. Binding Properties of Olfactory Proteins to Host Volatiles, Free Fatty Acids and Cuticular Hydrocarbons in the Termite Reticulitermes aculabialis. Insect Biochem. Mol. Biol. 2025, 176, 104211. [Google Scholar] [CrossRef] [PubMed]
- Calvello, M.; Guerra, N.; Brandazza, A.; D’Ambrosio, C.; Scaloni, A.; Dani, F.R.; Turillazzi, S.; Pelosi, P. Soluble Proteins of Chemical Communication in the Social Wasp Polistes dominulus. Cell. Mol. Life Sci. CMLS 2003, 60, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gong, W.C.; Ge, J.; Schinnerl, J.; Wang, B.; Sun, W.B. Variation in Floral Characters, Particularly Floral Scent, in Sapromyophilous stemona Species. J. Integr. Plant Biol. 2017, 59, 825–839. [Google Scholar] [CrossRef]
- Brodmann, J.; Twele, R.; Francke, W.; Yi-bo, L.; Xi-qiang, S.; Ayasse, M. Orchid Mimics Honey Bee Alarm Pheromone in Order to Attract Hornets for Pollination. Curr. Biol. 2009, 19, 1368–1372. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, L.; Wu, F.; Tan, J.; Wen, P.; Xu, W.; Li, H.L. A Dual Sensing Mechanism of Eastern Honeybee Apis cerana That Upregulates the Expression Level of Chemosensory Protein CSP1 and Enhances the Binding Affinity to Loquat Floral Volatiles at Low Temperature. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167601. [Google Scholar] [CrossRef]
- Yao, Q.; Quan, L.F.; Nie, J.; Xu, S.; Li, W.J.; Dong, Y.Z.; Chen, B.X. Molecular Cloning of Five Chemosensory Proteins from Conopomorpha sinensis Eggs and Gene Expression after Diflubenzuron Treatment. J. South China Agric. Univ. 2021, 42, 60–68. [Google Scholar]
- Li, J.; Gao, P.; Zhang, L. Identification and Expression Characteristics of Putative Chemosensory Proteins in the Peach Fruit Borer Carposina sasakii Matsumura (Lepidoptera: Carposinidae). Comp. Biochem. Physiol. Part D Genomics Proteomics 2021, 39, 100858. [Google Scholar] [CrossRef] [PubMed]


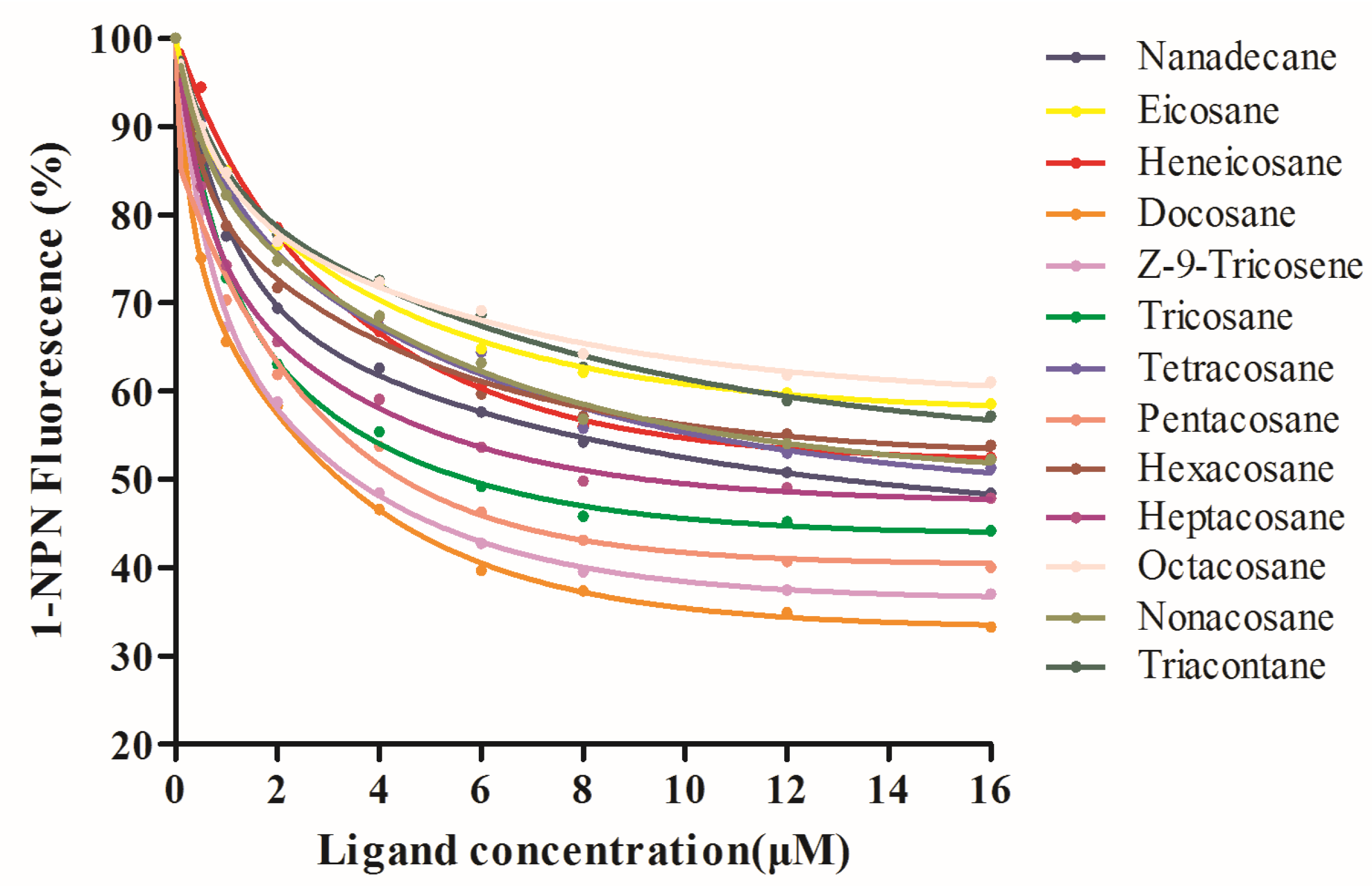

| No. | Compounds | VvelCSP1 | VvelCSP4 | ||
|---|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | ||
