Genetically Confirmed Familial Case of Nonsyndromic Cardiac Progeria Caused by the LMNA p.Asp300Asn Variant with Presumed Gonadal Mosaicism: Phenotypic Comparison with Previously Reported Patients
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-Gilford Progeria Syndrome: A premature aging disease caused by LMNA gene mutations. Ageing Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Plasilova, M.; Chattopadhyay, C.; Ghosh, A.; Wenzel, F.; Demougin, P.; Noppen, C.; Schaub, N.; Szinnai, G.; Terracciano, L.; Heinimann, K. Discordant Gene Expression Signatures and Related Phenotypic Differences in Lamin A- and A/C-Related Hutchinson-Gilford Progeria Syndrome (HGPS). PLoS ONE 2011, 6, e21433. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tan, D.; Song, D.; Zhang, X.; Chang, X.; Wang, Z.; Zhang, C.; Chan, S.H.-S.; Wu, Q.; Wu, L.; et al. Clinical spectrum and genetic variations of LMNA-related muscular dystrophies in a large cohort of Chinese patients. J. Med. Genet. 2021, 58, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, L.; Kahn, A.; Dhamija, R.; Vargas, H.E. Hepatic Steatosis Resulting from LMNA-Associated Familial Lipodystrophy. ACG Case Rep. J. 2020, 7, e00375. [Google Scholar] [CrossRef] [PubMed]
- Ferradini, V.; Cosma, J.; Romeo, F.; De Masi, C.; Michela, M.; Spitalieri, P.; Mannucci, S.; Parlapiano, G.; Di Lorenzo, F.; Martino, A.; et al. Clinical Features of LMNA-Related Cardiomyopathy in 18 Patients and Characterization of Two Novel Variants. J. Clin. Med. 2021, 10, 5075. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.F.; Karra, R.; Amos, K.; Landstrom, A.; Lakdawala, N.K.; Brezitski, K.; Kim, H.; DeVore, A.D. LMNA Cardiomyopathy: Important Considerations for the Heart Failure Clinician. J. Card. Fail. 2023, 29, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Mory, P.B.; Crispim, F.; Freire, M.B.S.; Salles, J.E.N.; Valério, C.M.; Godoy-Matos, A.F.; Dib, S.A.; Moisés, R.S. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. Eur. J. Endocrinol. 