Abstract
Background: An estimated 10–15% of all genetic diseases are attributable to variants in noncanonical splice sites, auxiliary splice sites and deep-intronic variants. Most of these unstudied variants are classified as variants of uncertain significance (VUS), which are not clinically actionable. This study investigated two novel splice-altering variants, CHM NM_000390.4:c.941-11T>G and CACNA1F NM_005183.4:c.2576+4_2576+5del implicated in choroideremia and cone dystrophy (COD), respectively, resulting in significant visual loss. Methods: Next-generation sequencing was employed to identify the candidate variants in CHM and CACNA1F, which were confirmed using Sanger sequencing. Cascade analysis was undertaken when additional family members were available. Functional analysis was conducted by cloning genomic regions of interest into gateway expression vectors, creating variant and wildtype midigenes, which were subsequently transfected into HEK293 cells. RNA was harvested and amplified by RT-PCR to investigate the splicing profile for each variant compared to the wildtype. Novel variants were reclassified according to ACMG/AMP and ClinGen SVI guidelines. Results: Midigene functional analysis confirmed that both variants disrupted splicing. The CHM NM_000390.4:c.941-11T>G variant caused exon 8 skipping, leading to a frameshift and the CACNA1F NM_005183.4:c.2576+4_2576+5del variant caused a multimodal splice defect leading to an in-frame insertion of seven amino acids and a frameshift. With this evidence, the former was upgraded to likely pathogenic and the latter to a hot VUS. Conclusions: This study adds to the mutational spectrum of splicing defects implicated in retinal degenerations by identifying and characterising two novel variants in CHM and CACNA1F. Our results highlight the importance of conducting functional analysis to investigate the consequences of intronic splice-altering variants and the significance of reclassifying VUS to confirm a genetic diagnosis.
1. Introduction
Choroideremia is a rare X-linked inherited retinal disease (IRD) affecting approximately 1 in 50,000 males (OMIM #303100) causing significant visual dysfunction [1]. Whilst female carriers are usually asymptomatic, they can show retinal pigment epithelial depigmentation to a variable degree due to skewed X chromosome inactivation [2]. Choroideremia is caused by variants in the CHM gene which encodes Rab escort protein 1 (REP-1) [3,4]. Loss of function variants in CHM reduce the prenylation of Rab GTPases which is believed to underpin the pathogenesis of this disease [5]. Symptoms of choroideremia typically begin in childhood and manifest as nyctalopia that gradually progresses to reduced visual acuity and visual field issues as observed in the participants of this study. To confirm a clinical diagnosis of choroideremia and enable access to future CHM gene therapy, identification of a likely pathogenic or pathogenic variant in the CHM gene is required.
Conversely, cone dystrophy (COD) is more clinically and genetically heterogeneous and is caused by variants in 40 different genes affecting approximately 1 in 40,000 people (RetNet, https://RetNet.org/ accessed on 25 April 2024) [6,7]. The participant in this study presented with progressive COD and next-generation sequencing (NGS) identified a potential splice-altering variant in CACNA1F. Likely pathogenic and pathogenic variants in this gene can cause progressive cone-rod dystrophy (CRD) (OMIM #300476), incomplete congenital stationary night Blindness (iCSNB) (OMIM #300071) and Åland island eye disease (AIED) (OMIM #300600) highlighting the clinical variability in IRDs [8,9,10]. The CACNA1F gene encodes a calcium channel α-1 subunit protein that is essential for neurotransmitter release in the phototransduction pathway [11]. Symptoms of COD include reduced visual acuity, photophobia, constriction of visual fields and colour vision defects. People with variants in CACNA1F may also present with nyctalopia, nystagmus, myopia, astigmatism, and a characteristic negative electroretinogram (ERG) commonly observed in CACNA1F-related iCSNB [12,13,14]. However, it was suggested that CACNA1F-related CRD is more similar to other X-linked CRDs than it is to iCSNB [8].
Splicing is an intricate process that involves converting precursor-mRNA into mature mRNA. When splicing is perturbed, aberrant transcripts may be translated into functional or partially functional proteins or more often, they may be degraded by nonsense-mediated decay (NMD). Approximately 10–15% of genetic diseases are attributable to variants that impair mRNA splicing by disrupting canonical, noncanonical, and auxiliary splice site sequence motifs [15,16]. Moreover, most non-canonical splice site variants are classified as variants of uncertain significance (VUS) due to their unknown splicing consequences and may explain a proportion of the genetically undiagnosed IRD cases. In silico tools are used to predict the splice-altering potential of variants with several studies reporting that SpliceAI outperforms many previously utilized tools [17,18,19]. However, to undertake a comprehensive screen for candidate splice-altering variants, a consensus approach using several tools may be optimal [19].
Variants are currently classified into five categories ranging from benign, likely benign, VUS, likely pathogenic and pathogenic according to guidelines developed by the American College of Medical Genetics and the Association for Molecular Pathology (ACMG/AMP) to establish the role of variants in an array of genetic conditions [20]. Updates and recommendations based on ACMG/AMP guidelines have been published by the ClinGen Sequence Variant Interpretation (SVI) working group to aid the variant interpretation process [21,22,23]. This involves investigation of population data, computational and predictive data, and functional data amongst other sources of evidence to assess the pathogenicity of such variants. Notably, current variant classification guidelines cap SpliceAI predictions at ACMG PP3 level for supporting evidence and is applied to variants with a score of >0.2 spliceogenicity [24]. This level of evidence may be insufficient to upgrade a VUS and, therefore, it is essential to validate in silico splice-altering predictions via functional analysis. Additionally, unpredicted multimodal splice defects can also arise providing rationale for functional studies to better understand the consequences of variants and their role in disease. Therefore, functional analysis using patient-derived RNA or mini/midigene assays is essential to reclassify these VUS as likely pathogenic/pathogenic making them clinically actionable.
This study investigated two novel variants in the X-linked genes CHM and CACNA1F, predicted from in silico analysis to be splice-altering, using midigene in vitro splice assays, a methodology that was used to interrogate candidate splice-altering variants causative of IRDs [25,26,27]. Our results confirmed that the CHM NM_000390.4:c.941-11T>G and CACNA1F NM_005183.4:c.2576+4_2576+5del variants resulted in aberrant splicing. Both variants were consequently upgraded from a tepid and cool VUS to likely pathogenic and hot VUS, respectively. Gathering evidence to upgrade variants is essential for patients to ensure accurate prognosis, management of the condition and eligibility for future trials and treatments.
