Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives
Abstract
1. Introduction
2. Critical Steps of Spermatogenesis
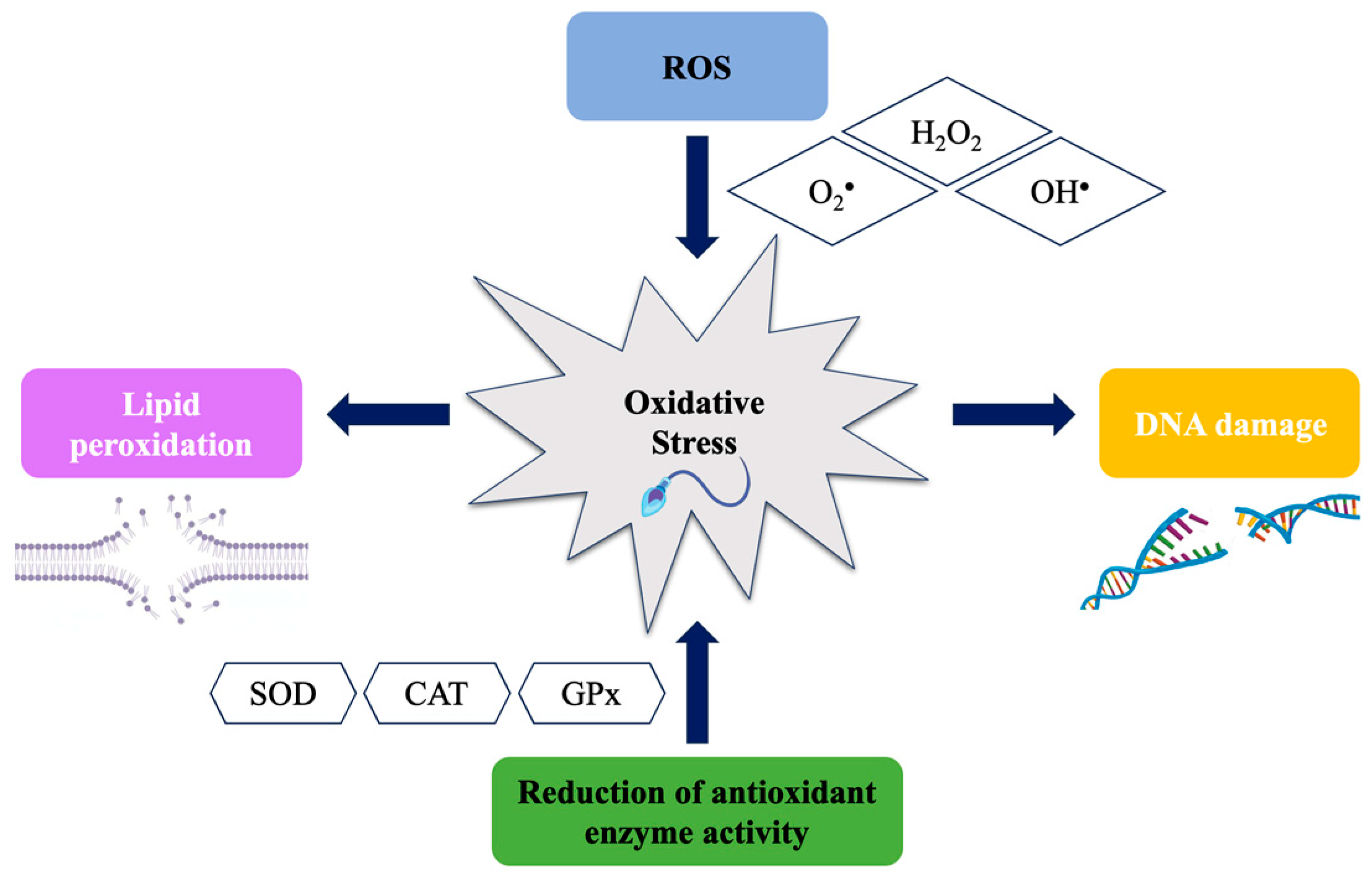
3. Male Oxidative Stress Infertility (MOSI) Biomarkers
4. Established Methods to Detect OS Biomarkers
5. New Oxidative Biomarkers in Male Infertility
5.1. NGS Polymorphisms
5.2. Differentially Expressed Genes (DEGs)
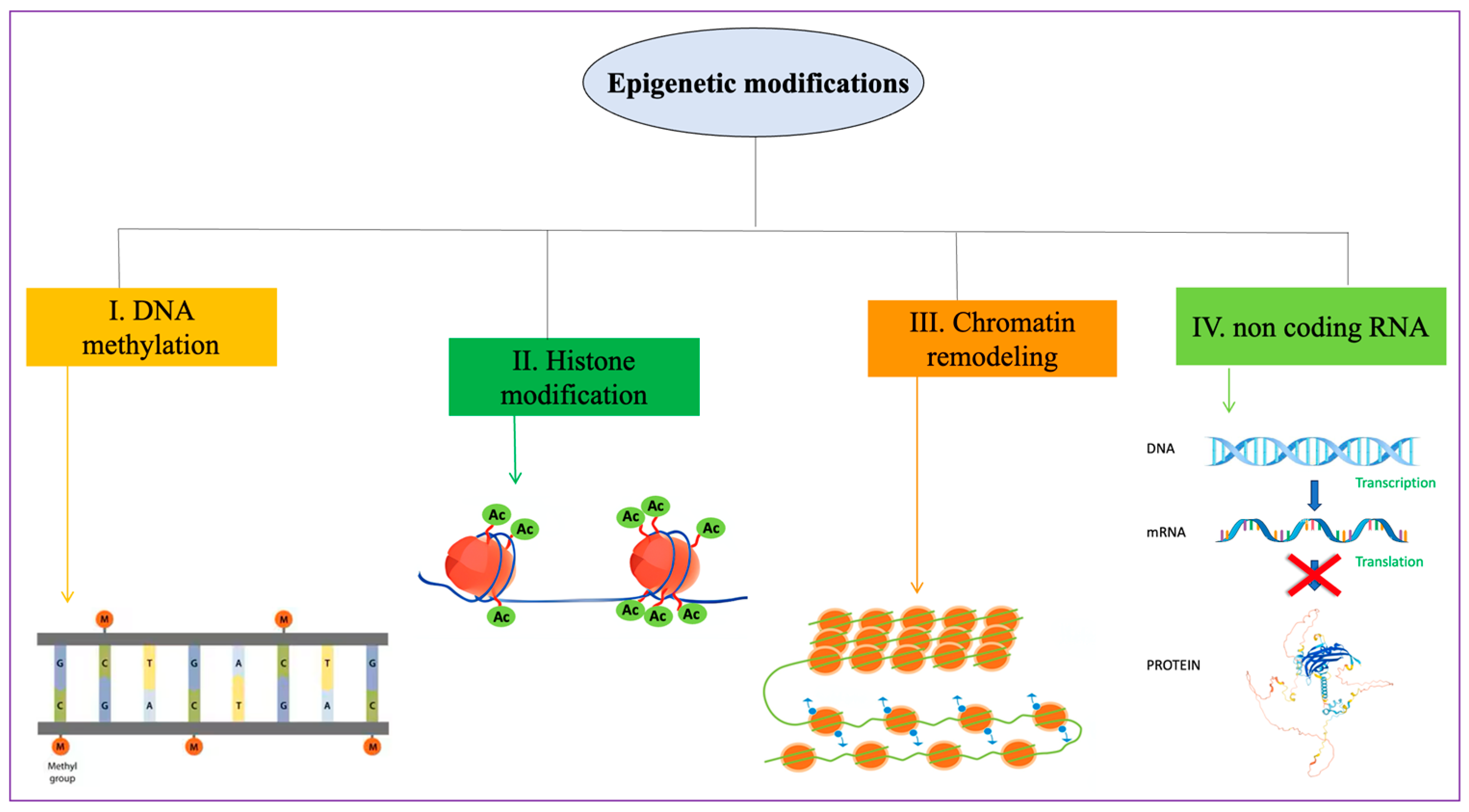
6. Epigenetic Mechanisms during Spermatogenesis and Epigenetic Factors in OS Affecting Male Infertility
7. Transgenerational Consequences of Epigenetic Changes in Sperm DNA
8. Insights into Future Redox Biomarker Research: Integrated Analysis of Omics Data for Male Infertility
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisht, S.; Dada, R. Oxidative stress: Major executioner in disease pathology, role in sperm DNA damage and preventive strategies. Front. Biosci. (Schol. Ed.) 2017, 9, 420–447. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. In Vitro Combination of Ascorbic and Ellagic Acids in Sperm Oxidative Damage Inhibition. Int. J. Mol. Sci. 2022, 23, 14751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fraga, C.G.; Motchnik, P.A.; Wyrobek, A.J.; Rempel, D.M.; Ames, B.N. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat. Res. 1996, 351, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Soto, E.; Medel-Flores, M.O.; Fernández-Martínez, E.; Oros-Pantoja, R.; Miranda-Covarrubias, J.C.; Sánchez-Monroy, V. High-Risk HPV with Multiple Infections Promotes CYP2E1, Lipoperoxidation and Pro-Inflammatory Cytokines in Semen of Asymptomatic Infertile Men. Antioxidants 2022, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, J.; Peng, Y.; Fan, W.; Han, S.; Peng, Q.; Xue, C.; Qin, X.; Liu, Y.; Ding, Z. Obesity-induced oxidative stress and mitochondrial dysfunction negatively affect sperm quality. FEBS Open Biol. 2023, 13, 763–778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocco, L.; Mottola, F.; Roychoudhury, S. Editorial: DNA damage and repair in reproductive and embryo cells. Front. Cell Dev. Biol. 2023, 11, 1274341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2021, 53, e13646. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of environmental factors on human semen quality and male fertility: A narrative review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dziewirska, E.; Radwan, M.; Wielgomas, B.; Klimowska, A.; Radwan, P.; Kałużny, P.; Hanke, W.; Słodki, M.; Jurewicz, J. Human Semen Quality, Sperm DNA Damage, and the Level of Urinary Concentrations of 1N and TCPY, the Biomarkers of Nonpersistent Insecticides. Am. J. Mens. Health 2019, 13, 1557988318816598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.J.; Liu, C.; Tu, Z.Z.; Lu, Q.; Messerlian, C.; Mustieles, V.; Sun, Y.; Lu, W.Q.; Pan, X.F.; Mao, C.; et al. Associations of Urinary Trichloroacetic Acid Concentrations with Spermatozoa Apoptosis and DNA Damage in a Chinese Population. Environ. Sci. Technol. 2022, 56, 6491–6499. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, Y.X.; Sun, L.; Chen, Y.J.; Liu, C.; Huang, L.L.; Lu, W.Q.; Zeng, Q. Urinary metabolites of polycyclic aromatic hydrocarbons, sperm DNA damage and spermatozoa apoptosis. J. Hazard. Mater. 2017, 329, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Onaolapo, M.C.; Omole, A.I.; Adeyemi, W.J.; Oluwole, D.T. Mechanism associated with changes in male reproductive functions during ageing process. Exp. Gerontol. 2023, 179, 112232. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, M.; San Gabriel, M.C.; Chan, D.; Behan, N.A.; Caron, M.; Pastinen, T.; Bourque, G.; MacFarlane, A.J.; Zini, A.; Trasler, J. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum. Mol. Genet. 2015, 24, 6301–6313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashapkin, V.; Suvorov, A.; Pilsner, J.R.; Krawetz, S.A.; Sergeyev, O. Age-associated epigenetic changes in mammalian sperm: Implications for offspring health and development. Hum. Reprod. Update 2023, 29, 24–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Gao, H.; Li, W.; Liu, C. Essential Role of Histone Replacement and Modifications in Male Fertility. Front. Genet. 