2012, 167, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yang, H.; Yuan, Y.; Bonnemann, C.; Chang, X.; Wang, S.; Wu, Y.; Wu, X.; Xiong, H. Phenotype–Genotype Analysis of Chinese Patients with Early-Onset LMNA-Related Muscular Dystrophy. PLoS ONE 2015, 10, e0129699. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J. Non-syndromic cardiac progeria in a patient with the rare pathogenic p.Asp300Asn variant in the LMNA gene. BMC Med. Genet. 2017, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Motegi, S.; Yokoyama, Y.; Uchiyama, A.; Ogino, S.; Takeuchi, Y.; Yamada, K.; Hattori, T.; Hashizume, H.; Ishikawa, Y.; Goto, M.; et al. First Japanese case of atypical progeroid syndrome/atypical Werner syndrome with heterozygous LMNA mutation. J. Dermatol. 2014, 41, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Renard, D.; Fourcade, G.; Milhaud, D.; Bessis, D.; Esteves-Vieira, V.; Boyer, A.; Roll, P.; Bourgeois, P.; Levy, N. Novel LMNA Mutation in Atypical Werner Syndrome Presenting with Ischemic Disease. Stroke 2009, 40, 531780. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; MacRae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J.; Spudich, S.; De Girolami, U.; et al. Missense Mutations in the Rod Domain of the Lamin A/C Gene as Causes of Dilated Cardiomyopathy and Conduction-System Disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, T.A.; Misteli, T. The lamin protein family. Genome Biol. 2011, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Gete, Y.G.; Koblan, L.W.; Mao, X.; Trappio, M.; Mahadik, B.; Fisher, J.P.; Liu, D.R.; Cao, K. Mechanisms of angiogenic incompetence in Hutchinson–Gilford progeria via downregulation of endothelial NOS. Aging Cell 2021, 20, 13388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Van de Peppel, I.P.; Rutten, J.W.; Jukema, J.W.; Aten, E.; Jazet, I.M.; Koopmann, T.T.; Barge-Schaapveld, D.Q.C.M.; Ajmone Marsan, N. Atypical Progeria Primarily Manifesting as Premature Cardiac Valvular Disease Segregates with LMNA-Gene Variants. J. Cardiovasc. Dev. Dis. 2024, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Doubaj, Y.; De Sandre-Giovannoli, A.; Vera, E.