Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders
Abstract
1. Introduction
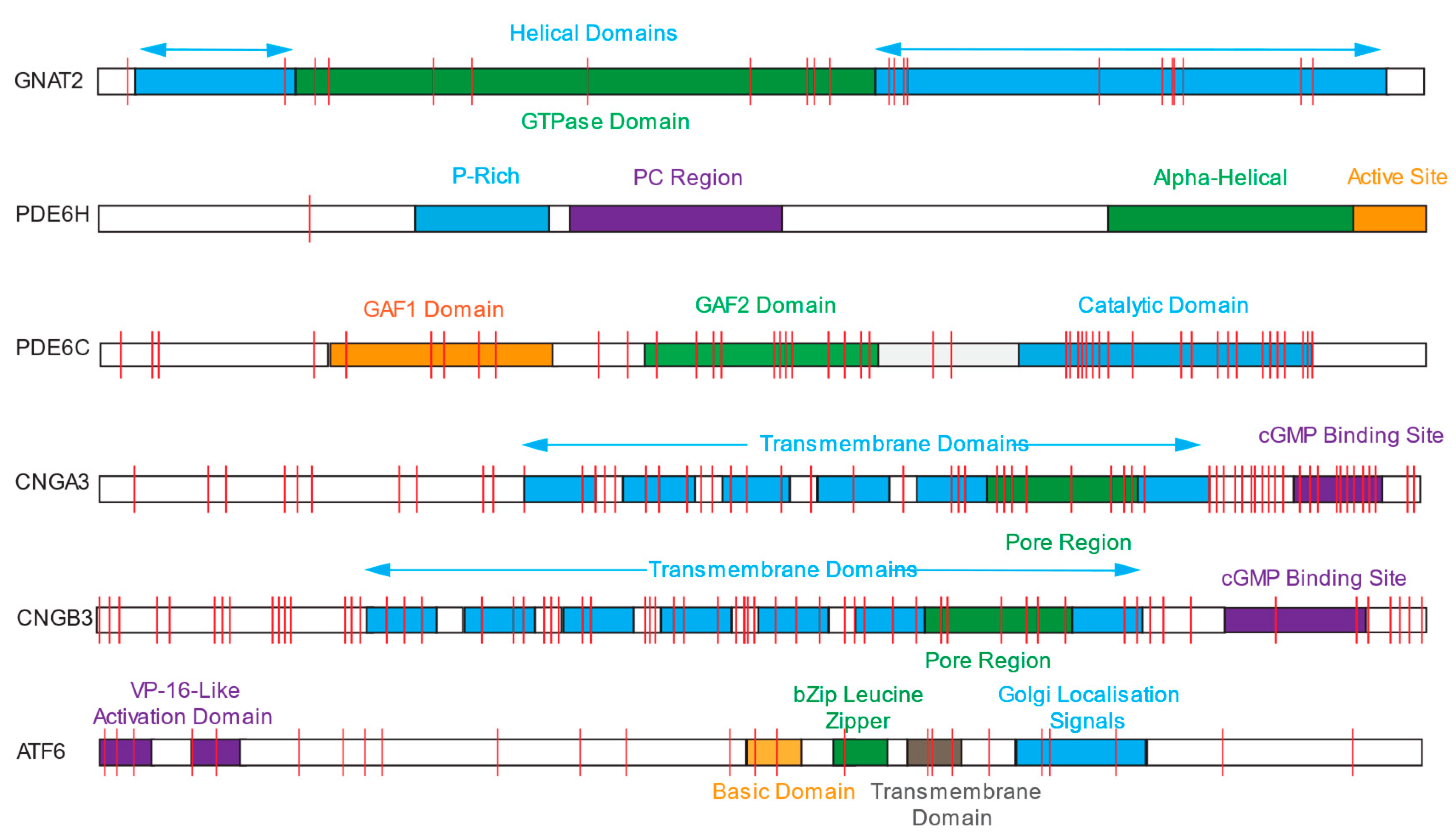
2. Achromatopsia
2.1. CNGB3 and CNGA
2.2. PDE6C and PDE6H
2.3. GNAT2
2.4. ATF6
2.5. Animal Models
3. Xp28-Associated Disorders
3.1. Blue-Cone Monochromatism
Animal Models
3.2. Bornholm Eye Disease
Animal Model
4. Cone and Cone–Rod Dystrophies
4.1. ABCA4
4.2. PRPH2
4.3. RPGR
4.4. Animal Model
5. Gene Therapies
| NCT Number | Study Title | Interventions | Phases | Start Date | Completion Date | Study Status |
|---|---|---|---|---|---|---|
| CNGA3 | ||||||
| NCT02610582 | Safety and Efficacy of rAAV.hCNGA3 Gene Therapy in Patients With CNGA3-linked Achromatopsia | rAAV.hCNGA3—gene therapy | PHASE1|PHASE2 | November 2015 | June 2027 | Active |
| NCT03278873 | Long-Term Follow-Up Gene Therapy Study for Achromatopsia CNGB3 and CNGA3 | AAV—CNGA3—gene therapy | PHASE1|PHASE2 | 29 June 2017 | 4 April 2024 | Completed—no results published |
| NCT03758404 | Gene Therapy for Achromatopsia (CNGA3) | AAV—CNGA3—gene therapy | PHASE1|PHASE2 | 12 August 2019 | 10 June 2021 | Completed—results published [98] |
| NCT02935517 | Safety and Efficacy Trial of AAV Gene Therapy in Patients With CNGA3 Achromatopsia (A Clarity Clinical Trial) | AGTC-402—gene therapy | PHASE1|PHASE2 | 3 August 2017 | August 2026 | Active |
| CNGB3 | ||||||
| NCT02599922 | Safety and Efficacy Trial of AAV Gene Therapy in Patients With CNGB3 Achromatopsia (A Clarity Clinical Trial) | rAAV2tYF-PR1.7-hCNGB3—gene therapy | PHASE1|PHASE2 | 11 April 2016 | July 2026 | Active |
| NCT03278873 | Long-Term Follow-Up Gene Therapy Study for Achromatopsia CNGB3 and CNGA3 | AAV—CNGB3—gene therapy | PHASE1|PHASE2 | 29 June 2017 | 4 April 2024 | Completed—no results published |
| NCT03001310 | Gene Therapy for Achromatopsia (CNGB3) | AAV—CNGB3—gene therapy | PHASE1|PHASE2 | 16 January 2017 | 25 October 2019 | Completed—results published [96] |
| RPGR | ||||||
| NCT04794101 | Follow-up Gene Therapy Trial for the Treatment of X-linked Retinitis Pigmentosa Associated With Variants in the RPGR Gene | AAV5-hRKp.RPGR—gene therapy | PHASE3 | 4 December 2020 | 19 December 2029 | Active |
| NCT03316560 | Safety and Efficacy of rAAV2tYF-GRK1-RPGR in Subjects With X-linked Retinitis Pigmentosa Caused by RPGR Mutations | rAAV2tYF-GRK1-RPGR—gene therapy | PHASE1|PHASE2 | 16 April 2018 | March 2025 | Active |
| NCT05874310 | Gene Therapy for Subjects With RPGR Mutation-associated X-linked Retinitis Pigmentosa | FT-002—gene therapy | EARLY_PHASE1 | 1 February 2023 | 1 November 2027 | Recruiting |