2019, 10, 962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; Baker, M.A. The Role of Genetics and Oxidative Stress in the Etiology of Male Infertility—A Unifying Hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Wang, R.; Yu, Y.; Zhu, H.; Li, L.; Yang, X.; Hu, X.; Liu, R. Non-Robertsonian translocations involving chromosomes 13, 14, or 15 in male infertility: 28 cases and a review of the literature. Medicine 2019, 98, e14730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clabout, T.; Maes, L.; Acke, F.; Wuyts, W.; Van Schil, K.; Coucke, P.; Janssens, S.; De Leenheer, E. Negative Molecular Diagnostics in Non-Syndromic Hearing Loss: What Next? Genes 2022, 14, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aitken, R.J.; De Iuliis, G.N.; Nixon, B. The Sins of Our Forefathers: Paternal Impacts on De Novo Mutation Rate and Development. Annu. Rev. Genet. 2020, 54, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Crafa, A.; Barbagallo, F.; Mongioì, L.M.; Condorelli, R.A.; Aversa, A.; Calogero, A.E.; La Vignera, S. Seminal Plasma Proteomic Biomarkers of Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 9113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimmermann, C.; Romero, Y.; Warnefors, M.; Bilican, A.; Borel, C.; Smith, L.B.; Kotaja, N.; Kaessmann, H.; Nef, S. Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS ONE 2014, 9, e107023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The cytoskeleton in spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens. Health 2020, 38, 412–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Barbăroșie, C.; Ambar, R.; Finelli, R. The Impact of Single- and Double-Strand DNA Breaks in Human Spermatozoa on Assisted Reproduction. Int. J. Mol. Sci. 2020, 21, 3882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de la Iglesia, A.; Jauregi, P.; Jodar, M.; Barrachina, F.; Ded, L.; Mallofré, C.; Rodríguez-Carunchio, L.; Corral, J.M.; Ballescà, J.L.; Komrskova, K.; et al. H4K5 Butyrylation Coexist with Acetylation during Human Spermiogenesis and Are Retained in the Mature Sperm Chromatin. Int. J. Mol. Sci. 2022, 23, 12398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dada, R.; Kumar, M.; Jesudasan, R.; Fernández, J.L.; Gosálvez, J.; Agarwal, A. Epigenetics and its role in male infertility. J. Assist. Reprod. Genet. 2012, 29, 213–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamagishi, S.I.; Edelstein, D.; Du, X.L.; Kaneda, Y.; Guzmán, M.; Brownlee, M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J. Biol. Chem. 2001, 276, 25096–25100. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crapster, J.A.; Rack, P.G.; Hellmann, Z.J.; Le, A.D.; Adams, C.M.; Leib, R.D.; Elias, J.E.; Perrino, J.; Behr, B.; Li, Y.; et al. HIPK4 is essential for murine spermiogenesis. eLife 2020, 9, e50209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Hu, C.; Xia, B.; He, Y.; Huang, J.; Yuan, Z.; Deng, J.; Duan, P. The Activated AMPK/mTORC2 Signaling Pathway Associated with Oxidative Stress in Seminal Plasma Contributes to Idiopathic Asthenozoospermia. Oxid. Med. Cell. Longev. 2022, 2022, 4240490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Sharma, R.; Roychoudhury, S.; Du Plessis, S.; Sabanegh, E. MiOXSYS: A novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil. Steril. 2016, 106, 566–573.e10. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Oliva, A.; Vergani, E.; Festa, R.; Silvestrini, A. The Dual Role of Oxidants in Male (In)fertility: Every ROSe Has a Thorn. Int. J. Mol. Sci. 2023, 24, 4994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baszyński, J.; Kamiński, P.; Bogdzińska, M.; Mroczkowski, S.; Szymański, M.; Wasilow, K.; Stanek, E.; Hołderna-Bona, K.; Brodzka, S.; Bilski, R.; et al. Enzymatic Antioxidant Defense and Polymorphic Changes in Male Infertility. Antioxidants 2022, 11, 817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Isa, M.L.M.; Alewi, N.A.M.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric oxide, 8-hydroxydeoxyguanosine, and total antioxidant capacity in human seminal plasma of infertile men and their relationship with sperm parameters. Clin. Exp. Reprod. Med. 2020, 47, 54–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Liu, C.; Cui, F.P.; Chen, P.P.; Deng, Y.L.; Luo, Q.; Miao, Y.; Sun, S.Z.; Li, Y.F.; Lu, W.Q.; et al. The role of oxidative stress in association between disinfection by-products exposure and semen quality: A mediation analysis among men from an infertility clinic. Chemosphere 2021, 268, 128856. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Bromfield, E.G.; Gibb, Z. OXIDATIVE STRESS AND REPRODUCTIVE FUNCTION: The impact of oxidative stress on reproduction: A focus on gametogenesis and fertilization. Reproduction 2022, 164, F79–F94. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, S.; Tsekhmistrenko, S.; Polishchuk, V.; Tsekhmistrenko, O.; Zdorovtseva, L.; Kotula-Balak, M.; Tarasiuk, K.; Ievstafiieva, Y.; Hutsol, T. Status of prooxidant and antioxidant systems in the sperm and seminal plasma of breeding boars of large white breed and SS23 synthetic line. J. Physiol. Pharmacol. 2022, 73, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Haghnazari, L.; Vaisi-Raygani, A.; Keshvarzi, F.; Ferdowsi, F.; Goodarzi, M.; Rahimi, Z.; Baniamerian, H.; Tavilani, H.; Vaisi-Raygani, H.; Vaisi-Raygani, H.; et al. Effect of Acetylcholinesterase and Butyrylcholinesterase on Intrauterine Insemination, Contribution to Inflammations, Oxidative Stress and Antioxidant Status; A Preliminary Report. J. Reprod. Infertil. 2016, 17, 157–162. [Google Scholar] [PubMed] [PubMed Central]
- Fallahi, S.; Rajaei, M.; Hesam, M.J.; Koolivand, M.; Malekzadeh, K. The effect of Phoenix dactylifera pollen on the expression of NRF2, SOD2, CAT, and GPX4 genes, and sperm parameters of fertile and infertile men: A controlled clinical trial. Int. J. Reprod. Biomed. 2021, 19, 545–558, Erratum in: Int. J. Reprod. Biomed. 2021, 19, 752. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Finelli, R.; Selvam, M.K.P.; Leisegang, K.; Majzoub, A.; Tadros, N.; Ko, E.; Parekh, N.; Henkel, R.; Durairajanayagam, D.; et al. A Global Survey of Reproductive Specialists to Determine the Clinical Utility of Oxidative Stress Testing and Antioxidant Use in Male Infertility. World J. Mens. Health 2021, 39, 470–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Panner Selvam, M.K.; Samanta, L.; Vij, S.C.; Parekh, N.; Sabanegh, E.; Tadros, N.N.; Arafa, M.; Sharma, R. Effect of Antioxidant Supplementation on the Sperm Proteome of Idiopathic Infertile Men. Antioxidants 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and ferulic acids protect arsenic-induced male reproductive toxicity via regulating Nfe2l2, Ppargc1a and StAR expressions in testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Said, T.M.; Grunewald, S.; Paasch, U.; Rasch, M.; Agarwal, A.; Glander, H.J. Effects of magnetic-activated cell sorting on sperm motility and cryosurvival rates. Fertil. Steril. 2005, 83, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Imai, H. Redox reactions in mammalian spermatogenesis and the potential targets of reactive oxygen species under oxidative stress. Spermatogenesis 2014, 4, e979108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noblanc, A.; Peltier, M.; Damon-Soubeyrand, C.; Kerchkove, N.; Chabory, E.; Vernet, P.; Saez, F.; Cadet, R.; Janny, L.; Pons-Rejraji, H.; et al. Epididymis response partly compensates for spermatozoa oxidative defects in snGPx4 and GPx5 double mutant mice. PLoS ONE 2012, 7, e38565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Flaherty, C.; de Souza, A.R. Hydrogen peroxide modifies human sperm peroxiredoxins in a dose-dependent manner. Biol. Reprod. 2011, 84, 238–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Panner Selvam, M.K.; Arafa, M.; Okada, H.; Homa, S.; Killeen, A.; Balaban, B.; Saleh, R.; Armagan, A.; Roychoudhury, S.; et al. Multi-center evaluation of oxidation-reduction potential by the MiOXSYS in males with abnormal semen. Asian J. Androl. 2019, 21, 565–569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henkel, R.; Morris, A.; Vogiatzi, P.; Saleh, R.; Sallam, H.; Boitrelle, F.; Garrido, N.; Arafa, M.; Gül, M.; Rambhatla, A.; et al. Predictive value of seminal oxidation-reduction potential analysis for reproductive outcomes of ICSI. Reprod. Biomed. Online 2022, 45, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.P.; Cheng, C.Y. Nitric oxide and cyclic nucleotides: Their roles in junction dynamics and spermatogenesis. Oxid. Med. Cell. Longev. 2008, 1, 25–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Chen, B.; An, T.; Zhang, H.; Xia, B.; Li, R.; Zhu, R.; Tian, Y.; Wang, L.; Zhao, D.; et al. BaZiBuShen alleviates altered testicular morphology and spermatogenesis and modulates Sirt6/P53 and Sirt6/NF-κB pathways in aging mice induced by D-galactose and NaNO2. J. Ethnopharmacol. 