2. Materials and Methods
2.1. Patient Recruitment
Probands and their families were recruited after clinical assessment at the Royal Victoria Eye and Ear Hospital (Dublin, Ireland) and the Royal Victoria Hospital (Belfast, Northern Ireland, UK). Following informed consent from all participants, routine clinical examination was conducted as previously described [28]. All clinical and laboratory testing was carried out in accordance with the tenets of the Declaration of Helsinki.
2.2. DNA Isolation and Target 5000 Next-Generation Sequencing
Patient DNA was isolated from peripheral blood using the DNA Blood Maxi Kit, Qiagen, Hilden, Germany. Sample preparation and Target Capture Next-Generation Sequencing (NGS) which encompasses the exons of 254 IRD-associated genes was performed for patients Pt-1 and Pt-7 as previously described [29]. Whole Exome Sequencing (WES) was performed for patient Pt-6. Identification of the causative variants was based on the genotype–phenotype correlation and variants were filtered as previously described to capture only those with a minor allele frequency of <1% in the Irish in-house and gnomAD (version 4) databases [30]. To confirm the candidate variants identified by NGS, the genomic regions of interest were amplified by polymerase chain reaction (PCR) using 5× FIREPol® Master Mix (Cat. no., 04-11-00S25; Solis BioDyne, Tartu, Estonia) according to the manufacturer’s protocol. All primers for PCR and Sanger sequencing were designed using Primer3plus software (version 4.1.0) and purchased from Merck, Darmstadt, Germany. Annealing temperatures were obtained using UCSC in silico PCR software (version V39x1) and all PCR primers are available in the Supplementary Materials. Direct Sanger sequencing of the PCR products was performed by Eurofins Genomics, Ebersberg, Germany and the chromatograms were manually analysed for the presence of each variant.
2.3. Cascade Analysis
For family A where additional family members were available, genomic regions of interest were PCR amplified using 5× FIREPol® Master Mix (Cat. no., 04-11-00S25; Solis BioDyne) according to the manufacturer’s protocols, Sanger sequenced (Eurofins Genomics, Ebersberg, Germany) and the chromatograms manually analysed for the presence or absence of each variant.
2.4. Variant Interpretation
Variants were classified before and after functional analysis according to ACMG/AMP criteria [20]. Variant interpretation recommendations and updates to support the use of ACMG/AMP guidelines published by the Association for Clinical Genomic Science (ACGS) and ClinGen Sequence Variant Interpretation (SVI) working group were also incorporated [21,22,23]. Sources of evidence included published population, computational, functional and segregation data. The population databases assessed were gnomAD (version 4) [30], LOVD (version 3.0) [31], ClinVar [32], Decipher (version 11.28) [33], UniProt (release 2024_06) [34] and dbSNP (build 156) [35]. The resulting candidate variants were then examined using in silico splice prediction tools which included SpliceSiteFinder-like [36], MaxEntScan [37], NNSPLICE (version 0.9) [38] and GENESplicer [39] accessed within Alamut Visual software version 2.1 (Interactive Biosoftware, Rouen, France; SOPHiA GENETICS, Lausanne, Switzerland) and SpliceAI (version 1.3.1) [40].
2.5. Midigene Generation
Variant and wildtype midigenes were generated by Long-Range PCR amplification of patient DNA using the Takara LA PCR™ Kit, Version 2.1 (Cat. no., RR013A; Takara Bio Inc., Saint-Germain-en-Laye, France) according to manufacturer’s instructions and primers with 5′ attB1 and attB2 tags to facilitate Gateway Cloning. Amplicons of 3.9 kb and 5.2 kb, respectively, were subsequently cloned into pDONOR221 vectors using Invitrogen™ Gateway Technology BP Clonase II Enzyme Mix (Cat. no., 11789021; Thermo Fisher Scientific Inc, Waltham, MA, USA) and transformed into DH10B™ competent E. coli (Cat. no., EC0113; Thermo Fisher Scientific Inc, Waltham, MA, USA). Plasmids were subject to diagnostic restriction digests using NheI and EcoRV and genotypes validated by Sanger sequencing. A total of 150 ng of each pDONOR221 construct was subsequently cloned into the destination vector pCI-NEO-RHO (Sangermano et al., 2016) using the Gateway LR Clonase II enzyme mix (Cat. no., 11791043; Thermo Fisher Scientific Inc., Waltham, MA, USA) [41]. Expression vectors were subject to digestion by XbaI and NheI (CHM midigene) and XhoI and NotI (CACNA1F midigene) to validate construct size and orientation and midigene inserts were Sanger sequenced.
2.6. Midigene Expression Analysis
HEK293 cells were maintained in prewarmed (37 °C) Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 1% L-Glutamine and 1% Sodium pyruvate in T75 flasks at 5% CO2. Cells were seeded in six well plates at a density of 5 × 105 cells/well and transfected once 70% confluent with variant and wildtype midigene constructs using FuGENE HD transfection reagent (Cat. no., 32042; Active Motif, Waterloo Belgium) according to manufacturer’s instructions. At 48 h post-transfection, mRNA was isolated using the RNeasy RNA isolation kit (Cat. no., 74104; QIAGEN, Hilden, Germany).
2.7. RT-PCR
cDNA was generated using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) and subsequent RT-PCR performed using AmpliTaq Gold 360 DNA Polymerase (Cat. no., 4398813; Thermo Fisher Scientific Inc., Waltham, MA, USA) according to the manufacturer’s protocol. Equal quantities of RT-PCR products were analysed by 2–4% agarose gel electrophoresis, visualised via a miniBIS Pro Bio-imaging system, purified using the GeneJET PCR Purification Kit (Cat. no., K0701; Thermo Fisher Scientific Inc., Waltham, MA, USA) and Sanger sequenced. For each assay, three independent experiments were performed to ensure reproducibility. RHO exon 5 and 309 bp of exon 4 of the β-actin housekeeping gene were amplified by RT-PCR to assess transfection efficiency and loading of equal amounts of PCR product. Primers used are available in Supplementary Materials.