| NCT06275620 | A Study Comparing Two Doses of AGTC-501 in Male Participants With X-linked Retinitis Pigmentosa Caused by RPGR Mutations (DAWN) | AGTC-501—gene therapy | PHASE2 | 14 November 2023 | August 2029 | Enrolling |
| NCT04671433 | Gene Therapy Trial for the Treatment of X-linked Retinitis Pigmentosa Associated with Variants in the RPGR Gene | AAV5-hRKp.RPGR—gene therapy | PHASE3 | 4 December 2020 | 20 September 2024 | Active |
| NCT04850118 | A Clinical Trial Evaluating the Safety and Efficacy of a Single Subretinal Injection of AGTC-501 in Participants With XLRP | rAAV2tYF-GRK1-hRPGRco G | PHASE2|PHASE3 | 14 March 2024 | October 2029 | Recruiting |
| NCT04517149 | 4D-125 in Patients With X-Linked Retinitis Pigmentosa (XLRP) | 4D-125—gene therapy | PHASE1|PHASE2 | 9 June 2020 | May 2029 | Active |
| NCT03584165 | Long-term Safety and Efficacy Follow-up of BIIB111 for the Treatment of Choroideremia and BIIB112 for the Treatment of X-Linked Retinitis Pigmentosa | BIIB111/BIIB112—gene therapy | PHASE3 | 4 June 2018 | 4 June 2026 | Enrolling |
| NCT03252847 | Gene Therapy for X-linked Retinitis Pigmentosa (XLRP)—Retinitis Pigmentosa GTPase Regulator (RPGR) | AAV2/5-RPGR—gene therapy | PHASE1|PHASE2 | 14 July 2017 | 18 November 2021 | Completed—no results published |
| NCT05926583 | A Study of AAV5-hRKp.RPGR for the Treatment of Japanese Participants With X-linked Retinitis Pigmentosa | AAV5-hRKp.RPGR—gene therapy | PHASE3 | 12 September 2023 | 9 October 2029 | Recruiting |
| NCT03116113 | A Clinical Trial of Retinal Gene Therapy for X-linked Retinitis Pigmentosa Using BIIB112 | BIIB112—gene therapy | PHASE1|PHASE2 | 8 March 2017 | 18 November 2020 | Completed—Results Published [85] |
| NCT06333249 | A Study Comparing Two Doses of AGTC-501 in Male Subjects With X-linked Retinitis Pigmentosa Caused by RPGR Mutations (SKYLINE) | rAAV2tYF-GRK1-RPGR—gene therapy | PHASE2 | 13 April 2021 | February 2027 | Active |
| ABCA4 | ||||||
| NCT01367444 | Phase I/IIA Study of SAR422459 in Participants With Stargardt’s Macular Degeneration | SAR422459—EIAV-ABCA4 gene therapy | PHASE1|PHASE2 | 8 June 2011 | 16 August 2019 | Terminated due to adverse effects [94,95] |
| NCT06300476 | Safety and Efficacy of a Single Subretinal Injection of JWK006 Gene Therapy in Subjects With Stargardt Disease (STGD1) | JWK006—AAV-ABCA4 gene therapy | PHASE1|PHASE2 | 20 November 2023 | 30 December 2029 | Active |
Clinical Heterogeneity
6. Conclusions
7. Limitations of the Review
Funding
Conflicts of Interest
References
- Ben-Yosef, T. Inherited Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 13467. [Google Scholar] [CrossRef] [PubMed]
- RetNet—Retinal Information Network. Available online: https://web.sph.uth.edu/RetNet/ (accessed on 14 November 2023).