2021, 271, 113810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Zhang, A.D.; Shi, Z.D.; Wang, L.G.; Qiu, Y. Changes in Levels of Seminal Nitric Oxide Synthase, Macrophage Migration Inhibitory Factor, Sperm DNA Integrity and Caspase-3 in Fertile Men after Scrotal Heat Stress. PLoS ONE 2015, 10, e0141320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of nitric oxide concentrations on human sperm motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Tamir, S.; Burney, S.; Tannenbaum, S.R. DNA damage by nitric oxide. Chem. Res. Toxicol. 1996, 9, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Türk, S.; Kilk, K.; Ausmees, K.; Punab, M.; Mändar, R. Increased levels of hydrogen peroxide and nitric oxide in male partners of infertile couples. Andrology 2013, 1, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Keyhan, H.; Dadvar, A.; Ansari, M.; Rafiee, K. Comparison of before and after varicocelectomy levels of nitric oxide in seminal fluid of infertile men. Nephrourol. Mon. 2012, 4, 629–632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moretti, E.; Collodel, G.; Fiaschi, A.I.; Micheli, L.; Iacoponi, F.; Cerretani, D. Nitric oxide, malondialdheyde and non-enzymatic antioxidants assessed in viable spermatozoa from selected infertile men. Reprod. Biol. 2017, 17, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Cabler, S.; McAlister, D.A.; Sabanegh, E.; Agarwal, A. The effect of obesity on sperm disorders and male infertility. Nat. Rev. Urol. 2010, 7, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.B.; Rubino, D.; Sinaii, N.; Ramsey, S.; Nieman, L.K. Cortisol, obesity, and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obesity 2013, 21, E105–E117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbasihormozi, S.H.; Babapour, V.; Kouhkan, A.; Niasari Naslji, A.; Afraz, K.; Zolfaghary, Z.; Shahverdi, A.H. Stress Hormone and Oxidative Stress Biomarkers Link Obesity and Diabetes with Reduced Fertility Potential. Cell J. 2019, 21, 307–313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aljaser, F.; Tabassum, H.; Fatima, S.; Abudawood, M.; Banu, N. Effect of trace elements on the seminal oxidative status and correlation to sperm motility in infertile Saudi males. Saudi J. Biol. Sci. 2021, 28, 4455–4460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, M.S.; Zaman, S.; Sajjad, M.; Shoaib, M.; Gilani, G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011, 1, 93–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Punjabi, U.; Goovaerts, I.; Peeters, K.; De Neubourg, D. Antioxidants in Male Infertility—If We Want to Get This Right We Need to Take the Bull by the Horns: A Pilot Study. Antioxidants 2023, 12, 1805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, L.; Guo, W.; Wu, S.; Liu, J.; Zhang, S.; Shi, L.; Ji, G.; Gu, A. Genetic variants in nitric oxide synthase genes and the risk of male infertility in a Chinese population: A case-control study. PLoS ONE 2014, 9, e115190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadia, K.; Sultan, S.; Khan, K.; Javeres, L.M.; Rumman, B.; Shah, S.T.A.; Batool, S.; Nurulain, S.M. Antioxidant enzymes and association of CAT SNP-21 A/T (rs7943316) with male infertility. Mol. Reprod. Dev. 2021, 88, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Seger, C.; Salzmann, L. After another decade: LC-MS/MS became routine in clinical diagnostics. Clin. Biochem. 2020, 82, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Neagu, A.N.; Jayathirtha, M.; Baxter, E.; Donnelly, M.; Petre, B.A.; Darie, C.C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27, 2411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.; Machalowski, T.; Harasny, P.; Kups, M.; Grabowska, M.; Duchnik, E.; Sipak, O.; Fraczek, M.; Kurpisz, M.; Kurzawa, R.; et al. Male Infertility Coexists with Decreased Sperm Genomic Integrity and Oxidative Stress in Semen Irrespective of Leukocytospermia. Antioxidants 2022, 11, 1987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potiris, A.; Voitse, A.; Mavrogianni, D.; Machairiotis, N.; Drakaki, E.; Papamentzelopoulou, M.; Karampitsakos, T.; Zikopoulos, A.; Evgeni, E.; Drakakis, P.; et al. Association of GSTM1 Polymorphism and Redox Potential with Idiopathic Male Infertility. J. Clin. Med. 2023, 12, 6775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farhadi-Azar, M.; Saei Ghare Naz, M.; Ghahremani, M.; Mousavi, M.; Azizi, F.; Ramezani Tehrani, F. Self-reported Male Infertility and Metabolic Disturbance: A Cross-Sectional Study. Int. J. Endocrinol. Metab. 2023, 21, e134895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karabulut, S.; Korkmaz, S.; Güneş, E.; Kabil, E.; Keskin, İ.; Usta, M.; Omurtag, G.Z. Seminal trace elements and their relationship with sperm parameters. Andrologia 2022, 54, e14610. [Google Scholar] [CrossRef] [PubMed]