2.8. AlphaFold Protein Modelling and InterPro Domains
The impact of splice-altering variants on protein structure was modelled using AlphaFold 3 via the AlphaFold server [42]. Hydrogen bonds and interresidue clashes in a 5Å radius were analysed using ChimeraX (version 1.6). To predict if the frameshift transcripts were likely to undergo nonsense-mediated decay, NMDescPredictor was used [43]. The variant and wildtype proteins were analysed using InterPro (version 102.0) to detect domains [44].
3. Results
In this study, three male probands underwent NGS to identify the variant underpinning their IRD. Two VUS were detected, CHM NM_000390.4:c.941-11T>G, and CACNA1F NM_005183.4:c.2576+4_2576+5del (Figure 1). Both VUS were intronic, absent from population databases and flagged for further investigation due to their predicted effect on splicing. Therefore, in vitro, midigene splice assays were performed to investigate potential splicing defects and confirm their likely association with disease.
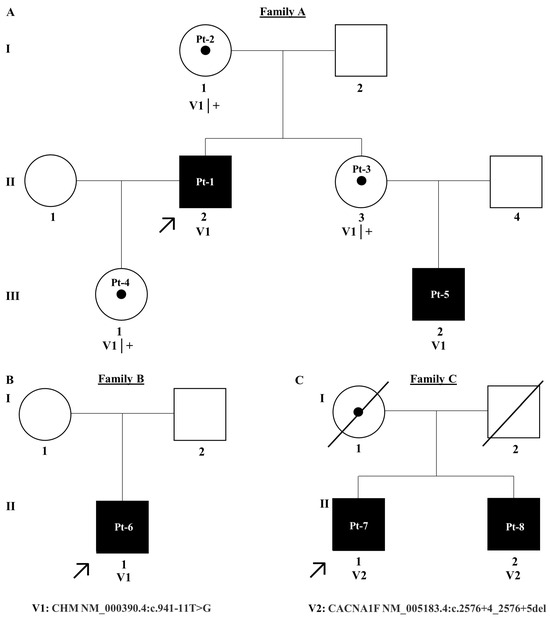
Figure 1.
Pedigree trees for families (A–C). A = family A, B = family B and C = Family C. Affected individuals are shaded black and unaffected individuals are unshaded. Patient IDs are written within circles for females and squares for males. Probands are denoted using a black arrow. Each generation in the pedigree is denoted by I–III.
3.1. CHM NM_000390.4:c.941-11T>G
Employing NGS a non-canonical splice site variant CHM NM_000390.4:c.941-11T>G in intron 7 of the gene, 11 base pairs upstream of the canonical splice acceptor site, was identified in families A and B. Pt-1 developed symptoms of choroideremia at age 11 experiencing night vision issues and photophobia, which further progressed as outlined in Table 1. The proband’s sister and daughter, Pt-3 and Pt-4, were identified as carriers of the c.941-11T>G variant. Despite being asymptomatic, fundus imaging for Pt-3 and Pt-4 shows patchy marked depigmentation and RPE disturbance which is not uncommon for female carriers possibly due to skewed X-chromosome inactivation (Figure 2). The proband’s nephew, Pt-5, developed symptoms by age 6 and was also confirmed to have the c.941-11T>G variant. Pt-6, assumed to be unrelated to family A, was also hemizygous for the c.941-11T>G variant and is similarly affected by choroideremia. Of note, c.941-11T>G was the only plausible candidate variant identified in CHM in families A and B who have classical choroideremia. Due to the specificity of this gene as causative of choroideremia, PP4 from ACMG guidelines could be applied at supporting strength (Table 2).

Table 1.
Clinical information for Pt-1, Pt-4 and Pt-5-Pt-7.
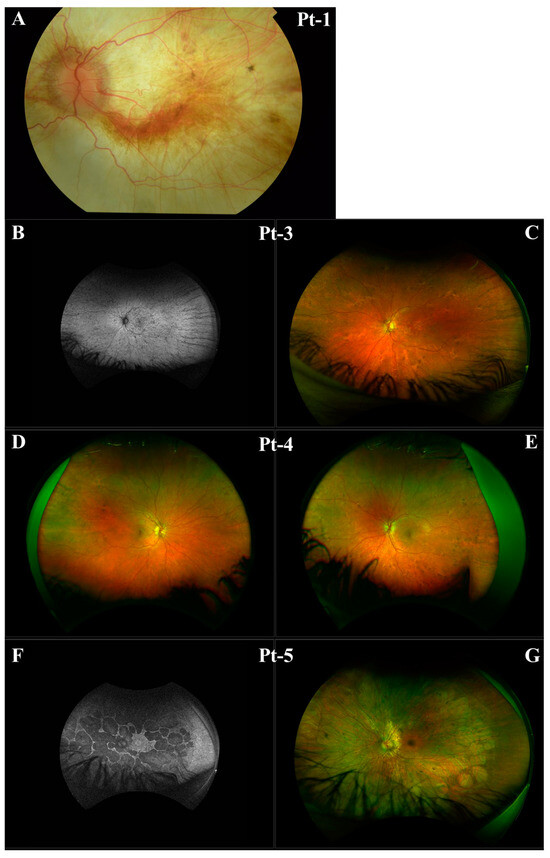
Figure 2.
Clinical imaging for Pt-1, Pt-3, Pt-4, and Pt-5 from Family A. (A) Left eye fundus of Pt-1. (B) Left eye fundus autofluorescence of Pt-3. (C) Left eye fundus of Pt-3. (D) Right eye fundus of Pt-4. (E) Left eye fundus of Pt-4. (F) Left eye fundus autofluorescence of Pt-5. (G) Left eye fundus of Pt-5.

Table 2.
Classification of variants according to ACGM/AMP and ClinGen SVI guidelines. For a detailed overview of the variant interpretation, see Supplementary Table S2.
CHM NM_000390.4:c.941-11T>G is absent from population databases and was flagged for investigation due to the high SpliceAI scores of 0.87 acceptor site loss at −11 bp, 0.45 donor site loss at −236 bp, and 0.34 acceptor site gain at −1 bp. Additionally, in silico tools such as SSFL, MES, NNSPLICE and GENESplicer predict that this variant is likely to perturb splicing as the acceptor site motif is destroyed.