- Mustafi, D.; Engel, A.H.; Palczewski, K. Structure of Cone Photoreceptors. Prog. Retin. Eye Res. 2009, 28, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Megaw, R.; Moye, A.; Zhang, Z.; Newton, F.; McPhie, F.; Murphy, L.C.; McKie, L.; He, F.; Jungnickel, M.K.; von Kriegsheim, A.; et al. Ciliary Tip Actin Dynamics Regulate Photoreceptor Outer Segment Integrity. Nat. Commun. 2024, 15, 4316. [Google Scholar] [CrossRef]
- Zhang, Z.; Moye, A.R.; He, F.; Chen, M.; Agosto, M.A.; Wensel, T.G. Centriole and Transition Zone Structures in Photoreceptor Cilia Revealed by Cryo-Electron Tomography. Life Sci. Alliance 2024, 7, e202302409. [Google Scholar] [CrossRef] [PubMed]
- Spencer, W.J.; Lewis, T.R.; Pearring, J.N.; Arshavsky, V.Y. Photoreceptor Discs: Built like Ectosomes. Trends Cell Biol. 2020, 30, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Terakita, A. The Opsins. Genome Biol. 2005, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A. General Structure of the Retina. Acta Ophthalmol. 2015, 93. [Google Scholar] [CrossRef]
- Masland, R.H. The Fundamental Plan of the Retina. Nat. Neurosci. 2001, 4, 877–886. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Geisert, E.E.; Nickerson, J.M. Introduction to the Retina. Prog. Mol. Biol. Transl. Sci. 2015, 134, 383–396. [Google Scholar] [CrossRef]
- Sechrest, E.R.; Chmelik, K.; Tan, W.D.; Deng, W.-T. Blue Cone Monochromacy and Gene Therapy. Vis. Res. 2023, 208, 108221. [Google Scholar] [CrossRef]
- Patterson, E.J.; Langlo, C.S.; Georgiou, M.; Kalitzeos, A.; Pennesi, M.E.; Neitz, J.; Hardcastle, A.J.; Neitz, M.; Michaelides, M.; Carroll, J. Comparing Retinal Structure in Patients with Achromatopsia and Blue Cone Monochromacy Using OCT. Ophthalmol. Sci. 2021, 1, 100047. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Robson, A.G.; Fujinami, K.; de Guimarães, T.A.C.; Fujinami-Yokokawa, Y.; Daich Varela, M.; Pontikos, N.; Kalitzeos, A.; Mahroo, O.A.; Webster, A.R.; et al. Phenotyping and Genotyping Inherited Retinal Diseases: Molecular Genetics, Clinical and Imaging Features, and Therapeutics of Macular Dystrophies, Cone and Cone-Rod Dystrophies, Rod-Cone Dystrophies, Leber Congenital Amaurosis, and Cone Dysfunction Syndromes. Prog. Retin. Eye Res. 2024, 100, 101244. [Google Scholar] [CrossRef] [PubMed]
- Szikra, T.; Trenholm, S.; Drinnenberg, A.; Jüttner, J.; Raics, Z.; Farrow, K.; Biel, M.; Awatramani, G.; Clark, D.A.; Sahel, J.-A.; et al. Rods in Daylight Act as Relay Cells for Cone-Driven Horizontal Cell–Mediated Surround Inhibition. Nat. Neurosci. 2014, 17, 1728–1735. [Google Scholar] [CrossRef]
- Rosenberg, T.; Baumann, B.; Kohl, S.; Zrenner, E.; Jorgensen, A.L.; Wissinger, B. Variant Phenotypes of Incomplete Achromatopsia in Two Cousins with GNAT2 Gene Mutations. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4256–4262. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; de la Camara, C.M.F.; Birtel, J.; Rehman, S.; McClements, M.E.; Issa, P.C.; Lotery, A.J.; MacLaren, R.E. Impaired Glutamylation of RPGRORF15 Underlies the Cone-Dominated Phenotype Associated with Truncating Distal ORF15 Variants. Proc. Natl. Acad. Sci. USA 2022, 119, e2208707119. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.C.; Liew, G.; Quan, Y.-H.; Ermetal, B.; Ueyama, H.; Davidson, A.E.; Schwarz, N.; Kanuga, N.; Chana, R.; Maher, E.R.; et al. Three Different Cone Opsin Gene Array Mutational Mechanisms; Genotype-Phenotype Correlation and Functional Investigation of Cone Opsin Variants. Hum. Mutat. 2014, 35, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Johnson, S.; Simunovic, M.P.; Bradshaw, K.; Holder, G.; Mollon, J.D.; Moore, A.T.; Hunt, D.M. Blue Cone Monochromatism: A Phenotype and Genotype Assessment with Evidence of Progressive Loss of Cone Function in Older Individuals. Eye 2005, 19, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Thiadens, A.A.H.J.; Phan, T.M.L.; Zekveld-Vroon, R.C.; Leroy, B.P.; van den Born, L.I.; Hoyng, C.B.; Klaver, C.C.W.; Roosing, S.; Pott, J.-W.R.; van Schooneveld, M.J.; et al. Clinical Course, Genetic Etiology, and Visual Outcome in Cone and Cone–Rod Dystrophy. Ophthalmology 2012, 119, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive Cone and Cone-Rod Dystrophies: Clinical Features, Molecular Genetics and Prospects for Therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef]
