Inherited Retinal Diseases with High Myopia: A Review
Abstract
1. Introduction
2. Methods
3. Collagen/Structural Integrity
3.1. COL2A1
3.2. COL9A1
3.3. COL11A1
3.4. COL18A1
3.5. P3H2/LEPREL1
4. Phototransduction and Visual Cycle
4.1. PDE6C
4.2. PDE6H
4.3. GUCY2D
4.4. ARR3
4.5. RBP3
5. Ciliary Trafficking and Microtubule-Associated Genes
5.1. RPGR
5.2. RP2
5.3. IFT140
5.4. CFAP418 (C8orf37)
5.5. FAM161A
6. Synaptic Ribbon and Bipolar Cell Signalling
6.1. NYX
6.2. CACNA1F
6.3. TRPM1
6.4. GRM6
6.5. LRIT3
6.6. GPR179
7. Opsin-Related Genes
7.1. OPN1LW
7.2. OPN1MW
8. Miscellaneous
8.1. COH1/VPS13B
8.2. ADAMTS18
8.3. LAMA1
9. Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben-Yosef, T. Inherited Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 13467. [Google Scholar] [CrossRef]
- Lamb, T.D. Photoreceptor Physiology and Evolution: Cellular and Molecular Basis of Rod and Cone Phototransduction. J. Physiol. 2022, 600, 4585–4601. [Google Scholar] [CrossRef]
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The Cell Biology of the Retinal Pigment Epithelium. Prog. Retin. Eye Res. 2020, 78, 100846. [Google Scholar] [CrossRef]
- Sahel, J.-A.; Marazova, K.; Audo, I. Clinical Characteristics and Current Therapies for Inherited Retinal Degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef] [PubMed]
- Prokofyeva, E.; Troeger, E.; Wilke, R.; Zrenner, E. Early Visual Symptom Patterns in Inherited Retinal Dystrophies. Ophthalmologica 2011, 226, 151–156. [Google Scholar] [CrossRef]
- Battu, R.; Ratra, D.; Gopal, L. Newer Therapeutic Options for Inherited Retinal Diseases: Gene and Cell Replacement Therapy. Indian J. Ophthalmol. 2022, 70, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Suppiej, A.; Marino, S.; Reffo, M.E.; Maritan, V.; Vitaliti, G.; Mailo, J.; Falsaperla, R. Early Onset Retinal Dystrophies: Clinical Clues to Diagnosis for Pediatricians. Ital. J. Pediatr. 2019, 45, 168. [Google Scholar] [CrossRef]
- Shih, Y.-F. Visual Outcomes for High Myopic Patients with or without Myopic Maculopathy: A 10 Year Follow up Study. Br. J. Ophthalmol. 2006, 90, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Deng, H.; Huang, X.; Yuan, L. Molecular Genetics of the COL2A1-Related Disorders. Mutat. Res./Rev. Mutat. Res. 2016, 768, 1–13. [Google Scholar] [CrossRef]
- Hoornaert, K.P.; Vereecke, I.; Dewinter, C.; Rosenberg, T.; Beemer, F.A.; Leroy, J.G.; Bendix, L.; Björck, E.; Bonduelle, M.; Boute, O.; et al. Stickler Syndrome Caused by COL2A1 Mutations: Genotype–Phenotype Correlation in a Series of 100 Patients. Eur. J. Hum. Genet. 2010, 18, 872–880. [Google Scholar] [CrossRef]
- Okazaki, S.; Meguro, A.; Ideta, R.; Takeuchi, M.; Yonemoto, J.; Teshigawara, T.; Yamane, T.; Okada, E.; Ideta, H.; Mizuki, N. Common Variants in the COL2A1 Gene Are Associated with Lattice Degeneration of the Retina in a Japanese Population. Mol. Vis. 2019, 25, 843–850. [Google Scholar] [PubMed]
- Shapiro, M.; Blair, M.; Solinski, M.; Zhang, D.; Jabbehdari, S. The Importance of Early Diagnosis of Stickler Syndrome: Finding Opportunities for Preventing Blindness. Taiwan J. Ophthalmol. 2018, 8, 189. [Google Scholar] [CrossRef]
- Chauvaud, D. Rhegmatogenous Retinal Detachment. Rev. Prat. 1996, 46, 1750–1755. [Google Scholar]
- Snead, M.P.; Payne, S.J.; Barton, D.E.; Yates, J.R.; Al-Imara, L.; Popp, F.M.; Scott, J.D. Stickler Syndrome: Correlation between Vitreoretinal Phenotypes and Linkage to Col 2A1. Eye 1994, 8, 609–614. [Google Scholar] [CrossRef]
- Liberfarb, R.M.; Levy, H.P.; Rose, P.S.; Wilkin, D.J.; Davis, J.; Balog, J.Z.; Griffith, A.J.; Szymko-Bennett, Y.M.; Johnston, J.J.; Francomano, C.A. The Stickler Syndrome: Genotype/Phenotype Correlation in 10 Families with Stickler Syndrome Resulting from Seven Mutations in the Type II Collagen Gene Locus COL2A1. Genet. Med. 2003, 5, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xing, X.; Li, D.; Yu, B. Restoration of Vision in KNIEST Dysplasia Patient Characterized by Retinal Detachment with Dialysis of the Ora Serrata: A Case Report. Medicine 2023, 102, e36090. [Google Scholar] [CrossRef] [PubMed]
- Hamidi-Toosi, S.; Maumenee, I.H. Vitreoretinal Degeneration in Spondyloepiphyseal Dysplasia Congenita. Arch. Ophthalmol. 1982, 100, 1104–1107. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Wang, Z.; Sun, L.; Li, S.; Zhang, T.; Luo, X.; Ding, X. Mutation Spectrum and De Novo Mutation Analysis in Stickler Syndrome Patients with High Myopia or Retinal Detachment. Genes 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Matsushita, I.; Nagata, T.; Hayashi, T.; Kakinoki, M.; Uchio, E.; Kondo, M.; Ohji, M.; Kusaka, S. Novel Mutations in the COL2A1 Gene in Japanese Patients with Stickler Syndrome. Hum. Genome Var. 2016, 3, 16018. [Google Scholar] [CrossRef]