Midigene functional analysis was conducted in HEK293 cells and transcripts of different sizes for the variant compared to wildtype identified via RT-PCR. In the variant sample (V1), a 456 bp product including exons 6–7 and RHO exon 5 was present. In contrast, a 682 bp wildtype product including exons 6–8 was present as expected. No residual wildtype transcript was present in the variant sample. Sanger sequencing of purified RT-PCR products confirmed that this variant results in the complete skipping of exon 8; exon skipping is commonly encountered when canonical splice acceptor sites are destroyed (Figure 3). Using HGVS nomenclature, the protein resulting from this variant is p.Tyr315CysfsTer18. The NMDescPredictor tool suggests that the transcript produced as a result of this variant is likely to undergo NMD.
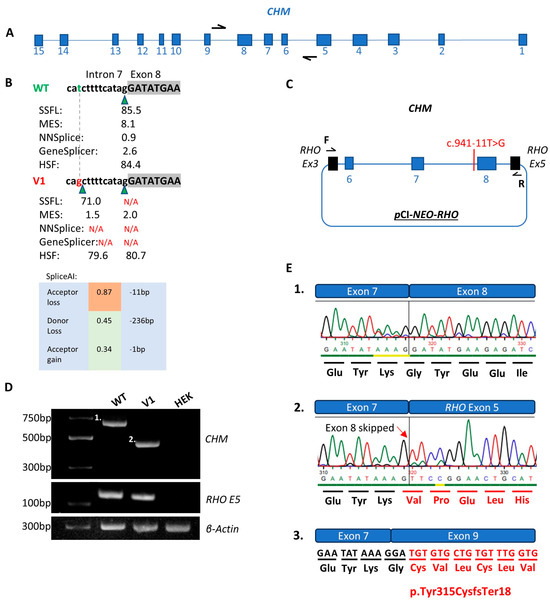
Figure 3.
Functional analysis of the CHM NM_000390.4:c.941-11T>G variant (V1) compared to wildtype (WT). (A) Illustrates the CHM gene in its antisense orientation with black arrows representing the primers used to amplify the region of interest. (B) Scores from in silico splice prediction tools for WT compared to V1. Green triangles represent splice acceptor sites. The green letter t represents WT and red g represents the c.941-11T>G variant. (C) Schematic of the expression clone including exons 6–8 of the CHM gene transfected into HEK293 cells. (D) Agarose gel illustrating the WT (band 1 of 682bp), V1 (band 2 of 456bp), RHO exon 5 control for WT, V1 and HEK cell only control and β-actin control for WT, V1 and HEK cell only control. (E.1) Sanger sequence chromatogram from the WT purified gel product in part D band 1. (E.2). V1 Sanger sequence chromatogram from the V1 purified gel product in part D band 2 with the altered amino acid sequence in red text and red arrow signifying the point at which the nucleotide sequence is altered. (E.3). Illustration of the protein product that would result from skipping of exon 8 denoted by the altered amino acid sequence in red text.
AlphaFold protein models were generated for wildtype and truncated versions of CHM (Figure 4). Although the number of hydrogen bonds remains stable at residue 315, there are obvious gross structural changes to the CHM protein and as described, it is highly likely that the transcript is degraded by NMD. Additionally, InterPro identified the RAE1/2 domain I and FAD/NAP(P)-binding domain in the wildtype amino acid sequence but could not identify any domains in the truncated protein p.Tyr315CysTer18 produced as a result of the c.941-11T>G variant (Figure 4). With this evidence, the c.941-11T>G variant was reclassified as likely pathogenic scoring 6 points (Table 2).
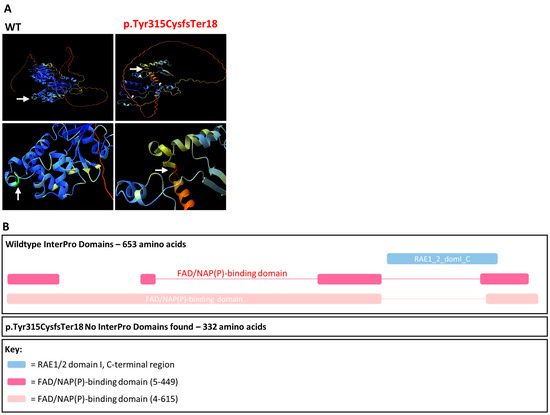
Figure 4.
AlphaFold protein models and InterPro domains. (A) AlphaFold protein folding predictions for wildtype (WT) compared to the amino acid sequence produced as a result of the CHM c.941-11T>G, p.Tyr315CysfsTer18 variant. The Tyr315 residue is green and Cys315 residue is red. Hydrogen bonds are denoted by the orange dashed line. (B) InterPro domains for wildtype compared to the amino acid sequence produced as a result of the CHM c.941-11T>G, p.Tyr315CysfsTer18 variant.
3.2. CACNA1F NM_005183.4:c.2576+4_2576+5del
Pt-7 presented in his early 20s with nystagmus and glare and was subsequently diagnosed with cone dystrophy (Table 1). A c.2543+4_2543+5delAG variant in intron 20 of the CACNA1F gene, four base pairs upstream of the canonical splice donor site, was identified by NGS. This variant is absent from population databases and was flagged for investigation due to the high SpliceAI scores for a 0.79 donor site loss at 6 bp and 0.89 for a donor site gain at −17 bp. Additionally, in silico tools such as SSFL, MES, NNSPLICE and GENESplicer predict that this variant is likely to perturb splicing as the splice donor site motif is destroyed. Midigene functional analysis confirmed that this variant, c.2543+4_2543+5delAG, results in a multimodal splice defect (Figure 5). In the wildtype, a product of 418 bp was evident as expected. In contrast, in the variant (V2), band 2 corresponded to a product of 439 bp due to a 21 nucleotide insertion, as predicted by SpliceAI and band 3 was a product of 365 bp due to a 53 nucleotide deletion in exon 20. Using HGVS nomenclature, the protein consequences could be defined as (p.P ro859_Leu860insCysAlaGlySerGlyArgGly) and (p.Val842AlafsTer31). Although the latter is predicted to undergo NMD, the resulting mature mRNA harbouring the additional seven amino acids would evade NMD. Notably, there was no residual wildtype transcript in the variant sample.