- Pontikos, N.; Arno, G.; Jurkute, N.; Schiff, E.; Ba-Abbad, R.; Malka, S.; Gimenez, A.; Georgiou, M.; Wright, G.; Armengol, M.; et al. Genetic Basis of Inherited Retinal Disease in a Molecularly Characterized Cohort of More Than 3000 Families from the United Kingdom. Ophthalmology 2020, 127, 1384–1394. [Google Scholar] [CrossRef]
- Mayer, A.K.; Van Cauwenbergh, C.; Rother, C.; Baumann, B.; Reuter, P.; De Baere, E.; Wissinger, B.; Kohl, S. CNGB3 Mutation Spectrum Including Copy Number Variations in 552 Achromatopsia Patients. Hum. Mutat. 2017, 38, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.K.G.; Bertelsen, M.; Grønskov, K.; Kohl, S.; Kessel, L. Genetic and Clinical Characterization of Danish Achromatopsia Patients. Genes 2023, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Genead, M.A.; Fishman, G.A.; Rha, J.; Dubis, A.M.; Bonci, D.M.O.; Dubra, A.; Stone, E.M.; Neitz, M.; Carroll, J. Photoreceptor Structure and Function in Patients with Congenital Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7298. [Google Scholar] [CrossRef]
- Daich Varela, M.; Ullah, E.; Yousaf, S.; Brooks, B.P.; Hufnagel, R.B.; Huryn, L.A. PDE6C: Novel Mutations, Atypical Phenotype, and Differences Among Children and Adults. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1. [Google Scholar] [CrossRef] [PubMed]
- Ansar, M.; Santos-Cortez, R.L.P.; Saqib, M.A.N.; Zulfiqar, F.; Lee, K.; Ashraf, N.M.; Ullah, E.; Wang, X.; Sajid, S.; Khan, F.S.; et al. Mutation of ATF6 Causes Autosomal Recessive Achromatopsia. Hum. Genet. 2015, 134, 941. [Google Scholar] [CrossRef]
- Mastey, R.R.; Georgiou, M.; Langlo, C.S.; Kalitzeos, A.; Patterson, E.J.; Kane, T.; Singh, N.; Vincent, A.; Moore, A.T.; Tsang, S.H.; et al. Characterization of Retinal Structure in ATF6-Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2631–2640. [Google Scholar] [CrossRef] [PubMed]
- Remmer, M.H.; Rastogi, N.; Ranka, M.P.; Ceisler, E.J. Achromatopsia. Curr. Opin. Ophthalmol. 2015, 26, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Solaki, M.; Baumann, B.; Reuter, P.; Andreasson, S.; Audo, I.; Ayuso, C.; Balousha, G.; Benedicenti, F.; Birch, D.; Bitoun, P.; et al. Comprehensive Variant Spectrum of the CNGA3 Gene in Patients Affected by Achromatopsia. Hum. Mutat. 2022, 43, 832–858. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Zobor, D.; Chiang, W.-C.; Weisschuh, N.; Staller, J.; Menendez, I.G.; Chang, S.; Beck, S.C.; Garrido, M.G.; Sothilingam, V.; et al. Mutations in the Unfolded Protein Response Regulator ATF6 Cause the Cone Dysfunction Disorder Achromatopsia. Nat. Genet. 2015, 47, 757–765. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A Genomic Mutational Constraint Map Using Variation in 76,156 Human Genomes. Nature 2023, 625, 92–100. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, Z.; Li, H.; Yang, J. Structure of the Human Cone Photoreceptor Cyclic Nucleotide-Gated Channel. Nat. Struct. Mol. Biol. 2022, 29, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Jägle, H.; Wissinger, B.; Zobor, D. Achromatopsia. In GeneReviews; University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- Kohl, S.; Varsanyi, B.; Antunes, G.A.; Baumann, B.; Hoyng, C.B.; Jägle, H.; Rosenberg, T.; Kellner, U.; Lorenz, B.; Salati, R.; et al. CNGB3 Mutations Account for 50% of All Cases with Autosomal Recessive Achromatopsia. Eur. J. Hum. Genet. 2005, 13, 302–308. [Google Scholar] [CrossRef] [PubMed]
- González-del Pozo, M.; Martín-Sánchez, M.; Bravo-Gil, N.; Méndez-Vidal, C.; Chimenea, Á.; Rodríguez-de la Rúa, E.; Borrego, S.; Antiñolo, G. Searching the Second Hit in Patients with Inherited Retinal Dystrophies and Monoallelic Variants in ABCA4, USH2A and CEP290 by Whole-Gene Targeted Sequencing. Sci. Rep. 2018, 8, 13312. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Robson, A.G.; Singh, N.; Pontikos, N.; Kane, T.; Hirji, N.; Ripamonti, C.; Rotsos, T.; Dubra, A.; Kalitzeos, A.; et al. Deep Phenotyping of PDE6C—Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5112. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Coppieters, F.; Meire, F.; Schaich, S.; Roosing, S.; Brennenstuhl, C.; Bolz, S.; van Genderen, M.M.; Riemslag, F.C.C.; Lukowski, R.; et al. A Nonsense Mutation in PDE6H Causes Autosomal-Recessive Incomplete Achromatopsia. Am. J. Hum. Genet. 2012, 91, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Brennenstuhl, C.; Tanimoto, N.; Burkard, M.; Wagner, R.; Bolz, S.; Trifunovic, D.; Kabagema-Bilan, C.; Paquet-Durand, F.; Beck, S.C.; Huber, G.; et al. Targeted Ablation of the Pde6h Gene in Mice Reveals Cross-Species Differences in Cone and Rod Phototransduction Protein Isoform Inventory. J. Biol. Chem. 2015, 290, 10242–10255. [Google Scholar] [CrossRef]