- Asl, A.J.; Sharifi, M.; Dashti, A.; Dashti, G.R. Relationship between long non-coding RNA MALAT1 and HOTAIR expression with sperm parameters, DNA and malondialdehyde levels in male infertility. Tissue Cell 2023, 85, 102248. [Google Scholar] [CrossRef] [PubMed]
- Barik, G.; Chaturvedula, L.; Bobby, Z. Role of Oxidative Stress and Antioxidants in Male Infertility: An Interventional Study. J. Hum. Reprod. Sci. 2019, 12, 204–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamiński, P.; Baszyński, J.; Jerzak, I.; Kavanagh, B.P.; Nowacka-Chiari, E.; Polanin, M.; Szymański, M.; Woźniak, A.; Kozera, W. External and Genetic Conditions Determining Male Infertility. Int. J. Mol. Sci. 2020, 21, 5274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbas, H.; Balkan, M.; Binici, M.; Gedik, A. The Possible Role of XRCC1 Gene Polymorphisms with Idiopathic Non-obstructive Azoospermia in Southeast Turkey. Urol. J. 2019, 16, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Hussein, M.M.A.; Saber, T.; Abd-Elhakim, Y.M. Palliative Effect of Resveratrol against Nanosized Iron Oxide-Induced Oxidative Stress and Steroidogenesis-Related Genes Dysregulation in Testicular Tissue of Adult Male Rats. Int. J. Environ. Res. Public Health 2022, 19, 8171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-Based Nanoparticles: Role of Reactive Oxygen Species in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Ali, H.A.; Saadeldin, I.M.; Ahmed, M.M. Querectin Alleviates Zinc Oxide Nanoreprotoxicity in Male Albino Rats. J. Biochem. Mol. Toxicol. 2016, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [CrossRef] [PubMed]
- Akerfelt, M.; Trouillet, D.; Mezger, V.; Sistonen, L. Heat shock factors at a crossroad between stress and development. Ann. N. Y. Acad. Sci. 2007, 1113, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Burnett, L. Automation of molecular-based analyses: A primer on massively parallel sequencing. Clin. Biochem. Rev. 2014, 35, 169–176. [Google Scholar] [PubMed] [PubMed Central]
- Cannarella, R.; Condorelli, R.A.; Paolacci, S.; Barbagallo, F.; Guerri, G.; Bertelli, M.; La Vignera, S.; Calogero, A.E. Next-generation sequencing: Toward an increase in the diagnostic yield in patients with apparently idiopathic spermatogenic failure. Asian J. Androl. 2021, 23, 24–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robay, A.; Abbasi, S.; Akil, A.; El-Bardisi, H.; Arafa, M.; Crystal, R.G.; Fakhro, K.A. A systematic review on the genetics of male infertility in the era of next-generation sequencing. Arab. J. Urol. 2018, 16, 53–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salvi, R.; Gawde, U.; Idicula-Thomas, S.; Biswas, B. Pathway Analysis of Genome Wide Association Studies (GWAS) Data Associated with Male Infertility. Reprod. Med. 2022, 3, 235–245. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Zhang, H.; Sun, J.; Sun, Y.; Wang, Z.; Liu, J.; Ding, Q.; Lu, S.; Shi, R.; et al. A genome-wide association study reveals that variants within the HLA region are associated with risk for nonobstructive azoospermia. Am. J. Hum. Genet. 2012, 90, 900–906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strange, R.C.; Spiteri, M.A.; Ramachandran, S.; Fryer, A.A. Glutathione-S-transferase family of enzymes. Mutat. Res. 2001, 482, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, Z.; Rezaee-Tazangi, F.; Zeidooni, L.; Alidadi, H.; Khorsandi, L. Protective effects of selenium on Bisphenol A-induced oxidative stress in mouse testicular mitochondria and sperm motility. JBRA Assist. Reprod. 2021, 25, 459–465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Mu, Y.; Liu, M.; Che, B.W.; Zhang, W.J.; Chen, K.H.; Tang, K.F. Glutathione S-transferase genetic polymorphisms and fluoride-induced reproductive toxicity in men with idiopathic infertility. Asian J. Androl. 