- Jacobson, A.; Besirli, C.G.; Bohnsack, B.L. Characteristics of a Three-Generation Family with Stickler Syndrome Type I. Genes 2023, 14, 847. [Google Scholar] [CrossRef]
- Metlapally, R.; Li, Y.J.; Tran-Viet, K.N.; Abbott, D.; Czaja, G.R.; Malecaze, F.; Calvas, P.; Mackey, D.; Rosenberg, T.; Paget, S.; et al. COL1A1 and COL2A1 Genes and Myopia Susceptibility: Evidence of Association and Suggestive Linkage to the COL2A1 Locus. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1310–1319. [Google Scholar]
- Sun, W.; Xiao, X.; Li, S.; Jia, X.; Zhang, Q. A Novel Deep Intronic Col2a1 Mutation in a Family with Early-onset High Myopia/Ocular-only Stickler Syndrome. Ophthalmic Physiol. Opt. 2020, 40, 281–288. [Google Scholar] [CrossRef]
- Muragaki, Y.; Kimura, T.; NInomiya, Y.; Olsen, B.R. The Complete Primary Structure of Two Distinct Forms of Human α(IX) Collagen Chains. Eur. J. Biochem. 1990, 192, 703–708. [Google Scholar] [CrossRef]
- Nikopoulos, K.; Schrauwen, I.; Simon, M.; Collin, R.W.; Veckeneer, M.; Keymolen, K.; Van Camp, G.; Cremers, F.P.; van den Born, L.I. Autosomal Recessive Stickler Syndrome in Two Families Is Caused by Mutations in the Col9a1 Gene. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4774. [Google Scholar] [CrossRef]
- Van Camp, G.; Snoeckx, R.L.; Hilgert, N.; van den Ende, J.; Fukuoka, H.; Wagatsuma, M.; Suzuki, H.; Erica Smets, R.M.; Vanhoenacker, F.; Declau, F.; et al. A New Autosomal Recessive Form of Stickler Syndrome Is Caused by a Mutation in the COL9A1 Gene. Am. J. Hum. Genet. 2006, 79, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, D.; Nixon, T.R.; Martin, H.; Richards, A.J.; McNinch, A.M.; Alexander, P.; Poulson, A.V.; Snead, M.P. Retinal Detachment in Type IX Collagen Recessive Stickler Syndrome. Eye 2024, 39, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Nixon, T.; Richards, A.J.; Lomas, A.; Abbs, S.; Vasudevan, P.; McNinch, A.; Alexander, P.; Snead, M.P. Inherited and de Novo Biallelic Pathogenic Variants in Col11a1 Result in Type 2 Stickler Syndrome with Severe Hearing Loss. Mol. Genet. Genom. Med. 2020, 8, e1354. [Google Scholar] [CrossRef]
- Richards, A.J.; McNinch, A.; Martin, H.; Oakhill, K.; Rai, H.; Waller, S.; Treacy, B.; Whittaker, J.; Meredith, S.; Poulson, A.; et al. Stickler Syndrome and the Vitreous Phenotype: Mutations in COL2A1 and COL11A1. Hum. Mutat. 2010, 31, E1461–E1471. [Google Scholar] [CrossRef]
- Boothe, M.; Morris, R.; Robin, N. Stickler Syndrome: A Review of Clinical Manifestations and the Genetics Evaluation. J. Pers. Med. 2020, 10, 105. [Google Scholar] [CrossRef]
- Guo, L.; Elcioglu, N.H.; Wang, Z.; Demirkol, Y.K.; Isguven, P.; Matsumoto, N.; Nishimura, G.; Miyake, N.; Ikegawa, S. Novel and Recurrent COL11A1 and COL2A1 Mutations in the Marshall–Stickler Syndrome Spectrum. Hum. Genome Var. 2017, 4, 17040. [Google Scholar] [CrossRef]
- Fujimoto, K.; Nagata, T.; Matsushita, I.; Oku, K.; Imagawa, M.; Kuniyoshi, K.; Hayashi, T.; Kimoto, K.; Ohji, M.; Kusaka, S.; et al. Ultra-Wide Field Fundus Autofluorescence Imaging of Eyes with Stickler Syndrome. Retina 2020, 41, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Rodriguez, S.H.; Blair, M.A.; Wroblewski, K.; Shapiro, M.J.; Blair, M.P. Laser prophylaxis in patients with Stickler syndrome. Ophthalmol. Retin. 2022, 6, 263–267. [Google Scholar] [CrossRef]
- Linton, E.; Jalil, A.; Sergouniotis, P.; Moussa, G.; Black, G.; Charles, S.; Ivanova, T. Laser prophylaxis in stickler syndrome: The Manchester protocol. Retina 2023, 43, 88. [Google Scholar] [CrossRef]
- Naravane, A.V.; Belin, P.J.; Pierce, B.; Quiram, P.A. Risk and prevention of retinal detachments in patients with Stickler syndrome. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 7–11. [Google Scholar] [CrossRef]
- Camp, D.A.; Bakhsh, S.R.; Torkashvand, A.; Jensen, N.R.; Naravane, A.V.; Belin, P.J.; Quiram, P.A.; Ivanova, T.; Wubben, T.J.; Besirli, C.G.; et al. Laser prophylaxis for retinal detachment in Stickler syndrome: A systematic review and meta-analysis. Acta Ophthalmol. 2025, 103, e364–e373. [Google Scholar] [CrossRef]
- Irene Díez García-Prieto, I.; Lopez-Martín, S.; Albert, J.; Jiménez de la Peña, M.; Fernández-Mayoralas, D.M.; Calleja-Pérez, B.; Gómez Fernández, M.T.; Álvarez, S.; Pihlajaniemi, T.; Izzi, V.; et al. Mutations in the COL18A1 Gene Associated with Knobloch Syndrome and Structural Brain Anomalies: A Novel Case Report and Literature Review of Neuroimaging Findings. Neurocase 2022, 28, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Määttä, M.; Heljasvaara, R.; Pihlajaniemi, T.; Uusitalo, M. Collagen XVIII/Endostatin Shows a Ubiquitous Distribution in Human Ocular Tissues and Endostatin-Containing Fragments Accumulate in Ocular Fluid Samples. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 245, 74–81. [Google Scholar] [CrossRef]