- Madeira, C.; Godinho, G.; Grangeia, A.; Falcão, M.; Silva, R.; Carneiro, Â.; Brandão, E.; Magalhães, A.; Falcão-Reis, F.; Estrela-Silva, S. Two Novel Disease-Causing Variants in the PDE6C Gene Underlying Achromatopsia. Case Rep. Ophthalmol. 2021, 12, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Singh, N.; Kane, T.; Robson, A.G.; Kalitzeos, A.; Hirji, N.; Webster, A.R.; Dubra, A.; Carroll, J.; Michaelides, M. Photoreceptor Structure in GNAT2—Associated Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 40. [Google Scholar] [CrossRef]
- Kroeger, H.; Grandjean, J.M.D.; Chiang, W.C.J.; Bindels, D.D.; Mastey, R.; Okalova, J.; Nguyen, A.; Powers, E.T.; Kelly, J.W.; Grimsey, N.J.; et al. ATF6 Is Essential for Human Cone Photoreceptor Development. Proc. Natl. Acad. Sci. USA 2021, 118, 10242–10255. [Google Scholar] [CrossRef]
- Biel, M.; Seeliger, M.; Pfeifer, A.; Kohler, K.; Gerstner, A.; Ludwig, A.; Jaissle, G.; Fauser, S.; Zrenner, E.; Hofmann, F. Selective Loss of Cone Function in Mice Lacking the Cyclic Nucleotide-Gated Channel CNG3. Proc. Natl. Acad. Sci. USA 1999, 96, 7553–7557. [Google Scholar] [CrossRef]
- Thapa, A.; Morris, L.; Xu, J.; Ma, H.; Michalakis, S.; Biel, M.; Ding, X.-Q. Endoplasmic Reticulum Stress-Associated Cone Photoreceptor Degeneration in Cyclic Nucleotide-Gated Channel Deficiency. J. Biol. Chem. 2012, 287, 18018–18029. [Google Scholar] [CrossRef] [PubMed]
- Ronning, K.E.; Allina, G.P.; Miller, E.B.; Zawadzki, R.J.; Pugh, E.N.; Herrmann, R.; Burns, M.E. Loss of Cone Function without Degeneration in a Novel Gnat2 Knock-out Mouse. Exp. Eye Res. 2018, 171, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal Degeneration Mutants in the Mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Grau, T.; Dangel, S.; Hurd, R.; Jurklies, B.; Sener, E.C.; Andreasson, S.; Dollfus, H.; Baumann, B.; Bolz, S.; et al. A Homologous Genetic Basis of the Murine Cpfl1 Mutant and Human Achromatopsia Linked to Mutations in the PDE6C Gene. Proc. Natl. Acad. Sci. USA 2009, 106, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Moshiri, A.; Chen, R.; Kim, S.; Harris, R.A.; Li, Y.; Raveendran, M.; Davis, S.; Liang, Q.; Pomerantz, O.; Wang, J.; et al. A Nonhuman Primate Model of Inherited Retinal Disease. J. Clin. Investig. 2019, 129, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Wissinger, B.; Baumann, B.; Buena-Atienza, E.; Ravesh, Z.; Cideciyan, A.V.; Stingl, K.; Audo, I.; Meunier, I.; Bocquet, B.; Traboulsi, E.I.; et al. The Landscape of Submicroscopic Structural Variants at the OPN1LW/OPN1MW Gene Cluster on Xq28 Underlying Blue Cone Monochromacy. Proc. Natl. Acad. Sci. USA 2022, 119, e2115538119. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.C.; Webb, T.R.; Kanuga, N.; Robson, A.G.; Holder, G.E.; Stockman, A.; Ripamonti, C.; Ebenezer, N.D.; Ogun, O.; Devery, S.; et al. X-Linked Cone Dystrophy Caused by Mutation of the Red and Green Cone Opsins. Am. J. Hum. Genet. 2010, 87, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Sumaroka, A.; Garafalo, A.V.; Cideciyan, A.V.; Charng, J.; Roman, A.J.; Choi, W.; Saxena, S.; Aksianiuk, V.; Kohl, S.; Wissinger, B.; et al. Blue Cone Monochromacy Caused by the C203R Missense Mutation or Large Deletion Mutations. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5762. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, A.L.; Duno, M.; Welinder, L.G. Blue Cone Monochromatism in a Female Due to Skewed X-Inactivation. Ophthalmic Genet. 2013, 34, 101–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, W.-T.; Du, W.; Zhu, P.; Li, J.; Xu, F.; Sun, J.; Gerstner, C.D.; Baehr, W.; Boye, S.L.; et al. Gene-Based Therapy in a Mouse Model of Blue Cone Monochromacy. Sci. Rep. 2017, 7, 6690. [Google Scholar] [CrossRef]
- Deng, W.-T.; Li, J.; Zhu, P.; Freedman, B.; Smith, W.C.; Baehr, W.; Hauswirth, W.W. Rescue of M-Cone Function in Aged Opn1mw −/− Mice, a Model for Late-Stage Blue Cone Monochromacy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3644. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Chmelik, K.; Sechrest, E.R.; Barbera, R.J.; Beahr, W.; Deng, W.-T. Gene Therapy Restores Vision to the All-Cone Nrl-/-/Opn1mw-/-/Opn1sw-/-Mouse Model of Blue Cone Monochromacy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 769. [Google Scholar]