| 1 | Nanadecane | 18.23 | 14.53 | 10.44 | 7.89 |
| 2 | Eicosane | - | - | - | - |
| 3 | Heneicosane | 11.27 | 9.02 | 19.76 | 14.74 |
| 4 | Docosane | - | - | 3.68 | 2.73 |
| 5 | Z-9-Tricosene | 17.35 | 13.85 | 3.97 | 2.97 |
| 6 | Tricosane | 3.85 | 3.07 | 5.09 | 3.85 |
| 7 | Tetracosane | 11.23 | 8.95 | 18.93 | 14.25 |
| 8 | Pentacosane | 4.83 | 3.87 | 4.65 | 3.49 |
| 9 | Hexacosane | 4.63 | 3.68 | - | - |
| 10 | Heptacosane | 5.53 | 4.40 | 6.20 | 4.60 |
| 11 | Octacosane | - | - | - | - |
| 12 | Nonacosane | 14.56 | 11.63 | 20.37 | 15.07 |
| 13 | Triacontane | - | - | - | - |
| Compounds | VvelCSP1 | VvelCSP4 |
|---|---|---|
| Binding Energy (kcal/mol) | Binding Energy (kcal/mol) | |
| Nanadecane | −5.86 | −7.14 |
| Heneicosane | −5.71 | −7.49 |
| Docosane | - | −8.22 |
| Z-9-Tricosene | −5.85 | −8.28 |
| Tricosane | −6.41 | −7.72 |
| Tetracosane | −6.1 | −4.74 |
| Pentacosane | −4.32 | −5.14 |
| Hexacosane | −4.18 | - |
| Heptacosane | −6.35 | −4.00 |
| Nonacosane | −3.70 | −5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, G.; Liu, Y.; Zhu, M.; Liu, K.; Xiao, Y.; Ai, H. The Chemoreceptive Molecular Mechanism Underlying CSP-Mediated Recognition of Seed Elaiosome from Stemona tuberosa by Hornets. Genes 2025, 16, 1265. https://doi.org/10.3390/genes16111265
Long G, Liu Y, Zhu M, Liu K, Xiao Y, Ai H. The Chemoreceptive Molecular Mechanism Underlying CSP-Mediated Recognition of Seed Elaiosome from Stemona tuberosa by Hornets. Genes. 2025; 16(11):1265. https://doi.org/10.3390/genes16111265
Chicago/Turabian StyleLong, Guangyan, Yuying Liu, Mengyao Zhu, Kaiyu Liu, Yutao Xiao, and Hui Ai. 2025. "The Chemoreceptive Molecular Mechanism Underlying CSP-Mediated Recognition of Seed Elaiosome from Stemona tuberosa by Hornets" Genes 16, no. 11: 1265. https://doi.org/10.3390/genes16111265
APA StyleLong, G., Liu, Y., Zhu, M., Liu, K., Xiao, Y., & Ai, H. (2025). The Chemoreceptive Molecular Mechanism Underlying CSP-Mediated Recognition of Seed Elaiosome from Stemona tuberosa by Hornets. Genes, 16(11), 1265. https://doi.org/10.3390/genes16111265