- Suzuki, O.T.; Sertié, A.L.; Der Kaloustian, V.M.; Kok, F.; Carpenter, M.; Murray, J.; Czeizel, A.E.; Kliemann, S.E.; Rosemberg, S.; Monteiro, M.; et al. Molecular Analysis of Collagen XVIII Reveals Novel Mutations, Presence of a Third Isoform, and Possible Genetic Heterogeneity in Knobloch Syndrome. Am. J. Hum. Genet. 2002, 71, 1320–1329. [Google Scholar] [CrossRef]
- Vasilyeva, T.; Kadyshev, V.; Khalanskaya, O.; Kuznetsova, S.; Ionova, S.; Marakhonov, A.; Zinchenko, R. Clinical and Molecular Findings in Patients with Knobloch Syndrome 1: Case Series Report. Genes 2024, 15, 1295. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Arno, G.; Ku, C.A.; Ge, Z.; Waseem, N.; Chandra, A.; Webster, A.R.; Robson, A.G.; Michaelides, M.; Weleber, R.G.; et al. Molecular and Clinical Findings in Patients with Knobloch Syndrome. JAMA Ophthalmol. 2016, 134, 753. [Google Scholar] [CrossRef]
- Chong, S.C.; Yuen, Y.-P.; Cao, Y.; Fan, S.-S.; Leung, T.Y.; Chan, E.K.; Zhu, X.L. Case Report: Novel Biallelic Variants in the COL18A1 Gene in a Chinese Family with Knobloch Syndrome. Front. Neurol. 2022, 13, 853918. [Google Scholar] [CrossRef]
- Menzel, O.; Bekkeheien, R.C.J.; Reymond, A.; Fukai, N.; Boye, E.; Kosztolanyi, G.; Aftimos, S.; Deutsch, S.; Scott, H.S.; Olsen, B.R.; et al. Knobloch Syndrome: Novel Mutations in Col18a1, Evidence for Genetic Heterogeneity, and a Functionally Impaired Polymorphism in Endostatin. Hum. Mutat. 2004, 23, 77–84. [Google Scholar] [CrossRef]
- Schnur, R.E.; Dvořáček, L.; Kalsner, L.; Shapiro, F.L.; Grebeňová, D.; Yanni, D.; Wasserman, B.N.; Dyer, L.M.; Antonarakis, S.E.; Kuželová, K. New Kinase-deficient Pak2 Variants Associated with Knobloch Syndrome Type 2. Clin. Genet. 2024, 106, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.J.; Farnand, A.W.; Traeger, G.R.; Weis, M.A.; Eyre, D.R. A Role for Prolyl 3-Hydroxylase 2 in Post-Translational Modification of Fibril-Forming Collagens. J. Biol. Chem. 2011, 286, 30662–30669. [Google Scholar] [CrossRef]
- Tiainen, P.; Pasanen, A.; Sormunen, R.; Myllyharju, J. Characterization of Recombinant Human Prolyl 3-Hydroxylase Isoenzyme 2, an Enzyme Modifying the Basement Membrane Collagen IV. J. Biol. Chem. 2008, 283, 19432–19439. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Johnson, T.V. The Internal Limiting Membrane: Roles in Retinal Development and Implications for Emerging Ocular Therapies. Exp. Eye Res. 2021, 206, 108545. [Google Scholar] [CrossRef] [PubMed]
- Mordechai, S.; Gradstein, L.; Pasanen, A.; Ofir, R.; El Amour, K.; Levy, J.; Belfair, N.; Lifshitz, T.; Joshua, S.; Narkis, G.; et al. High Myopia Caused by a Mutation in LEPREL1, Encoding Prolyl 3-Hydroxylase 2. Am. J. Hum. Genet. 2011, 89, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Stingl, K.; Audo, I.; Biskup, S.; Bocquet, B.; Branham, K.; Burstedt, M.S.; De Baere, E.; De Vries, M.J.; Golovleva, I.; et al. Mutations in the Gene PDE6C Encoding the Catalytic Subunit of the Cone Photoreceptor Phosphodiesterase in Patients with Achromatopsia. Hum. Mutat. 2018, 39, 1366–1371. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Singh, N.; Pontikos, N.; Kane, T.; Hirji, N.; Ripamonti, C.; Rotsos, T.; Dubra, A.; Kalitzeos, A.; et al. Deep Phenotyping of Pde6c-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5112. [Google Scholar] [CrossRef]
- Daich Varela, M.; Ullah, E.; Yousaf, S.; Brooks, B.P.; Hufnagel, R.B.; Huryn, L.A. PDE6C: Novel Mutations, Atypical Phenotype, and Differences among Children and Adults. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef]
- Eshel, Y.M.; Abaev, O.; Yahalom, C. Achromatopsia: Long term visual performance and clinical characteristics. Eur. J. Ophthalmol. 2023, 34, 986–991. [Google Scholar] [CrossRef]
- Kohl, S.; Coppieters, F.; Meire, F.; Schaich, S.; Roosing, S.; Brennenstuhl, C.; Bolz, S.; van Genderen, M.M.; Riemslag, F.C.C.; Lukowski, R.; et al. A Nonsense Mutation in PDE6H Causes Autosomal-Recessive Incomplete Achromatopsia. Am. J. Hum. Genet. 2012, 91, 527–532. [Google Scholar] [CrossRef]
- Andersen, M.K.; Bertelsen, M.; Grønskov, K.; Kohl, S.; Kessel, L. Genetic and Clinical Characterization of Danish Achromatopsia Patients. Genes 2023, 14, 690. [Google Scholar] [CrossRef]
- Dizhoor, A. The Human Photoreceptor Membrane Guanylyl Cyclase, Retgc, Is Present in Outer Segments and Is Regulated by Calcium and a Soluble Activator. Neuron 1994, 12, 1345–1352. [Google Scholar] [CrossRef]