- Haim, M.; Fledelius, H.C. Skarsholm X-linked Myopia in Danish Family. Acta Ophthalmol. 1988, 66, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited Retinal Diseases: Therapeutics, Clinical Trials and End Points—A Review. Clin. Exp. Ophthalmol. 2021, 49, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Johnson, S.; Bradshaw, K.; Holder, G.E.; Simunovic, M.P.; Mollon, J.D.; Moore, A.T.; Hunt, D.M. X-Linked Cone Dysfunction Syndrome with Myopia and Protanopia. Ophthalmology 2005, 112, 1448–1454. [Google Scholar] [CrossRef]
- Holmquist, D.; Epstein, D.; Olsson, M.; Wissinger, B.; Kohl, S.; Hengstler, J.; Tear-Fahnehjelm, K. Visual and Ocular Findings in a Family with X-Linked Cone Dysfunction and Protanopia. Ophthalmic Genet. 2021, 42, 570–576. [Google Scholar] [CrossRef]
- Neitz, M.; Neitz, J. Intermixing the OPN1LW and OPN1MW Genes Disrupts the Exonic Splicing Code Causing an Array of Vision Disorders. Genes 2021, 12, 1180. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, S.H.; Kuchenbecker, J.A.; Neitz, M.; Neitz, J. A Mouse Model of Cone Dystrophy Caused by a Toxic Opsin Variant. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4643. [Google Scholar]
- Greenwald, S.H.; Kuchenbecker, J.A.; Rowlan, J.S.; Neitz, J.; Neitz, M. Role of a Dual Splicing and Amino Acid Code in Myopia, Cone Dysfunction and Cone Dystrophy Associated with L/M Opsin Interchange Mutations. Transl. Vis. Sci. Technol. 2017, 6, 2. [Google Scholar] [CrossRef]
- Greenwald, S.H.; Kuchenbecker, J.A.; Roberson, D.K.; Neitz, M.; Neitz, J. S-Opsin Knockout Mice with the Endogenous M-Opsin Gene Replaced by an L-Opsin Variant. Vis. Neurosci. 2014, 31, 25–37. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Stone, E.; Latchney, L.; Williams, D.; Dubra, A.; Chung, M. Phenotypic Diversity in Autosomal-Dominant Cone-Rod Dystrophy Elucidated by Adaptive Optics Retinal Imaging. Br. J. Ophthalmol. 2018, 102, 136–141. [Google Scholar] [CrossRef]
- Creel, D.J. Electroretinograms. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 481–493. [Google Scholar]
- Han, J.H.; Rodenburg, K.; Hayman, T.; Calzetti, G.; Kaminska, K.; Quinodoz, M.; Marra, M.; Wallerich, S.; Allon, G.; Nagy, Z.Z.; et al. Loss-of-Function Variants in UBAP1L Cause Autosomal Recessive Retinal Degeneration. Genet. Med. 2024, 2024, 101106. [Google Scholar] [CrossRef]
- Zeitz, C.; Navarro, J.; Azizzadeh Pormehr, L.; Méjécase, C.; Neves, L.M.; Letellier, C.; Condroyer, C.; Albadri, S.; Amprou, A.; Antonio, A.; et al. Variants in UBAP1L Lead to Autosomal Recessive Rod-Cone and Cone-Rod Dystrophy. Genet. Med. 2024, 2024, 101081. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Liao, Y.; Chen, C.; Liao, C.; He, D.; Chen, J.; Ma, J.; Liu, Z.; Wu, Y. Conversion of All-Trans-Retinal into All-Trans-Retinal Dimer Reflects an Alternative Metabolic/Antidotal Pathway of All-Trans-Retinal in the Retina. J. Biol. Chem. 2018, 293, 14507–14519. [Google Scholar] [CrossRef] [PubMed]
- Al-Khuzaei, S.; Broadgate, S.; Foster, C.R.; Shah, M.; Yu, J.; Downes, S.M.; Halford, S. An Overview of the Genetics of ABCA4 Retinopathies, an Evolving Story. Genes 2021, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kasilian, M.; Mahroo, O.A.R.; Tanna, P.; Kalitzeos, A.; Robson, A.G.; Tsunoda, K.; Iwata, T.; Moore, A.T.; Fujinami, K.; et al. Early Patterns of Macular Degeneration in ABCA4-Associated Retinopathy. Ophthalmology 2018, 125, 735–746. [Google Scholar] [CrossRef]
- Maugeri, A.; Klevering, B.J.; Rohrschneider, K.; Blankenagel, A.; Brunner, H.G.; Deutman, A.F.; Hoyng, C.B.; Cremers, F.P.M. Mutations in the ABCA4 (ABCR) Gene Are the Major Cause of Autosomal Recessive Cone-Rod Dystrophy. Am. J. Hum. Genet. 2000, 67, 960–966. [Google Scholar] [CrossRef]
- Ducroq, D.; Rozet, J.-M.; Gerber, S.; Perrault, I.; Barbet, F.; Hanein, S.; Hakiki, S.; Dufier, J.-L.; Munnich, A.; Hamel, C.; et al. The ABCA4 Gene in Autosomal Recessive Cone-Rod Dystrophies. Am. J. Hum. Genet. 2002, 71, 1480–1482. [Google Scholar] [CrossRef]
- Bianco, L.; Arrigo, A.; Antropoli, A.; Saladino, A.; Spiga, I.; Patricelli, M.G.; Bandello, F.; Carrera, P.; Battaglia Parodi, M. PRPH2-Associated Retinopathy. Ophthalmol. Retin. 2023, 7, 450–461. [Google Scholar] [CrossRef]
- Chakraborty, D.; Strayve, D.G.; Makia, M.S.; Conley, S.M.; Kakahel, M.; Al-Ubaidi, M.R.; Naash, M.I. Novel Molecular Mechanisms for Prph2-associated Pattern Dystrophy. FASEB J. 2020, 34, 1211–1230. [Google Scholar] [CrossRef]