-V.; Navarro, C.L.; Elalaoui, S.C.; Tajir, M.; Lévy, N. Abdelaziz Sefiani An inherited LMNA gene mutation in atypical Progeria syndrome. Am. J. Med. Genet.—Part A 2012, 158A, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.; Biervliet, M.; Reyniers, E.; D’Apice, M.R.; Novelli, G.; Storm, K. Somatic and gonadal mosaicism in Hutchinson-Gilford progeria. Am. J. Med. Genet. A 2005, 135A, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Luo, J.; Zhong, J.; Liu, X.; Tang, J.; Lan, D. Detection of gonosomal mosaicism by ultra-deep sequencing and droplet digital PCR in patients with Emery–Dreifuss muscular dystrophy. Mol. Genet. Genom. Med. 2023, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hou, Y.; Lv, X.; Yan, C.; Lin, P. Somatic and germinal mosaicism in a Han Chinese family with laminopathies. Eur. J. Hum. Genet. EJHG 2023, 31, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
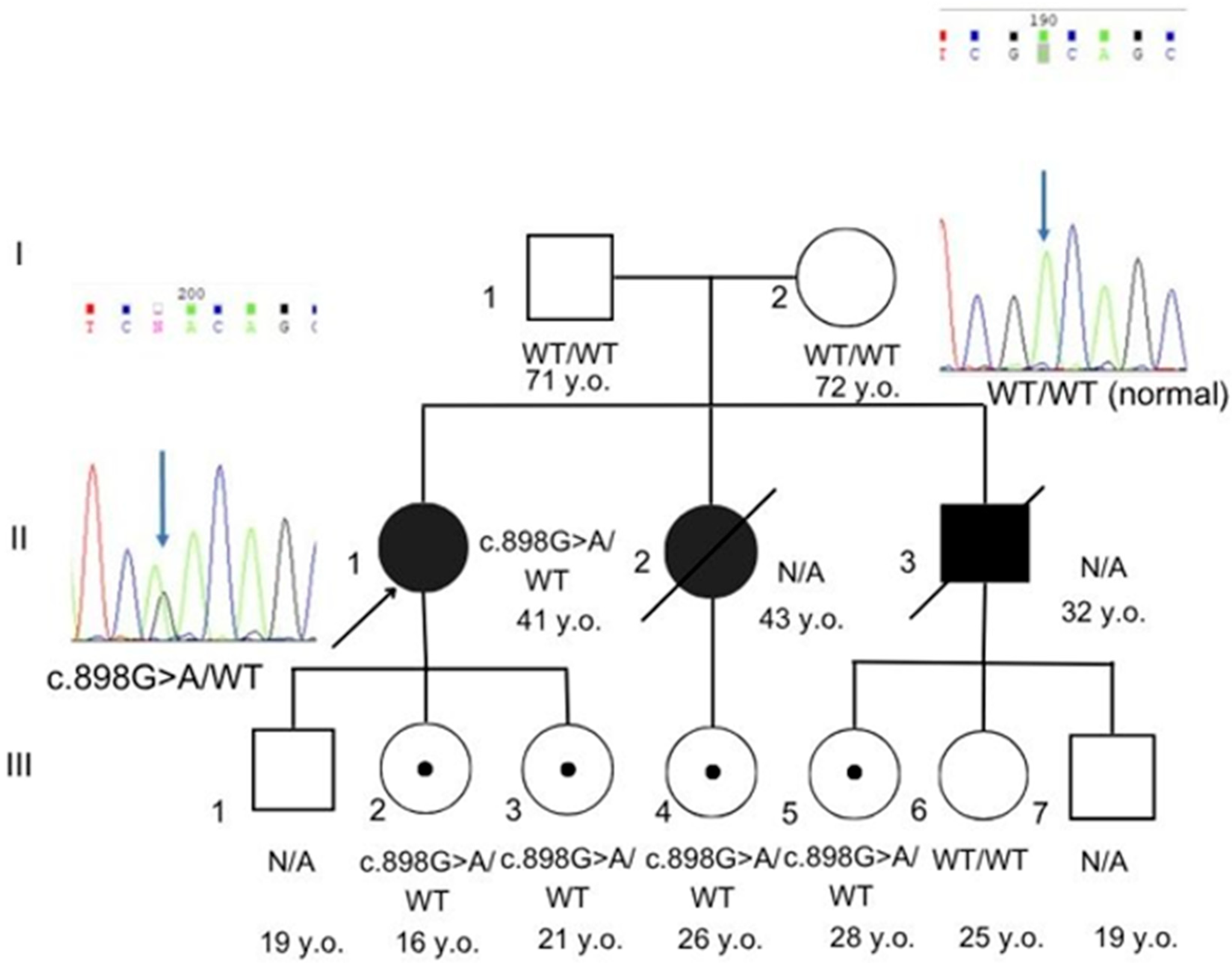
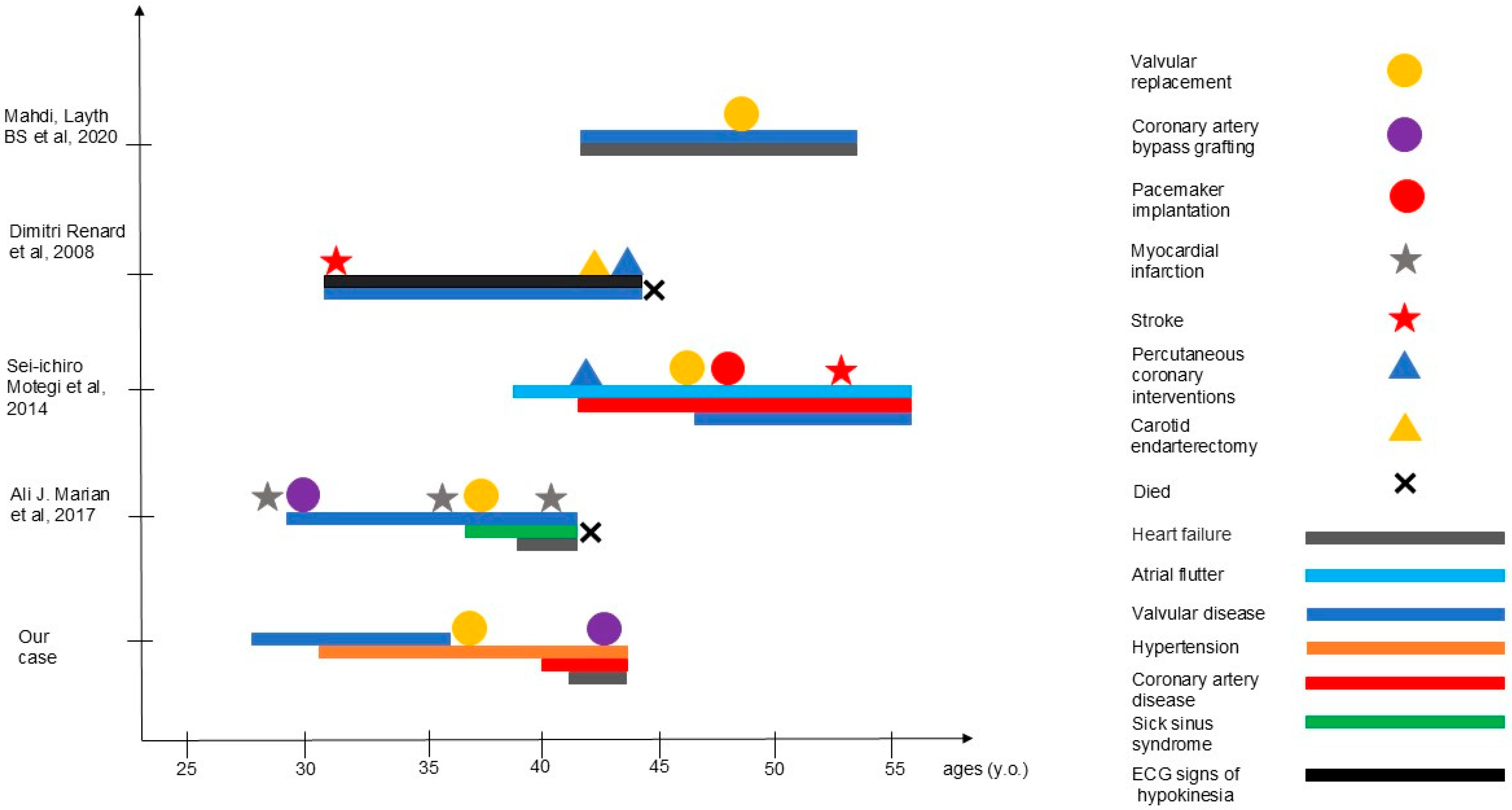
| Characteristics | Our Case | Mahdi et al., 2020 [4] | Marian et al., 2017 [9] | Motegi et al, 2014 [10] | Renard et al., 2009 [11] |
|---|---|---|---|---|---|
| Sex | f | f | f | m | m |
| BMI (kg/m2) | 16.6 | 19 | 18.3 | 14.1 | 14.9 |
| Hypertension | yes | yes | no | no | yes |
| Family history | father—(CABG) at 63 y sister—aortic stenosis, CAD, died at 43 y; brother—pulmonary embolism at 32y, died at 40 y | father—valvular heart disease, lean body habitus | N/D | sister—progeroid features, AVR, stroke at 47 y father and paternal relatives—stroke/MI, liver failure in midlife | father—Werner syndrome, MI at 52 y |
| Facial/phenotypic features | No phenotypic features | Lean body habitus with well-defined truncal and extremity musculature | No phenotypic features | High-pitched voice, pigmentation, sclerodactyly | Bird-like face, lipodystrophy, scleroderma-like skin |
| Smoking | no | no | no | no | no |
| Diabetes mellitus | no | no | no | no | no |