- Lazar, C.H.; Mutsuddi, M.; Kimchi, A.; Zelinger, L.; Mizrahi-Meissonnier, L.; Marks-Ohana, D.; Boleda, A.; Ratnapriya, R.; Sharon, D.; Swaroop, A.; et al. Whole Exome Sequencing Reveals GUCY2D as a Major Gene Associated with Cone and Cone-Rod Dystrophy in Israel. Investig. Ophthalmol. Vis. Sci. 2014, 56, 420–430. [Google Scholar] [CrossRef]
- Wimberg, H.; Lev, D.; Yosovich, K.; Namburi, P.; Banin, E.; Sharon, D.; Koch, K.-W. Photoreceptor Guanylate Cyclase (GUCY2D) Mutations Cause Retinal Dystrophies by Severe Malfunction of Ca2+-Dependent Cyclic GMP Synthesis. Front. Mol. Neurosci. 2018, 11, 348. [Google Scholar] [CrossRef]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber Congenital Amaurosis/Early-Onset Severe Retinal Dystrophy: Clinical Features, Molecular Genetics and Therapeutic Interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, C.; Martín-Merida, I.; Blanco-Kelly, F.; Trujillo-Tiebas, M.J.; Avila-Fernandez, A.; Riveiro-Alvarez, R.; del Pozo-Valero, M.; Perea-Romero, I.; Swafiri, S.T.; Zurita, O.; et al. Comprehensive Genotyping and Phenotyping Analysis of Gucy2d-Associated Rod- and Cone-Dominated Dystrophies. Am. J. Ophthalmol. 2023, 254, 87–103. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; Black, A.; Bennett, J.; Cremers, F.P.M. Lighting a Candle in the Dark: Advances in Genetics and Gene Therapy of Recessive Retinal Dystrophies. J. Clin. Investig. 2010, 120, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Haarman, A.E.G.; Thiadens, A.A.; van Tienhoven, M.; Loudon, S.E.; de Klein, J.E.M.M.A.; Brosens, E.; Polling, J.R.; van der Schoot, V.; Bouman, A.; Kievit, A.J.A.; et al. Whole-Exome Sequencing of Known Eye Genes Reveals Genetic Causes for High Myopia. Hum. Mol. Genet. 2022, 31, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-M.; Wei, J.; Wang, J.; Zhang, G.; Bi, J.; Ye, L. Advances in the Study of ARR3 in Myopia. Front. Cell Dev. Biol. 2025, 13, 1551135. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.; Li, X.; Yi, Z.; Jiang, Y.; Zhang, F.; Zhou, L.; Li, S.; Jia, X.; Sun, W.; et al. Genetic and Clinical Landscape of Arr3-Associated MYP26: The Most Common Cause of Mendelian Early-Onset High Myopia with a Unique Inheritance. Br. J. Ophthalmol. 2022, 107, 1545–1553. [Google Scholar] [CrossRef]
- Mazijk, R.; Haarman, A.E.G.; Hoefsloot, L.H.; Polling, J.R.; Tienhoven, M.; Klaver, C.C.W.; Verhoeven, V.J.M.; Loudon, S.E.; Thiadens, A.A.H.J.; Kievit, A.J.A. Early Onset X-linked Female Limited High Myopia in Three Multigenerational Families Caused by Novel Mutations in the Arr3 Gene. Hum. Mutat. 2022, 43, 380–388. [Google Scholar] [CrossRef]
- Edwards, R.B.; Adler, A.J. IRBP Enhances Removal of 11- Cis -Retinaldehyde from Isolated RPE Membranes. Exp. Eye Res. 2000, 70, 235–245. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; McGee, T.L.; Ziviello, C.; Banfi, S.; Dryja, T.P.; Gonzalez-Fernandez, F.; Ghosh, D.; Berson, E.L. A Homozygous Missense Mutation in the Irbp Gene (Rbp3) Associated with Autosomal Recessive Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1864. [Google Scholar] [CrossRef]
- Arno, G.; Hull, S.; Robson, A.G.; Holder, G.E.; Cheetham, M.E.; Webster, A.R.; Plagnol, V.; Moore, A.T. Lack of Interphotoreceptor Retinoid Binding Protein Caused by Homozygous Mutation of RBP3 Is Associated with High Myopia and Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2358–2365. [Google Scholar] [CrossRef]
- Georgiou, M.; Fujinami, K.; Robson, A.G.; Fujinami-Yokokawa, Y.; Shakarchi, A.F.; Ji, M.H.; Uwaydat, S.H.; Kim, A.; Kolesnikova, M.; Arno, G.; et al. RBP3-Retinopathy—Inherited High Myopia and Retinal Dystrophy: Genetic Characterization, Natural History, and Deep Phenotyping. Am. J. Ophthalmol. 2024, 258, 119–129. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.A.; Atkins, S.J.; Peranen, J.; Swaroop, A.; Khanna, H. Interaction of Retinitis Pigmentosa GTPase Regulator (RPGR) with Rab8a GTPase: Implications for Cilia Dysfunction and Photoreceptor Degeneration. Hum. Mol. Genet. 2010, 19, 3591–3598. [Google Scholar] [CrossRef]
- Nassisi, M.; De Bartolo, G.; Mohand-Said, S.; Condroyer, C.; Antonio, A.; Lancelot, M.-E.; Bujakowska, K.; Smirnov, V.; Pugliese, T.; Neidhardt, J.; et al. Retrospective Natural History Study of RPGR-Related Cone- and Cone-Rod Dystrophies While Expanding the Mutation Spectrum of the Disease. Int. J. Mol. Sci. 2022, 23, 7189. [Google Scholar] [CrossRef] [PubMed]