- Salinas, R.Y.; Pearring, J.N.; Ding, J.-D.; Spencer, W.J.; Hao, Y.; Arshavsky, V.Y. Photoreceptor Discs Form through Peripherin-Dependent Suppression of Ciliary Ectosome Release. J. Cell Biol. 2017, 216, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Gocho, K.; Akeo, K.; Itoh, N.; Kameya, S.; Hayashi, T.; Katagiri, S.; Gekka, T.; Ohkuma, Y.; Tsuneoka, H.; Takahashi, H. High-Resolution Adaptive Optics Retinal Image Analysis at Early Stage Central Areolar Choroidal Dystrophy with PRPH2 Mutation. Ophthalmic Surg Lasers Imaging Retin. 2016, 47, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Holder, G.E.; Bradshaw, K.; Hunt, D.M.; Moore, A.T. Cone–Rod Dystrophy, Intrafamilial Variability, and Incomplete Penetrance Associated with the R172W Mutation in the Peripherin/RDS Gene. Ophthalmology 2005, 112, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.P.; Hughbanks-Wheaton, D.K.; Birch, D.G.; Sullivan, L.S.; Conneely, K.N.; Bowne, S.J.; Stone, E.M.; Daiger, S.P. Autosomal Dominant Retinal Dystrophies Caused by a Founder Splice Site Mutation, c.828+3A>T, in PRPH2 and Protein Haplotypes in Trans as Modifiers. Investig. Ophthalmol. Vis. Sci. 2016, 57, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Beltran, W.A.; Hammond, P.; Acland, G.M.; Aguirre, G.D. A Frameshift Mutation in RPGR Exon ORF15 Causes Photoreceptor Degeneration and Inner Retina Remodeling in a Model of X-Linked Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Vössing, C.; Atigbire, P.; Eilers, J.; Markus, F.; Stieger, K.; Song, F.; Neidhardt, J. The Major Ciliary Isoforms of RPGR Build Different Interaction Complexes with INPP5E and RPGRIP1L. Int. J. Mol. Sci. 2021, 22, 3583. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef] [PubMed]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its Role in Photoreceptor Physiology, Human Disease, and Future Therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef]
- Megaw, R.; Abu-Arafeh, H.; Jungnickel, M.; Mellough, C.; Gurniak, C.; Witke, W.; Zhang, W.; Khanna, H.; Mill, P.; Dhillon, B.; et al. Gelsolin Dysfunction Causes Photoreceptor Loss in Induced Pluripotent Cell and Animal Retinitis Pigmentosa Models. Nat. Commun. 2017, 8, 271. [Google Scholar] [CrossRef]
- Talib, M.; Van Schooneveld, M.J.; Thiadens, A.A.; Fiocco, M.; Wijnholds, J.; Florijn, R.J.; Schalij-Delfos, N.E.; Van Genderen, M.M.; Putter, H.; Cremers, F.P.M.; et al. CLINICAL and GENETIC CHARACTERISTICS of MALE PATIENTS with RPGR-ASSOCIATED RETINAL DYSTROPHIES: A Long-Term Follow-up Study. Retina 2019, 39, 1186–1189. [Google Scholar] [CrossRef]
- Hadalin, V.; Buscarino, M.; Sajovic, J.; Meglič, A.; Jarc-Vidmar, M.; Hawlina, M.; Volk, M.; Fakin, A. Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy. Int. J. Mol. Sci. 2023, 24, 3840. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial Results from a First-in-Human Gene Therapy Trial on X-Linked Retinitis Pigmentosa Caused by Mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Park, J.H.; Gumerson, J.; Wu, Z.; Swaroop, A.; Qian, H.; Roll-Mecak, A.; Li, T. Loss of RPGR Glutamylation Underlies the Pathogenic Mechanism of Retinal Dystrophy Caused by TTLL5 Mutations. Proc. Natl. Acad. Sci. USA 2016, 113, E2925–E2934. [Google Scholar] [CrossRef] [PubMed]
- Brunner, S.; Skosyrski, S.; Kirschner-Schwabe, R.; Knobeloch, K.-P.; Neidhardt, J.; Feil, S.; Glaus, E.; Luhmann, U.F.O.; Rüther, K.; Berger, W. Cone versus Rod Disease in a Mutant Rpgr Mouse Caused by Different Genetic Backgrounds. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1106. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Q.; Nour, M.; Ritter, L.M.; Goldberg, A.F.X.; Fliesler, S.J.; Naash, M.I. The R172W Mutation in Peripherin/Rds Causes a Cone-Rod Dystophy in Transgenic Mice. Hum. Mol. Genet. 2004, 13, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Conley, S.M.; Zulliger, R.; Naash, M.I. The K153Del PRPH2 Mutation Differentially Impacts Photoreceptor Structure and Function. Hum. Mol. Genet. 2016, 25, 3500–3514. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S. ATP-Binding Cassette Transporter ABCA4: Molecular Properties and Role in Vision and Macular Degeneration. J. Bioenergy Biomembr. 2007, 39, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, T.; Golczak, M.; Palczewski, K. Retinopathy in Mice Induced by Disrupted All-Trans-Retinal Clearance. J. Biol. Chem. 2008, 283, 26684–26693. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Waheed, N.; Laich, Y.; Yang, P.; Fujinami-Yokokawa, Y.; Higgins, J.J.; Lu, J.T.; Curtiss, D.; Clary, C.; Michaelides, M. Stargardt Macular Dystrophy and Therapeutic Approaches. Br. J. Ophthalmol. 2023, 108, 495–505. [Google Scholar] [CrossRef]