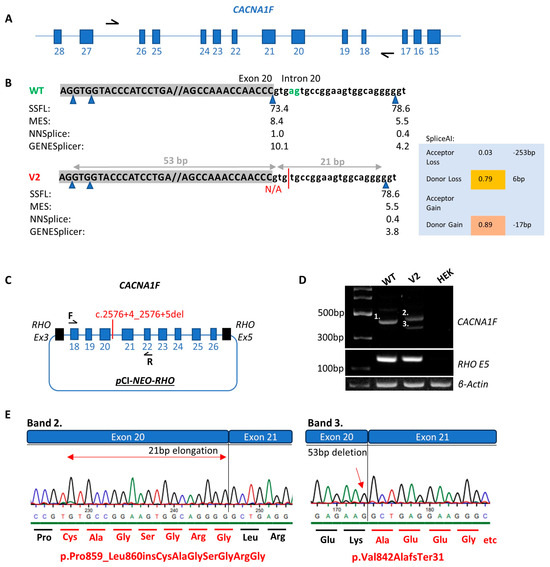
Figure 5.
Functional analysis of the CACNA1F c.2576+4_2576+5del variant (V2) compared to wildtype (WT). (A) Illustrates the CACNA1F gene in its antisense orientation with black arrows representing the primers used to amplify the region of interest. (B) Scores from in silico splice prediction tools for WT compared to V2. Blue triangles represent splice donor sites. The green letters ag represent the WT nucleotide sequence and the red line represents the V2 nucleotide sequence as a result of the c.2576+4_2576+5del variant. (C) Schematic of the expression clone including exons 18–26 of the CACNA1F gene which was transfected into HEK293 cells. (D) Agarose gel illustrating the WT (band 1 of 418 bp), V2 (band 2 of 439 bp), V2 (band 3 of 365 bp), RHO exon 5 control for WT, V2 and HEK cell only control and β-actin control for WT, V2 and HEK cell only control. (E) Sanger sequence chromatograms from the V2 band 2 and V2 band 3 purified gel products. The altered amino acid sequence as a result of the c.2576+4_2575+5del variant is illustrated by the red text. The red arrow signifies the point at which the nucleotide sequence is altered.
AlphaFold protein models were generated representing the wildtype and aberrant versions of CACNA1F that arose due to the c.2543+4_2543+5delAG variant (Figure 6). It is clear that the incorporation of the additional seven amino acids results in a dramatic change to the overall structure of the protein and it increases the level of uncertainty in terms of predicting protein folding. However, the seven amino acids are incorporated into the cytoplasmic topological domain and are not predicted to affect the transmembrane domain or any other relevant domains when compared to the wildtype protein sequence according to UniProt and InterPro (Figure 6). Conversely, the truncated protein p.Val842AlafsTer31 results in the loss of the Isoleucine-glutamine domain, two voltage-dependent channels, two ion transporters, the L-type calcium channel, and the voltage-gated calcium channel subunit α domains. With this evidence, the c.2543+4_2543+5delAG variant was reclassified as a hot VUS scoring 5 points with the application of PVS1 at strong strength.
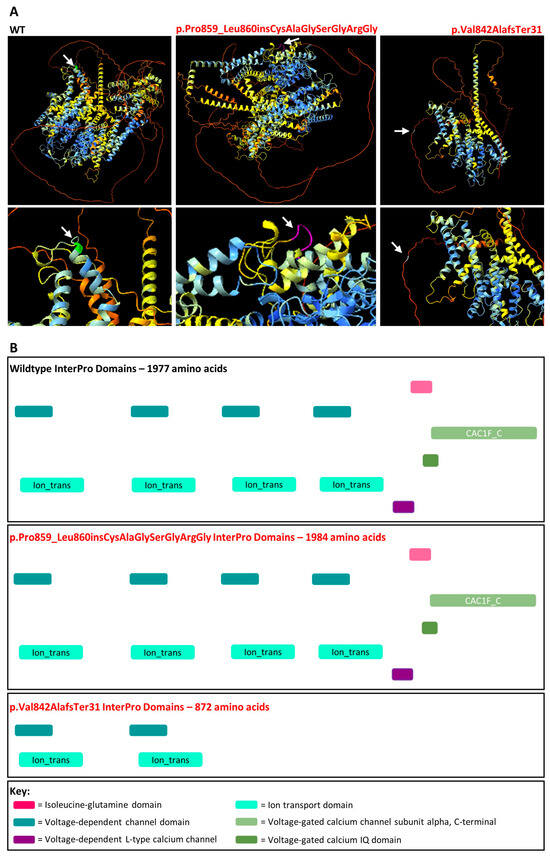
Figure 6.
AlphaFold protein models and InterPro domains. (A) AlphaFold protein folding predictions for wildtype (WT) compared to the amino acid sequence produced as a result of the CACNA1F c.2576+4_2576+5del,p.Pro859_Leu860insCysAlaGlySerGlyArgGly or p.Val842AlafsTer31 protein change. The Pro859 residue is coloured green, Pro859_Leu860insCysAlaGlySerGlyArgGly residues are coloured purple and Ala842 residue is coloured white. The white arrows represent the region of the protein altered as a result of the protein change. (B) InterPro domains for wildtype compared to the amino acid sequences produced as a result of the CACNA1F c.2576+4_2576+5del,p.Pro859_Leu860insCysAlaGlySerGlyArgGly/p.Val842AlafsTer31 protein change.
4. Discussion
In this study, two novel VUS, CHM c.941-11T>G and CACNA1F c.2576+4_2576+5del, were investigated to assess their splice-altering potential and association with the IRDs choroideremia and cone dystrophy. Functional analysis using in vitro midigene splice assays confirmed that both variants altered splicing and likely underpin the cause of disease for the probands in families A, B and C.
The CHM c.941-11T>G variant caused exon 8 skipping which was predicted by SpliceAI with a score of 0.87 acceptor site loss. This variant also creates a cryptic splice acceptor site with a SpliceAI score of 0.34. Using RT-PCR primers that bind in the expression clone backbone (Supplementary Table S1), the use of this cryptic splice acceptor site was not observed in the V1 sample. Notably, there was no residual wildtype transcript in the V1 sample (as evaluated using the exon 6–8 construct) due to the demise of the splice acceptor site illustrating the detrimental consequences of non-canonical splice site variants. The complete loss of CHM due to predicted NMD likely explains the severe phenotype for Pt-1. Furthermore, even if NMD was evaded, this truncated protein lacked the critical RAE1/2 domain I and FAD/NAP(P)-binding domains required for the REP-1 function [45,46]. Unfortunately, with the limited construct size of 12 kb, evaluation of the splicing of exon 7–9 was impossible because exons 8 and 9 are separated by >48 kb. For this reason, PVS1 as per latest ACMG guidelines was applied at strong strength which upgraded this variant to likely pathogenic. Therefore, obtaining patient mRNA from blood and repeating the RT-PCR would be optimal to further validate this defect to apply PVS1 at full strength.