| Serum cholesterol (mg/dL) | 64.62 (ref: 63–93.6) | Normal | 130 (ref: <200) | High | Normal |
| Serum Triglyceride (mg/dL) | 22.32 (ref: 9–31.5) | 353, elevated (ref. N/D) | 155 (ref: <150) | High | Normal |
| Serum LDL (mg/dL) | 37.44 (ref: 1.44–72) | normal | 75 (ref: <100) | High | Normal |
| Serum HDL (mg/dL) | 17.1 (ref: 16.2–34.02) | 31 reduced (ref. N/D) | 24 (ref: 40–60) | N/D | N/D |
| Other manifestations | Hepatic steatosis Pancreatitis Sleep apnea Barrett’s esophagus | Progeroid features, basal squamous cell carcinomas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzhnaya, E.; Sharova, M.; Chubykina, U.; Orlova, A.; Vorontsova, E.; Vasiliev, P. Genetically Confirmed Familial Case of Nonsyndromic Cardiac Progeria Caused by the LMNA p.Asp300Asn Variant with Presumed Gonadal Mosaicism: Phenotypic Comparison with Previously Reported Patients. Genes 2025, 16, 1250. https://doi.org/10.3390/genes16111250
Nuzhnaya E, Sharova M, Chubykina U, Orlova A, Vorontsova E, Vasiliev P. Genetically Confirmed Familial Case of Nonsyndromic Cardiac Progeria Caused by the LMNA p.Asp300Asn Variant with Presumed Gonadal Mosaicism: Phenotypic Comparison with Previously Reported Patients. Genes. 2025; 16(11):1250. https://doi.org/10.3390/genes16111250
Chicago/Turabian StyleNuzhnaya, Ekaterina, Margarita Sharova, Uliana Chubykina, Anna Orlova, Ekaterina Vorontsova, and Peter Vasiliev. 2025. "Genetically Confirmed Familial Case of Nonsyndromic Cardiac Progeria Caused by the LMNA p.Asp300Asn Variant with Presumed Gonadal Mosaicism: Phenotypic Comparison with Previously Reported Patients" Genes 16, no. 11: 1250. https://doi.org/10.3390/genes16111250
APA StyleNuzhnaya, E., Sharova, M., Chubykina, U., Orlova, A., Vorontsova, E., & Vasiliev, P. (2025). Genetically Confirmed Familial Case of Nonsyndromic Cardiac Progeria Caused by the LMNA p.Asp300Asn Variant with Presumed Gonadal Mosaicism: Phenotypic Comparison with Previously Reported Patients. Genes, 16(11), 1250. https://doi.org/10.3390/genes16111250