2023, 25, 404–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vojtech, L.; Woo, S.; Hughes, S.; Levy, C.; Ballweber, L.; Sauteraud, R.P.; Strobl, J.; Westerberg, K.; Gottardo, R.; Tewari, M.; et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014, 42, 7290–7304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslani, F.; Modarresi, M.H.; Soltanghoraee, H.; Akhondi, M.M.; Shabani, A.; Lakpour, N.; Sadeghi, M.R. Seminal molecular markers as a non-invasive diagnostic tool for the evaluation of spermatogenesis in non-obstructive azoospermia. Syst. Biol. Reprod. Med. 2011, 57, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Houle, E.; Pilsner, J.R. Extracellular vesicle cargo of the male reproductive tract and the paternal preconception environment. Syst. Biol. Reprod. Med. 2021, 67, 103–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aliakbari, F.; Eshghifar, N.; Mirfakhraie, R.; Pourghorban, P.; Azizi, F. Coding and Non-Coding RNAs, as Male Fertility and Infertility Biomarkers. Int. J. Fertil. Steril. 2021, 15, 158–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omolaoye, T.S.; Hachim, M.Y.; du Plessis, S.S. Using publicly available transcriptomic data to identify mechanistic and diagnostic biomarkers in azoospermia and overall male infertility. Sci. Rep. 2022, 12, 2584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y.; Wu, X.; Li, Q.; Huang, X.; Wang, J.; Zhao, L.; Zhang, T.; Xuan, X. Identification and Potential Value of Candidate Genes in Patients with Non-obstructive Azoospermia. Urology 2022, 164, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ou, N.; Song, Y.; Hu, R.; Zhang, W.; Liang, Z.; Yang, Y.; Liu, X. Identification and verification of key genes in varicocele rats through high-throughput sequencing and bioinformatics analysis. Andrologia 2020, 52, e13662. [Google Scholar] [CrossRef] [PubMed]
- Kadiyska, T.; Tourtourikov, I.; Dabchev, K.; Madzharova, D.; Tincheva, S.; Spandidos, D.A.; Zoumpourlis, V. Role of testis-specific serine kinase 1B in undiagnosed male infertility. Mol. Med. Rep. 2022, 25, 204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saetan, U.; Chotigeat, W. Differentially expressed genes in the testes from early to mature development of banana shrimp (Fenneropenaeus merguiensis). PLoS ONE 2023, 18, e0292127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, H.; Zhou, X.; Li, D.K.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS ONE 2017, 12, e0178535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarvis, S.; Gethings, L.A.; Samanta, L.; Pedroni, S.M.A.; Withers, D.J.; Gray, N.; Plumb, R.S.; Winston, R.M.L.; Williamson, C.; Bevan, C.L. High fat diet causes distinct aberrations in the testicular proteome. Int. J. Obes. 2020, 44, 1958–1969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Gugliandolo, A.; Diomede, F.; Pizzicannella, J.; Trubiani, O.; Iori, R.; Tardiolo, G.; Guarnieri, S.; Bramanti, P.; Mazzon, E. Moringin Pretreatment Inhibits the Expression of Genes Involved in Mitophagy in the Stem Cell of the Human Periodontal Ligament. Molecules 2019, 24, 3217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Li, X.; Ma, Q.; Zhu, Y.; Zhao, W.; Yang, Y.; Xiao, W.; Huang, D.; Cai, F.; Chan, D.Y.L.; et al. Testis cell pyroptosis mediated by CASP1 and CASP4: Possible sertoli cell-only syndrome pathogenesis. Reprod. Biol. Endocrinol. 2023, 21, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nieto, S.J.; Kosten, T.A. Who’s your daddy? Behavioral and epigenetic consequences of paternal drug exposure. Int. J. Dev. Neurosci. 2019, 78, 109–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tahmasbpour Marzouni, E.; Ilkhani, H.; Beigi Harchegani, A.; Shafaghatian, H.; Layali, I.; Shahriary, A. Epigenetic Modifications, A New Approach to Male Infertility Etiology: A Review. Int. J. Fertil. Steril. 2022, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krzastek, S.C.; Smith, R.P.; Kovac, J.R. Future diagnostics in male infertility: Genomics, epigenetics, metabolomics and proteomics. Transl. Androl. Urol. 2020, 9 (Suppl. 2), S195–S205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Wang, Z. microRNA-21 and hypertension. Hypertens. Res. 2018, 41, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Maze, I.; Nestler, E.J. The epigenetic landscape of addiction. Ann. N. Y. Acad. Sci. 2011, 1216, 99–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abballe, L.; Miele, E. Epigenetic modulators for brain cancer stem cells: Implications for anticancer treatment. World J. Stem Cells 2021, 13, 670–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aigner, G.P.; Nenning, P.; Fiechtner, B.; Šrut, M.; Höckner, M. DNA Methylation and Detoxification in the Earthworm Lumbricus terrestris Exposed to Cadmium and the DNA Demethylation Agent 5-aza-2′-deoxycytidine. Toxics 2022, 10, 100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Aston, K.I.; Meyer, T.D.; Hotaling, J.M.; Shamsi, M.B.; Johnstone, E.B.; Cox, K.J.; Stanford, J.B.; Porucznik, C.A.; Carrell, D.T. Decreased fecundity and sperm DNA methylation patterns. Fertil. Steril. 2016, 105, 51–57.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aston, K.I.; Uren, P.J.; Jenkins, T.G.; Horsager, A.; Cairns, B.R.; Smith, A.D.; Carrell, D.T. Aberrant sperm DNA methylation predicts male fertility status and embryo quality. Fertil. Steril. 2015, 104, 1388–1397.e5. [Google Scholar] [CrossRef] [PubMed]