The CACNA1F c.2576+4_2576+5del variant caused a multimodal splice defect as evident in Figure 5. Of note, band 3 in the V2 sample was not predicted by the in silico tools which underscores the importance of conducting functional analysis to fully understand the consequences of splice-altering variants. There was no wildtype transcript present in the V2 sample and the smaller band 3 resulting in the protein change p.Val842AlafsTer31 is predicted to undergo NMD (Figure 5 and Figure 6). However, the larger band 2 in the V2 sample incorporating seven additional amino acids, p.Pro848_Leu849insCysAlaGlySerGlyArgGly, is not predicted to undergo NMD. Additionally, there is no evidence that this change disrupts a critical domain in this protein as the same domains were identified by InterPro for the wildtype and p.Pro848_Leu849insCysAlaGlySerGlyArgGly amino acid sequences. Despite this, the seven additional amino acids at this position did disrupt the conformation of the folded protein compared to wildtype (Figure 6). Furthermore, a missense variant in this region, CACNA1F NM_005183.3:c.2579T>C,p.Leu860Pro, was classified as likely pathogenic on LOVD and observed in a patient with iCSNB [47]. Therefore, further investigation into the effect(s) of these seven additional amino acids in this region is required to explain the severe COD Pt-7 experiences. For this reason, PVS1 was applied at strong strength as the transcript resulting in p.Pro848_Leu849insCysAlaGlySerGlyArgGly may potentially be partially functional and is predicted to evade NMD.
It is important to note that the proportion of different transcripts expressed from a wildtype or mutant gene can vary between cell types due to the presence or absence of auxiliary splicing factors. Thus, it is possible that the truncated CACNA1F p.Val842AlafsTer31 may dominate the in-frame transcript in retinal cells which may possibly explain the progressive COD in Pt-7. However, similarly, the in-frame CACNA1F p.Pro848_Leu849insCysAlaGlySerGlyArgGly may have detrimental effects that have not as yet been fully elucidated. Due to the presence of universal splicing factors in this cell line and ease of transfection, HEK293 cells were used to elucidate the role of many splice-altering variants in IRDs [25,26,27]. A limitation of using HEK293 cells is that they do not exactly recapitulate the splicing process in retinal cells. For example, Albert et al., differentiated fibroblasts that were taken from patients with Stargardt Disease into photoreceptor precursor cells (PPCs) to investigate two deep intronic variants (ABCA4 c.4539+2001G>A and c.4539+2028C>T) which cause a 345 nucleotide pseudoexon insertion resulting in a frameshift (p.Arg1514Leufs*36) [48]. Of note, this defect was not apparent in experiments using HEK293 cells. It could, therefore, be advantageous to use patient-derived PPCs or retinal organoids if available. These retinal models would also be of use for assessing VUS in genes such as CHM with large introns that surpass the cloning size limit.
Interestingly, Pt-7 is myopic with congenital nystagmus while their sibling, Pt-8, was found to have a characteristic electronegative ERG. These features are typical of CACNA1F-related iCSNB, yet the clinical diagnosis in family C was COD. Therefore, it appears the clinical features in this family are not as distinct as previously described for CACNA1F-related CRD [8]. This may in part be due to the multimodal splice defect caused by the CACNA1F c.2576+4_2576+5del variant; however, the clinical overlap between iCSNB, CRD and AIED was reported previously with no known clear genotype–phenotype correlations [13,49,50]. Identifying modifiers and better understanding the genotype–phenotype correlations in this gene is essential to offer a clear prognosis to patients and their families.
In summary, conducting functional analysis and recruiting additional family members is of paramount importance to better understand the physiological consequences of variants and potentially upgrade VUS. Based on our investigations, the CHM c.941-11T>G variant was reclassified as likely pathogenic and the CACNA1F c.2576+4_2576+5del variant as a hot VUS. Additional family members will be required to conduct segregation analysis and/or investigation into the functionality of the p.Pro848_Leu849insCysAlaGlySerGlyArgGly protein to upgrade the latter variant to likely pathogenic. Reclassifying variants in this way is essential to ensure their clinical actionability with regard to family planning and precision medicines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16010025/s1. Table S1. Primers; Table S2. Variant Interpretation before and after functional analysis.
Author Contributions
Conceptualization, G.J.F., A.R.R. and L.K.F.; methodology, A.R.R., C.S., L.K.F. and E.K.; software, A.R.R. and L.K.F.; validation, G.J.F., L.K.F. and N.C.; investigation, A.R.R., C.S., L.K.F., R.L., E.M., A.D., E.K., L.W., C.K., G.S., J.T., D.J.K., S.M.-W., N.C., E.D. and P.F.K.; resources, G.J.F., D.J.K., G.S. and P.F.K.; writing, A.R.R., G.J.F., N.C. and L.K.F.; writing—review and editing, A.R.R., C.S., L.K.F., R.L., E.M., A.D., E.K., L.W., C.K., G.S., J.T., D.J.K., S.M.-W., N.C., E.D., P.F.K. and G.J.F.; visualization, A.R.R. and L.K.F.; supervision, G.J.F., L.K.F., N.C. and P.F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fighting Blindness Ireland (FB22FAR, FB16FAR), Fighting Blindness Ireland/Health Research Board of Ireland—Health Research Charities Ireland (MRCG-2016-14, HRCI-HRB-2020-007), Science Foundation Ireland (16/IA/4452 and 22/FFP-A/10544) and the Irish Research Council (GOIPG/2017/1631).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of The Royal Victoria Eye and Ear Hospital (RVEEH), Dublin, Ireland.