- Fahim, A.T.; Sullivan, L.S.; Bowne, S.J.; Jones, K.D.; Wheaton, D.K.; Khan, N.W.; Heckenlively, J.R.; Jayasundera, K.T.; Branham, K.H.; Andrews, C.A.; et al. X-Chromosome Inactivation Is a Biomarker of Clinical Severity in Female Carriers of RPGR-Associated X-Linked Retinitis Pigmentosa. Ophthalmol. Retin. 2020, 4, 510–520. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, L.; Ouyang, J.; Xiao, X.; Sun, W.; Li, S.; Zhang, Q. Genotype–Phenotype Analysis of RPGR Variations: Reporting of 62 Chinese Families and a Literature Review. Front. Genet. 2021, 12, 600210. [Google Scholar] [CrossRef]
- Di Iorio, V.; Karali, M.; Melillo, P.; Testa, F.; Brunetti-Pierri, R.; Musacchia, F.; Condroyer, C.; Neidhardt, J.; Audo, I.; Zeitz, C.; et al. Spectrum of Disease Severity in Patients with X-Linked Retinitis Pigmentosa Due to Rpgr Mutations. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lai, Y.; Sun, L.; Li, S.; Ding, X. High Myopia Is Common in Patients with X-Linked Retinopathies. Retina 2024, 44, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-P. Myopia with X-Linked Retinitis Pigmentosa Results from a Novel Gross Deletion of RPGR Gene. Int. J. Ophthalmol. 2020, 13, 1306–1311. [Google Scholar] [CrossRef]
- Mawatari, G.; Fujinami, K.; Liu, X.; Yang, L.; Yokokawa, Y.F.; Komori, S.; Ueno, S.; Terasaki, H.; Katagiri, S.; Hayashi, T.; et al. Clinical and genetic characteristics of 14 patients from 13 Japanese families with RPGR-associated retinal disorder: Report of eight novel variants. Hum. Genome Var. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Veltel, S.; Gasper, R.; Eisenacher, E.; Wittinghofer, A. The Retinitis Pigmentosa 2 Gene Product Is a GTPase-Activating Protein for ARF-like 3. Nat. Struct. Mol. Biol. 2008, 15, 373–380. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Uwaydat, S.H.; Ji, M.H.; Shakarchi, A.F.; Pontikos, N.; Mahroo, O.A.; Cheetham, M.E.; Webster, A.R.; Hardcastle, A.J.; et al. Rp2-Associated X-Linked Retinopathy: Clinical Findings, Molecular Genetics, and Natural History in a Large Cohort of Female Carriers. Am. J. Ophthalmol. 2024, 261, 112–120. [Google Scholar] [CrossRef]
- Jayasundera, T. RP2 Phenotype and Pathogenetic Correlations in X-Linked Retinitis Pigmentosa. Arch. Ophthalmol. 2010, 128, 915. [Google Scholar] [CrossRef]
- Hull, S.; Owen, N.; Islam, F.; Tracey-White, D.; Plagnol, V.; Holder, G.E.; Michaelides, M.; Carss, K.; Raymond, F.L.; Rozet, J.-M.; et al. Nonsyndromic Retinal Dystrophy Due to Bi-Allelic Mutations in the Ciliary Transport Gene Ift140. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1053. [Google Scholar] [CrossRef]
- Helm, B.M.; Willer, J.R.; Sadeghpour, A.; Golzio, C.; Crouch, E.; Vergano, S.S.; Katsanis, N.; Davis, E.E. Partial Uniparental Isodisomy of Chromosome 16 Unmasks a Deleterious Biallelic Mutation in IFT140 That Causes Mainzer-SALDINO Syndrome. Hum. Genom. 2017, 11, 16. [Google Scholar] [CrossRef]
- Estrada-Cuzcano, A.; Neveling, K.; Kohl, S.; Banin, E.; Rotenstreich, Y.; Sharon, D.; Falik-Zaccai, T.C.; Hipp, S.; Roepman, R.; Wissinger, B.; et al. Mutations in C8ORF37, Encoding a Ciliary Protein, Are Associated with Autosomal-Recessive Retinal Dystrophies with Early Macular Involvement. Am. J. Hum. Genet. 2012, 90, 102–109. [Google Scholar] [CrossRef]
- Katagiri, S.; Hayashi, T.; Yoshitake, K.; Akahori, M.; Ikeo, K.; Gekka, T.; Tsuneoka, H.; Iwata, T. Novel C8orf37 Mutations in Patients with Early-Onset Retinal Dystrophy, Macular Atrophy, Cataracts, and High Myopia. Ophthalmic Genet. 2014, 37, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Singh, K.; Uppal, A.; Vanita, V. A Nonsense Mutation in C8ORF37 Linked with Retinitis Pigmentosa, Early Macular Degeneration, Cataract, and Myopia in an ARRP Family from North India. BMC Ophthalmol. 2023, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Bandah-Rozenfeld, D.; Mizrahi-Meissonnier, L.; Farhy, C.; Obolensky, A.; Chowers, I.; Pe’er, J.; Merin, S.; Ben-Yosef, T.; Ashery-Padan, R.; Banin, E.; et al. Homozygosity Mapping Reveals Null Mutations in FAM161A as a Cause of Autosomal-Recessive Retinitis Pigmentosa. Am. J. Hum. Genet. 2010, 87, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.A.; Letteboer, S.J.F.; Kostic, C.; Bandah-Rozenfeld, D.; Hetterschijt, L.; Sharon, D.; Arsenijevic, Y.; Roepman, R.; Rivolta, C. FAM161A, Associated with Retinitis Pigmentosa, Is a Component of the Cilia-Basal Body Complex and Interacts with Proteins Involved in Ciliopathies. Hum. Mol. Genet. 2012, 21, 5174–5184. [Google Scholar] [CrossRef]
- Beryozkin, A.; Khateb, S.; Idrobo-Robalino, C.A.; Khan, M.I.; Cremers, F.P.; Obolensky, A.; Hanany, M.; Mezer, E.; Chowers, I.; Newman, H.; et al. Unique Combination of Clinical Features in a Large Cohort of 100 Patients with Retinitis Pigmentosa Caused by FAM161A Mutations. Sci. Rep. 2020, 10, 15156. [Google Scholar] [CrossRef]