- García-Herrero, S.; Meseguer, M.; Martínez-Conejero, J.A.; Remohí, J.; Pellicer, A.; Garrido, N. The transcriptome of spermatozoa used in homologous intrauterine insemination varies considerably between samples that achieve pregnancy and those that do not. Fertil. Steril. 2010, 94, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Denomme, M.M.; McCallie, B.R.; Parks, J.C.; Booher, K.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Inheritance of epigenetic dysregulation from male factor infertility has a direct impact on reproductive potential. Fertil. Steril. 2018, 110, 419–428.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Cheng, X.; He, Y.; Xie, Y.; Xu, F.; Xu, Y.; Huang, W. Function and mechanism of histone β-hydroxybutyrylation in health and disease. Front. Immunol. 2022, 13, 981285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaoqin, G.; Zhenghui, Z.; Xueqian, Z.; Yuan, H. Epigenetic modifications in human spermatozoon and its potential role in embryonic development. Yi Chuan 2014, 36, 439–446. (In Chinese) [Google Scholar] [PubMed]
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aoki, V.W.; Emery, B.R.; Liu, L.; Carrell, D.T. Protamine levels vary between individual sperm cells of infertile human males and correlate with viability and DNA integrity. J. Androl. 2006, 27, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Kotaja, N. MicroRNAs and spermatogenesis. Fertil. Steril. 2014, 101, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Olotu, O.; Ahmedani, A.; Kotaja, N. Small Non-Coding RNAs in Male Reproduction. Semin. Reprod. Med. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, A.; Gagliardi, A.; Piaggeschi, G.; Tarallo, S.; Cordero, F.; Pensa, R.G.; Impeduglia, A.; Caviglia, G.P.; Ribaldone, D.G.; Gallo, G.; et al. Faecal miRNA profiles associated with age, sex, BMI, and lifestyle habits in healthy individuals. Sci. Rep. 2021, 11, 20645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarallo, S.; Ferrero, G.; De Filippis, F.; Francavilla, A.; Pasolli, E.; Panero, V.; Cordero, F.; Segata, N.; Grioni, S.; Pensa, R.G.; et al. Stool microRNA profiles reflect different dietary and gut microbiome patterns in healthy individuals. Gut 2022, 71, 1302–1314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrero, G.; Festa, R.; Follia, L.; Lettieri, G.; Tarallo, S.; Notari, T.; Giarra, A.; Marinaro, C.; Pardini, B.; Marano, A.; et al. Small noncoding RNAs and sperm nuclear basic proteins reflect the environmental impact on germ cells. Mol. Med. 2024, 30, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becker, L.S.; Al Smadi, M.A.; Koch, H.; Abdul-Khaliq, H.; Meese, E.; Abu-Halima, M. Towards a More Comprehensive Picture of the MicroRNA-23a/b-3p Impact on Impaired Male Fertility. Biology 2023, 12, 800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luk, A.C.; Chan, W.Y.; Rennert, O.M.; Lee, T.L. Long noncoding RNAs in spermatogenesis: Insights from recent high-throughput transcriptome studies. Reproduction 2014, 147, R131–R141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Li, X.; Zhang, P.; Wang, J.; Zhu, D.; Chen, X.; Ye, L. Low long non-coding RNA HOTAIR expression is associated with down-regulation of Nrf2 in the spermatozoa of patients with asthenozoospermia or oligoasthenozoospermia. Int. J. Clin. Exp. Pathol. 2015, 8, 14198–14205. [Google Scholar] [PubMed] [PubMed Central]