Informed Consent Statement
Informed consent was obtained from all patients prior to DNA analysis in this study.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the affected individuals and their families for participating in this study and our funding bodies.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Waldock, W.J.; Taylor, L.J.; Sperring, S.; Staurenghi, F.; Martinez-Fernandez de la Camara, C.; Whitfield, J.; Clouston, P.; Yusuf, I.H.; MacLaren, R.E. A hypomorphic variant of choroideremia is associated with a novel intronic mutation that leads to exon skipping. Ophthalmic Genet. 2024, 45, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Gocuk, S.A.; Lancaster, J.; Su, S.; Jolly, J.K.; Edwards, T.L.; Hickey, D.G.; Ritchie, M.E.; Blewitt, M.E.; Ayton, L.N.; Gouil, Q. Measuring X-Chromosome inactivation skew for X-linked diseases with adaptive nanopore sequencing. Genome Res. 2024, 34, 1954–1965. [Google Scholar] [CrossRef]
- Cremers, F.P.; Brunsmann, F.; van de Pol, T.J.; Pawlowitzki, I.H.; Paulsen, K.; Wieringa, B.; Ropers, H.H. Deletion of the DXS165 locus in patients with classical choroideremia. Clin. Genet. 1987, 32, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Seabra, M.C.; Brown, M.S.; Goldstein, J.L. Retinal degeneration in choroideremia: Deficiency of rab geranylgeranyl transferase. Science 1993, 259, 377–381. [Google Scholar] [CrossRef]
- Raeker, M.; Perera, N.D.; Karoukis, A.J.; Chen, L.; Feathers, K.L.; Ali, R.R.; Thompson, D.A.; Fahim, A.T. Reduced Retinal Pigment Epithelial Autophagy Due to Loss of Rab12 Prenylation in a Human iPSC-RPE Model of Choroideremia. Cells 2024, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P.; Rossiter, B.J.F.; Greenberg, J.; Christoffels, A.; Hide, W. Data Services and Software for Identifying Genes and Mutations Causing Retinal Degeneration. 1998. Available online: https://web.sph.uth.edu/RetNet/help.htm?csrt=9550839127900483002#q4 (accessed on 25 April 2024).
- Hamel, C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 2, 7. [Google Scholar] [CrossRef]
- Jalkanen, R.; Mäntyjärvi, M.; Tobias, R.; Isosomppi, J.; Sankila, E.M.; Alitalo, T.; Bech-Hansen, N.T. X linked cone-rod dystrophy, CORDX3, is caused by a mutation in the CACNA1F gene. J. Med. Genet. 2006, 43, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.M.; Nyakatura, G.; Apfelstedt-Sylla, E.; Hellebrand, H.; Lorenz, B.; Weber, B.H.; Wutz, K.; Gutwillinger, N.; Rüther, K.; Drescher, B.; et al. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, R.; Bech-Hansen, N.T.; Tobias, R.; Sankila, E.M.; Mäntyjärvi, M.; Forsius, H.; de la Chapelle, A.; Alitalo, T. A novel CACNA1F gene mutation causes Aland Island eye disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Bech-Hansen, N.T.; Naylor, M.J.; Maybaum, T.A.; Pearce, W.G.; Koop, B.; Fishman, G.A.; Mets, M.; Musarella, M.A.; Boycott, K.M. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat. Genet. 1998, 19, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Sallah, S.R.; Sergouniotis, P.I.; Barton, S.; Ramsden, S.; Taylor, R.L.; Safadi, A.; Kabir, M.; Ellingford, J.M.; Lench, N.; Lovell, S.C.; et al. Using an integrative machine learning approach utilising homology modelling to clinically interpret genetic variants: CACNA1F as an exemplar. Eur. J. Hum. Genet. 2020, 28, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Méjécase, C.; Ali, S.M.A.; Moosajee, M.; Kozak, I. A Novel Splice-Site Variant in CACNA1F Causes a Phenotype Synonymous with Åland Island Eye Disease and Incomplete Congenital Stationary Night Blindness. Genes 2021, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Mahroo, O.A. Negative electroretinograms: Genetic and acquired causes, diagnostic approaches and physiological insights. Eye 2021, 35, 2419–2437. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Shaw, K.; Phillips, A.; Cooper, D.N. The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum. Genet. 2014, 133, 1–9. [Google Scholar] [CrossRef]
- Krawczak, M.; Thomas, N.S.; Hundrieser, B.; Mort, M.; Wittig, M.; Hampe, J.; Cooper, D.N. Single base-pair substitutions in exon-intron junctions of human genes: Nature, distribution, and consequences for mRNA splicing. Hum. Mutat. 2007, 28, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Park, J.; Chae, H.; Kim, M. Comparison of In Silico Tools for Splice-Altering Variant Prediction Using Established Spliceogenic Variants: An End-User’s Point of View. Int. J. Genom. 2022, 2022, 5265686. [Google Scholar] [CrossRef]
- Wai, H.A.; Lord, J.; Lyon, M.; Gunning, A.; Kelly, H.; Cibin, P.; Seaby, E.G.; Spiers-Fitzgerald, K.; Lye, J.; Ellard, S.; et al. Blood RNA analysis can increase clinical diagnostic rate and resolve variants of uncertain significance. Genet. Med. 2020, 22, 1005–1014. [Google Scholar] [CrossRef]
- Rowlands, C.; Thomas, H.B.; Lord, J.; Wai, H.A.; Arno, G.; Beaman, G.; Sergouniotis, P.; Gomes-Silva, B.; Campbell, C.; Gossan, N.; et al. Comparison of in silico strategies to prioritize rare genomic variants impacting RNA splicing for the diagnosis of genomic disorders. Sci. Rep. 2021, 11, 20607. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Ellard, S.; Baple, E.; Berry, I.; Forrester, N.; Turnbull, C.; Owens, M.; Eccles, D.; Abbs, S.; Scott, R.; Deans, Z.; et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020; Association for Clinical Genomic Science: Edinburgh, UK, 2020. [Google Scholar]
- Durkie, M.; Cassify, E.-J.; Berry, I.; Owens, M.; Turnbull, C.; Scott, R.H.; Taylor, R.W.; Deans, Z.C.; Ellard, S.; Baple, E.L.; et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2024. Available online: https://www.acgs.uk.com/quality/best-practice-guidelines/ (accessed on 25 April 2024).