- Gregg, R.G.; Kamermans, M.; Klooster, J.; Lukasiewicz, P.D.; Peachey, N.S.; Vessey, K.A.; McCall, M.A. NYCTALOPIN Expression in Retinal Bipolar Cells Restores Visual Function in a Mouse Model of Complete X-Linked Congenital Stationary Night Blindness. J. Neurophysiol. 2007, 98, 3023–3033. [Google Scholar] [CrossRef]
- Poels, M.M.; de Wit, G.C.; Bijveld, M.M.; van Genderen, M.M. Natural Course of Refractive Error in Congenital Stationary Night Blindness: Implications for Myopia Treatment. Investig. Ophthalmol. Vis. Sci. 2024, 65, 9. [Google Scholar] [CrossRef]
- Huang, L.; Bai, X.; Xie, Y.; Zhou, Y.; Wu, J.; Li, N. Clinical and Genetic Studies for a Cohort of Patients with Congenital Stationary Night Blindness. Orphanet J. Rare Dis. 2024, 19, 101. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, X.; Guo, X.; Li, S.; Yang, Z.; Zhang, Q. CSNB1 in Chinese Families Associated with Novel Mutations in Nyx. J. Hum. Genet. 2006, 51, 634–640. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Li, S.; Jia, X.; Yang, Z.; Huang, S.; Caruso, R.C.; Guan, T.; Sergeev, Y.; Guo, X.; et al. Mutations in NYX of Individuals with High Myopia, but without Night Blindness. Mol. Vis. 2007, 13, 330–336. [Google Scholar]
- Strom, T.M.; Nyakatura, G.; Apfelstedt-Sylla, E.; Hellebrand, H.; Lorenz, B.; Weber, B.H.; Wutz, K.; Gutwillinger, N.; Rüther, K.; Drescher, B.; et al. An L-Type Calcium-Channel Gene Mutated in Incomplete X-Linked Congenital Stationary Night Blindness. Nat. Genet. 1998, 19, 260–263. [Google Scholar] [CrossRef]
- Wang, P. Phenotype and Genotype of CACNA1F-Related Diseases. Investig. Ophthalmol. Vis. Sci. 2024, 65, 5267. [Google Scholar]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-Function Mutations in a Calcium-Channel A1-Subunit Gene in XP11.23 Cause Incomplete X-Linked Congenital Stationary Night Blindness. Nat. Genet. 1998, 19, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.; Maybaum, T.; Naylor, M.; Weleber, R.; Robitaille, J.; Miyake, Y.; Bergen, A.; Pierpont, M.; Pearce, W.; Bech-Hansen, N. A Summary of 20 CACNA1F Mutations Identified in 36 Families with Incomplete X-Linked Congenital Stationary Night Blindness, and Characterization of Splice Variants. Hum. Genet. 2001, 108, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hauser, B.M.; Place, E.; Huckfeldt, R.; Vavvas, D.G. A novel homozygous nonsense variant in CABP4 causing stationary cone/rod synaptic dysfunction. Ophthalmic Genet. 2024, 45, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O.; Alrashed, M.; Alkuraya, F.S. Clinical characterisation of the CABP4-related retinal phenotype. Br. J. Ophthalmol. 2013, 97, 262–265. [Google Scholar] [CrossRef]
- Katta, M.; de Guimaraes, T.A.C.; Fujinami-Yokokawa, Y.; Fujinami, K.; Georgiou, M.; Mahroo, O.A.; Webster, A.R.; Michaelides, M. Congenital Stationary Night Blindness: Structure, Function and Genotype-Phenotype Correlations in a Cohort of 122 Patients. Ophthalmol Retin. 2024, 8, 932–941. [Google Scholar] [CrossRef]
- Li, Z.; Sergouniotis, P.I.; Michaelides, M.; Mackay, D.S.; Wright, G.A.; Devery, S.; Moore, A.T.; Holder, G.E.; Robson, A.G.; Webster, A.R. Recessive Mutations of the Gene TRPM1 Abrogate on Bipolar Cell Function and Cause Complete Congenital Stationary Night Blindness in Humans. Am. J. Hum. Genet. 2009, 85, 711–719. [Google Scholar] [CrossRef]
- Al-Hujaili, H.; Taskintuna, I.; Neuhaus, C.; Bergmann, C.; Schatz, P. Long-Term Follow-Up of Retinal Function and Structure in TRPM1-Associated Complete Congenital Stationary Night Blindness. Mol. Vis. 2019, 25, 851–858. [Google Scholar]
- Miraldi Utz, V.; Pfeifer, W.; Longmuir, S.Q.; Olson, R.J.; Wang, K.; Drack, A.V. Presentation of Trpm1-Associated Congenital Stationary Night Blindness in Children. JAMA Ophthalmol. 2018, 136, 389. [Google Scholar] [CrossRef]
- Dryja, T.P.; McGee, T.L.; Berson, E.L.; Fishman, G.A.; Sandberg, M.A.; Alexander, K.R.; Derlacki, D.J.; Rajagopalan, A.S. Night Blindness and Abnormal Cone Electroretinogram on Responses in Patients with Mutations in the Grm6 Gene Encoding Mglur6. Proc. Natl. Acad. Sci. USA 2005, 102, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Guo, R.; Huang, D.; Ji, J.; Liu, W. Compound Heterozygous Mutations in GRM6 Causing Complete Schubert-Bornschein Type Congenital Stationary Night Blindness. Heliyon 2024, 10, e27039. [Google Scholar] [CrossRef]
- Sergouniotis, P.I.; Robson, A.G.; Li, Z.; Devery, S.; Holder, G.E.; Moore, A.T.; Webster, A.R. A phenotypic study of congenital stationary night blindness (CSNB) associated with mutations in the GRM6 gene. Acta Ophthalmol. 2012, 90, e192–e197. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Xiao, X.; Wang, P.; Guo, X.; Zhang, Q. Sequence variations of GRM6 in patients with high myopia. Mol. Vis. 2009, 15, 2094–2100. [Google Scholar]