- Wang, R.; McClard, C.K.; Laswell, S.; Mahmoudzadeh, R.; Salabati, M.; Ammar, M.; Vannavong, J.; Aziz, A.A.; Ewald, A.; Calvanese, A.V.; et al. Quantifying Burden of Intravitreal Injections: Questionnaire Assessment of Life Impact of Treatment by Intravitreal Injections (QUALITII). BMJ Open Ophthalmol. 2022, 7, e001188. [Google Scholar] [CrossRef]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Ségaut-Prévost, C.; et al. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients With ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1-5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Choi, D.; Erker, L.R.; Pennesi, M.E.; Yang, P.; Chegarnov, E.N.; Steinkamp, P.N.; Schlechter, C.L.; Dhaenens, C.-M.; Mohand-Said, S.; et al. Test–Retest Variability of Functional and Structural Parameters in Patients with Stargardt Disease Participating in the SAR422459 Gene Therapy Trial. Transl. Vis. Sci. Technol. 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-Year Results of Phase I Retinal Gene Therapy Trial for CNGA3-Mutated Achromatopsia: Results of a Non Randomised Controlled Trial. Br. J. Ophthalmol. 2022, 106, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, M.; Anderson, E.J.; Maimon-Mor, R.O.; Rider, A.; Greenwood, J.A.; Hirji, N.; Zaman, S.; Jones, P.R.; Schwarzkopf, D.S.; Rees, G.; et al. A Demonstration of Cone Function Plasticity after Gene Therapy in Achromatopsia. Brain 2022, 145, 3803–3815. [Google Scholar] [CrossRef]
- Michaelides, M.; Hirji, N.; Wong, S.C.; Besirli, C.G.; Zaman, S.; Kumaran, N.; Georgiadis, A.; Smith, A.J.; Ripamonti, C.; Gottlob, I.; et al. First-in-Human Gene Therapy Trial of AAV8-HCARp.HCNGB3 in Adults and Children with CNGB3-Associated Achromatopsia. Am. J. Ophthalmol. 2023, 253, 243–251. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 21 May 2024).
- Sun, D.; Sun, W.; Gao, S.-Q.; Lehrer, J.; Naderi, A.; Wei, C.; Lee, S.; Schilb, A.L.; Scheidt, J.; Hall, R.C.; et al. Effective Gene Therapy of Stargardt Disease with PEG-ECO/PGRK1-ABCA4-S/MAR Nanoparticles. Mol. Ther. Nucleic Acids 2022, 29, 823–835. [Google Scholar] [CrossRef]
- Li, R.; Jing, Q.; She, K.; Wang, Q.; Jin, X.; Zhao, Q.; Su, J.; Song, L.; Fu, J.; Wu, X.; et al. Split AAV8 Mediated ABCA4 Expression for Gene Therapy of Mouse Stargardt Disease (STGD1). Hum. Gene Ther. 2023, 34, 616–628. [Google Scholar] [CrossRef]
- Barroso-Gil, M.; Olinger, E.; Ramsbottom, S.A.; Molinari, E.; Miles, C.G.; Sayer, J.A. Update of Genetic Variants in CEP120 and CC2D2A —With an Emphasis on Genotype-phenotype Correlations, Tissue Specific Transcripts and Exploring Mutation Specific Exon Skipping Therapies. Mol. Genet. Genom. Med. 2021, 9, e1603. [Google Scholar] [CrossRef]
- Méjécase, C.; Mohand-Said, S.; Andrieux, C.; Hummel, A.; El Shamieh, S.; Antonio, A.; Boyard, F.; Condroyer, C.; Michiels, C.; Blanchard, S.; et al. CC2D2A Mutations Lead to Variable Phenotypes in a Family with Retinal Dystrophy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 573. [Google Scholar]
- Fadaie, Z.; Whelan, L.; Dockery, A.; Li, C.H.Z.; Van Den Born, L.I.; Hoyng, C.B.; Gilissen, C.; Corominas, J.; Rowlands, C.; Megaw, R.; et al. BBS1 Branchpoint Variant Is Associated with Non-Syndromic Retinitis Pigmentosa. J. Med. Genet. 2022, 59, 438–444. [Google Scholar] [CrossRef]
- Scheidecker, S.; Hull, S.; Perdomo, Y.; Studer, F.; Pelletier, V.; Muller, J.; Stoetzel, C.; Schaefer, E.; Defoort-Dhellemmes, S.; Drumare, I.; et al. Predominantly Cone-System Dysfunction as Rare Form of Retinal Degeneration in Patients with Molecularly Confirmed Bardet-Biedl Syndrome. Am. J. Ophthalmol. 2015, 160, 364–372. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brotherton, C.; Megaw, R. Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders. Genes 2024, 15, 727. https://doi.org/10.3390/genes15060727
Brotherton C, Megaw R. Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders. Genes. 2024; 15(6):727. https://doi.org/10.3390/genes15060727
Chicago/Turabian StyleBrotherton, Chloe, and Roly Megaw. 2024. "Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders" Genes 15, no. 6: 727. https://doi.org/10.3390/genes15060727
APA StyleBrotherton, C., & Megaw, R. (2024). Molecular Mechanisms Governing Sight Loss in Inherited Cone Disorders. Genes, 15(6), 727. https://doi.org/10.3390/genes15060727