- Rehm, H.L.; Berg, J.S.; Brooks, L.D.; Bustamante, C.D.; Evans, J.P.; Landrum, M.J.; Ledbetter, D.H.; Maglott, D.R.; Martin, C.L.; Nussbaum, R.L.; et al. ClinGen—The Clinical Genome Resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef]
- Walker, L.C.; Hoya, M.; Wiggins, G.A.R.; Lindy, A.; Vincent, L.M.; Parsons, M.T.; Canson, D.M.; Bis-Brewer, D.; Cass, A.; Tchourbanov, A.; et al. Using the ACMG/AMP framework to capture evidence related to predicted and observed impact on splicing: Recommendations from the ClinGen SVI Splicing Subgroup. Am. J. Hum. Genet. 2023, 110, 1046–1067. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, Z.; Whelan, L.; Dockery, A.; Li, C.H.Z.; van den Born, L.I.; Hoyng, C.B.; Gilissen, C.; Corominas, J.; Rowlands, C.; Megaw, R.; et al. BBS1 branchpoint variant is associated with non-syndromic retinitis pigmentosa. J. Med. Genet. 2022, 59, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, Z.; Whelan, L.; Ben-Yosef, T.; Dockery, A.; Corradi, Z.; Gilissen, C.; Haer-Wigman, L.; Corominas, J.; Astuti, G.D.N.; de Rooij, L.; et al. Whole genome sequencing and in vitro splice assays reveal genetic causes for inherited retinal diseases. NPJ Genom. Med. 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Weisschuh, N.; Buena-Atienza, E.; Wissinger, B. Splicing mutations in inherited retinal diseases. Prog. Retin. Eye Res. 2021, 80, 100874. [Google Scholar] [CrossRef]
- Stephenson, K.A.J.; Zhu, J.; Wynne, N.; Dockery, A.; Cairns, R.M.; Duignan, E.; Whelan, L.; Malone, C.P.; Dempsey, H.; Collins, K.; et al. Target 5000: A standardized all-Ireland pathway for the diagnosis and management of inherited retinal degenerations. Orphanet J. Rare Dis. 2021, 16, 200. [Google Scholar] [CrossRef]
- Dockery, A.; Stephenson, K.; Keegan, D.; Wynne, N.; Silvestri, G.; Humphries, P.; Kenna, P.F.; Carrigan, M.; Farrar, G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes 2017, 8, 304. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Fokkema, I.; Kroon, M.; López Hernández, J.A.; Asscheman, D.; Lugtenburg, I.; Hoogenboom, J.; den Dunnen, J.T. The LOVD3 platform: Efficient genome-wide sharing of genetic variants. Eur. J. Hum. Genet. 2021, 29, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Riley, G.R.; Jang, W.; Rubinstein, W.S.; Church, D.M.; Maglott, D.R. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014, 42, D980–D985. [Google Scholar] [CrossRef] [PubMed]
- Firth, H.V.; Richards, S.M.; Bevan, A.P.; Clayton, S.; Corpas, M.; Rajan, D.; Van Vooren, S.; Moreau, Y.; Pettett, R.M.; Carter, N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009, 84, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Uniprot Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.B.; Senapathy, P. RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987, 15, 7155–7174. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.; Burge, C.B. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J. Comput. Biol. 2004, 11, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved splice site detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Lin, X.; Salzberg, S.L. GeneSplicer: A new computational method for splice site prediction. Nucleic Acids Res. 2001, 29, 1185–1190. [Google Scholar] [CrossRef]
- de Sainte Agathe, J.M.; Filser, M.; Isidor, B.; Besnard, T.; Gueguen, P.; Perrin, A.; Van Goethem, C.; Verebi, C.; Masingue, M.; Rendu, J.; et al. SpliceAI-visual: A free online tool to improve SpliceAI splicing variant interpretation. Hum. Genom. 2023, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, R.; Bax, N.M.; Bauwens, M.; van den Born, L.I.; De Baere, E.; Garanto, A.; Collin, R.W.; Goercharn-Ramlal, A.S.; den Engelsman-van Dijk, A.H.; Rohrschneider, K.; et al. Photoreceptor Progenitor mRNA Analysis Reveals Exon Skipping Resulting from the ABCA4 c.5461-10T→C Mutation in Stargardt Disease. Ophthalmology 2016, 123, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Coban-Akdemir, Z.; White, J.J.; Song, X.; Jhangiani, S.N.; Fatih, J.M.; Gambin, T.; Bayram, Y.; Chinn, I.K.; Karaca, E.; Punetha, J.; et al. Identifying Genes Whose Mutant Transcripts Cause Dominant Disease Traits by Potential Gain-of-Function Alleles. Am. J. Hum. Genet. 2018, 103, 171–187. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef]
- Pylypenko, O.; Rak, A.; Reents, R.; Niculae, A.; Sidorovitch, V.; Cioaca, M.D.; Bessolitsyna, E.; Thomä, N.H.; Waldmann, H.; Schlichting, I.; et al. Structure of Rab escort protein-1 in complex with Rab geranylgeranyltransferase. Mol. Cell 2003, 11, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Pylypenko, O.; Niculae, A.; Pyatkov, K.; Goody, R.S.; Alexandrov, K. Structure of the Rab7:REP-1 complex: Insights into the mechanism of Rab prenylation and choroideremia disease. Cell 2004, 117, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Wutz, K.; Sauer, C.; Zrenner, E.; Lorenz, B.; Alitalo, T.; Broghammer, M.; Hergersberg, M.; de la Chapelle, A.; Weber, B.H.; Wissinger, B.; et al. Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur. J. Hum. Genet. 2002, 10, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Garanto, A.; Sangermano, R.; Khan, M.; Bax, N.M.; Hoyng, C.B.; Zernant, J.; Lee, W.; Allikmets, R.; Collin, R.W.J.; et al. Identification and Rescue of Splice Defects Caused by Two Neighboring Deep-Intronic ABCA4 Mutations Underlying Stargardt Disease. Am. J. Hum. Genet. 2018, 102, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Phenotype and genotype of CACNA1F related diseases. Investig. Ophthalmol. Vis. Sci. 2024, 65, 5267. [Google Scholar]
- Vincent, A.; Wright, T.; Day, M.A.; Westall, C.A.; Héon, E. A novel p.Gly603Arg mutation in CACNA1F causes Åland island eye disease and incomplete congenital stationary night blindness phenotypes in a family. Mol. Vis. 2011, 17, 3262–3270. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).