- Neuillé, M.; Morgans, C.W.; Cao, Y.; Orhan, E.; Michiels, C.; Sahel, J.; Audo, I.; Duvoisin, R.M.; Martemyanov, K.A.; Zeitz, C. Lrit3 Is Essential to Localize Trpm1 to the Dendritic Tips of Depolarizing Bipolar Cells and May Play a Role in Cone Synapse Formation. Eur. J. Neurosci. 2015, 42, 1966–1975. [Google Scholar] [CrossRef]
- Zeitz, C.; Jacobson, S.G.; Hamel, C.P.; Bujakowska, K.; Neuillé, M.; Orhan, E.; Zanlonghi, X.; Lancelot, M.-E.; Michiels, C.; Schwartz, S.B.; et al. Whole-Exome Sequencing Identifies LRIT3 Mutations as a Cause of Autosomal-Recessive Complete Congenital Stationary Night Blindness. Am. J. Hum. Genet. 2013, 92, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Song, X.; Li, J.; Xing, Y.; Li, T. Mutation screening of the LRIT3, CABP4, and GPR179 genes in Chinese patients with Schubert-Bornschein congenital stationary night blindness. Ophthalmic Genet. 2017, 38, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Jeong, H.; Laboute, T.; Martemyanov, K.A.; Lee, H.H. Cryo-EM Structure of Human Class C Orphan GPCR GPR179 Involved in Visual Processing. Nat. Commun. 2024, 15, 8299. [Google Scholar] [CrossRef]
- Audo, I.; Bujakowska, K.; Orhan, E.; Poloschek, C.M.; Defoort-Dhellemmes, S.; Drumare, I.; Kohl, S.; Luu, T.D.; Lecompte, O.; Zrenner, E.; et al. Whole-Exome Sequencing Identifies Mutations in GPR179 Leading to Autosomal-Recessive Complete Congenital Stationary Night Blindness. Am. J. Hum. Genet. 2012, 90, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Klooster, J.; van Genderen, M.M.; Yu, M.; Florijn, R.J.; Riemslag, F.C.; Bergen, A.A.; Gregg, R.G.; Peachey, N.S.; Kamermans, M. Ultrastructural localization of GPR179 and the impact of mutant forms on retinal function in CSNB1 patients and a mouse model. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6973–6981. [Google Scholar] [CrossRef] [PubMed]
- Nathans, J.; Thomas, D.; Hogness, D.S. Molecular Genetics of Human Color Vision: The Genes Encoding Blue, Green, and Red Pigments. Science 1986, 232, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Sechrest, E.R.; Chmelik, K.; Tan, W.D.; Deng, W.-T. Blue Cone Monochromacy and Gene Therapy. Vis. Res. 2023, 208, 108221. [Google Scholar] [CrossRef]
- Neitz, M.; Wagner-Schuman, M.; Rowlan, J.S.; Kuchenbecker, J.A.; Neitz, J. Insight from OPN1LW Gene Haplotypes into the Cause and Prevention of Myopia. Genes 2022, 13, 942. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, W.; Xiao, X.; Jiang, Y.; Ouyang, J.; Wang, J.; Yi, Z.; Li, S.; Jia, X.; Wang, P.; et al. Unique Haplotypes in Opn1lw as a Common Cause of High Myopia with or without Protanopia: A Potential Window into Myopic Mechanism. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Katagiri, S.; Iwasa, M.; Hayashi, T.; Hosono, K.; Yamashita, T.; Kuniyoshi, K.; Ueno, S.; Kondo, M.; Ueyama, H.; Ogita, H.; et al. Genotype Determination of the OPN1LW/OPN1MW Genes: Novel Disease-Causing Mechanisms in Japanese Patients with Blue Cone Monochromacy. Sci. Rep. 2018, 8, 11507. [Google Scholar] [CrossRef]
- Michaelides, M.; Johnson, S.; Simunovic, M.P.; Bradshaw, K.; Holder, G.; Mollon, J.D.; Moore, A.T.; Hunt, D.M. Blue Cone Monochromatism: A Phenotype and Genotype Assessment with Evidence of Progressive Loss of Cone Function in Older Individuals. Eye 2004, 19, 2–10. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Carroll, J.; Hardcastle, A.J.; Michaelides, M. The Cone Dysfunction Syndromes: Table 1. Br. J. Ophthalmol. 2015, 100, 115–121. [Google Scholar] [CrossRef]
- Orosz, O.; Rajta, I.; Vajas, A.; Takács, L.; Csutak, A.; Fodor, M.; Kolozsvári, B.; Resch, M.; Sényi, K.; Lesch, B.; et al. Myopia and Late-Onset Progressive Cone Dystrophy Associate to LVAVA/MVAVA Exon 3 Interchange Haplotypes of Opsin Genes on Chromosome X. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1834. [Google Scholar] [CrossRef]
- Gardner, J.C.; Liew, G.; Quan, Y.-H.; Ermetal, B.; Ueyama, H.; Davidson, A.E.; Schwarz, N.; Kanuga, N.; Chana, R.; Maher, E.R.; et al. Three Different Cone Opsin Gene Array Mutational Mechanisms; Genotype-Phenotype Correlation and Functional Investigation of Cone Opsin Variants. Hum. Mutat. 2014, 35, 1354–1362. [Google Scholar] [CrossRef]
- Haer-Wigman, L.; den Ouden, A.; van Genderen, M.M.; Kroes, H.Y.; Verheij, J.; Smailhodzic, D.; Hoekstra, A.S.; Vijzelaar, R.; Blom, J.; Derks, R.; et al. Diagnostic analysis of the highly complex OPN1LW/OPN1MW gene cluster using long-read sequencing and MLPA. NPJ Genom. Med. 2022, 7, 65. [Google Scholar] [CrossRef]
- Seifert, W.; Kühnisch, J.; Maritzen, T.; Horn, D.; Haucke, V.; Hennies, H.C. Cohen Syndrome-Associated Protein, Coh1, Is a Novel, Giant Golgi Matrix Protein Required for Golgi Integrity. J. Biol. Chem. 2011, 286, 37665–37675. [Google Scholar] [CrossRef]
- Hennies, H.C.; Rauch, A.; Seifert, W.; Schumi, C.; Moser, E.; Al-Taji, E.; Tariverdian, G.; Chrzanowska, K.H.; Krajewska-Walasek, M.; Rajab, A.; et al. Allelic Heterogeneity in the COH1 Gene Explains Clinical Variability in Cohen Syndrome. Am. J. Hum. Genet. 2004, 75, 138–145. [Google Scholar] [CrossRef]
- Kolehmainen, J.; Wilkinson, R.; Lehesjoki, A.-E.; Chandler, K.; Kivitie-Kallio, S.; Clayton-Smith, J.; Träskelin, A.-L.; Waris, L.; Saarinen, A.; Khan, J.; et al. Delineation of Cohen Syndrome Following a Large-Scale Genotype-Phenotype Screen. Am. J. Hum. Genet. 2004, 75, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Nasser, F.; Kurtenbach, A.; Biskup, S.; Weidensee, S.; Kohl, S.; Zrenner, E. Ophthalmic Features of Retinitis Pigmentosa in Cohen Syndrome Caused by Pathogenic Variants in the Vps13b Gene. Acta Ophthalmol. 2019, 98, E316–E321. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Kim, J. Ophthalmic Findings in Cohen Syndrome Patient without Subjective Ophthalmic Complaints: A Case Report. Medicine 2023, 102, e35945. [Google Scholar] [CrossRef]
- Aldahmesh, M.A.; Alshammari, M.J.; Khan, A.O.; Mohamed, J.Y.; Alhabib, F.A.; Alkuraya, F.S. The Syndrome of Microcornea, Myopic Chorioretinal Atrophy, and Telecanthus (MMCAT) Is Caused by Mutations in Adamts18. Hum. Mutat. 2013, 34, 1195–1199. [Google Scholar] [CrossRef]
- Khan, A.O. Microcornea with myopic chorioretinal atrophy, telecanthus and posteriorly-rotated ears: A distinct clinical syndrome. Ophthalmic Genet. 2012, 33, 196–199. [Google Scholar] [CrossRef]
- Faizi, N.; Casteels, I.; Termote, B.; Coucke, P.; De Baere, E.; De Bruyne, M.; Balikova, I. High Myopia and Vitreal Veils in a Patient with Poretti– Boltshauser Syndrome Due to a Novel Homozygous Lama1 Mutation. Ophthalmic Genet. 2022, 43, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Go, M.; Kelly, M.P.; Holgado, S.; Toth, C.A. Ocular Manifestations of Poretti-Boltshauser Syndrome: Findings from Multimodal Imaging and Electrophysiology. Retin. Cases Brief Rep. 2020, 16, 270–274. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Zhang, F.J.; Zhu, S.Q.; Duan, H.; Li, Y.; Zhou, Z.J.; Ma, W.X.; Wang, N.L. The Association of a Single Nucleotide Polymorphism in the Promoter Region of the LAMA1 Gene with Susceptibility to Chinese High Myopia. Mol. Vis. 2011, 17, 1003–1010. [Google Scholar] [PubMed]
- Martínez-Albert, N.; Bueno-Gimeno, I.; Gené-Sampedro, A. Risk Factors for Myopia: A Review. J. Clin. Med. 2023, 12, 6062. [Google Scholar] [CrossRef] [PubMed]
- Landreneau, J.R. Review on the Myopia Pandemic: Epidemiology, Risk Factors, and Prevention. Mo Med. 2021, 118, 156–163. [Google Scholar] [PubMed]
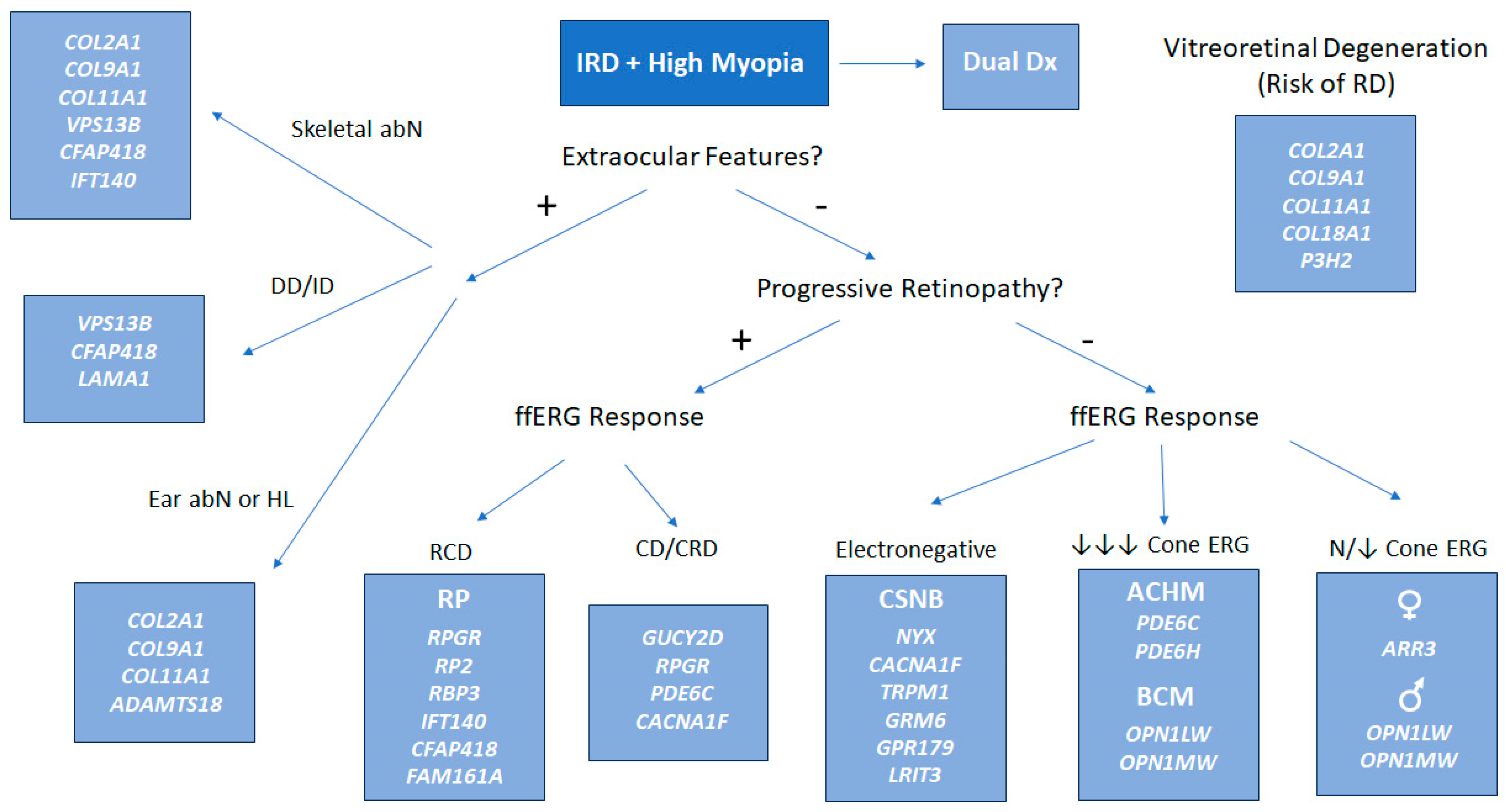
| Monogenic IRDs and High Myopia Functional Categories |
|---|
| Collagen/Structural Integrity |
| COL2A1 (AD; OMIM: 120140) |
| COL9A1 (AR; OMIM: 120210) |
| COL11A1 (AD; OMIM: 120280) |
| COL18A1 (AR; OMIM: 120328) |
| P3H2 (AR; OMIM: 610341) |
| Phototransduction and Visual Cycle |
| PDE6C (AR; OMIM: 600827) |
| PDE6H (AR; OMIM: 601190) |
| GUCY2D (AD/AR; OMIM: 600179) |
| ARR3 (XL; OMIM: 301770) |
| RBP3 (AR; OMIM: 180290) |
| Ciliary Trafficking and Microtubule-Associated Proteins |
| RPGR (XL; OMIM: 312610) |
| RP2 (XL; OMIM: 300757) |
| IFT140 (AR; OMIM: 614620) |
| CFAP418 (AR; OMIM: 614477) |
| FAM161A (AR; OMIM: 613596) |
| Synaptic Ribbon and Bipolar Cell Signalling |
| NYX (XL; OMIM: 300278) |
| CACNA1F (XL; OMIM: 300110) |
| TRPM1 (AR; OMIM: 603576) |
| GRM6 (AR; OMIM: 604096) |
| LRIT3 (AR; OMIM: 615004) |
| GPR179 (AR; OMIM: 614515) |
| Opsin-Related Proteins |
| OPN1LW (XL; OMIM: 300822) |
| OPN1MW (XL; OMIM: 300821) |
| Miscellaneous |
| VPS13B (AR; OMIM: 607817) |
| ADAMTS18 (AR; OMIM: 607512) |
| LAMA1 (AR; OMIM: 150320) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Sheri, N.; Benson, M.D. Inherited Retinal Diseases with High Myopia: A Review. Genes 2025, 16, 1183. https://doi.org/10.3390/genes16101183
Liu C, Sheri N, Benson MD. Inherited Retinal Diseases with High Myopia: A Review. Genes. 2025; 16(10):1183. https://doi.org/10.3390/genes16101183
Chicago/Turabian StyleLiu, Cyndy, Narin Sheri, and Matthew D. Benson. 2025. "Inherited Retinal Diseases with High Myopia: A Review" Genes 16, no. 10: 1183. https://doi.org/10.3390/genes16101183
APA StyleLiu, C., Sheri, N., & Benson, M. D. (2025). Inherited Retinal Diseases with High Myopia: A Review. Genes, 16(10), 1183. https://doi.org/10.3390/genes16101183