- Chen, K.; Mai, Z.; Zhou, Y.; Gao, X.; Yu, B. Low NRF2 mRNA expression in spermatozoa from men with low sperm motility. Tohoku J. Exp. Med. 2012, 228, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.M. Histone acetylation and control of gene expression. J. Cell Sci. 1991, 99 Pt 1, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R. Epigenetics and maternal nutrition: Nature v. nurture. Proc. Nutr. Soc. 2011, 70, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Ravel, C.; Benkhalifa, M.; Cohen-Bacrie, P.; Berthaut, I.; Bashamboo, A.; McElreavey, K. Methylation changes in mature sperm deoxyribonucleic acid from oligozoospermic men: Assessment of genetic variants and assisted reproductive technology outcome. Fertil. Steril. 2013, 100, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef] [PubMed]
- Urdinguio, R.G.; Bayón, G.F.; Dmitrijeva, M.; Toraño, E.G.; Bravo, C.; Fraga, M.F.; Bassas, L.; Larriba, S.; Fernández, A.F. Aberrant DNA methylation patterns of spermatozoa in men with unexplained infertility. Hum. Reprod. 2015, 30, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, N.; Puiu, M.; Niculescu, M. Effects of Dietary Nutrients on Epigenetic Changes in Cancer. Methods Mol. Biol. 2018, 1856, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Estill, M.S.; Krawetz, S.A. The Epigenetic Consequences of Paternal Exposure to Environmental Contaminants and Reproductive Toxicants. Curr. Environ. Health Rep. 2016, 3, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Buisan, M.; Mecca, R.; Jones, C.; Coward, K.; Yeste, M. Contribution of semen to early embryo development: Fertilization and beyond. Hum. Reprod. Update 2023, 29, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.G.; Aston, K.I.; James, E.R.; Carrell, D.T. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 2017, 63, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, male infertility, and the sperm epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.A.; Jenkins, T.G. Epigenetic mechanisms within the sperm epigenome and their diagnostic potential. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101481. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, S.; Liew, F.F.; Dhawan, V.; Das, B.; Mottola, F.; Slama, P.; Rocco, L.; Roychoudhury, S. Environmental and Genetic Traffic in the Journey from Sperm to Offspring. Biomolecules 2023, 13, 1759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donkin, I.; Barrès, R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meeker, J.D. Exposure to environmental endocrine disruptors and child development. Arch. Pediatr. Adolesc. Med. 2012, 166, E1–E7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020, 112, 1308–1325. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Diao, L.; Liu, J.; Jiang, N.; Zhang, J.; Wang, H.; Zhou, W.; Huang, G.; Ma, D. Paternal ethanol exposure and behavioral abnormities in offspring: Associated alterations in imprinted gene methylation. Neuropharmacology 2014, 81, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Meccariello, R. Cannabis and Paternal Epigenetic Inheritance. Int. J. Environ. Res. Public Health 2023, 20, 5663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Pabarja, A.; Ganjalikhan Hakemi, S.; Musanejad, E.; Ezzatabadipour, M.; Nematollahi-Mahani, S.N.; Afgar, A.; Afarinesh, M.R.; Haghpanah, T. Genetic and epigenetic modifications of F1 offspring’s sperm cells following in utero and lactational combined exposure to nicotine and ethanol. Sci. Rep. 2021, 11, 12311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omolaoye, T.S.; Omolaoye, V.A.; Kandasamy, R.K.; Hachim, M.Y.; Du Plessis, S.S. Omics and Male Infertility: Highlighting the Application of Transcriptomic Data. Life 2022, 12, 280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.; Zhou, L.; Shi, J.; Cheng, C.Y.; Sun, F. Multiomics analysis of male infertility. Biol. Reprod. 2022, 107, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Carrell, D.T.; Aston, K.I.; Oliva, R.; Emery, B.R.; De Jonge, C.J. The “omics” of human male infertility: Integrating big data in a systems biology approach. Cell Tissue Res. 2016, 363, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Ogrinc Wagner, A.; Turk, A.; Kunej, T. Towards a Multi-Omics of Male Infertility. World J. Mens. Health 2023, 41, 272–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehrparavar, B.; Minai-Tehrani, A.; Arjmand, B.; Gilany, K. Metabolomics of Male Infertility: A New Tool for Diagnostic Tests. J. Reprod. Infertil. 2019, 20, 64–69. [Google Scholar] [PubMed] [PubMed Central]
- Minai-Tehrani, A.; Jafarzadeh, N.; Gilany, K. Metabolomics: A state-of-the-art technology for better understanding of male infertility. Andrologia 2016, 48, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.L.H.; Gadella, B.M.; Sutherland, J.M.; Nixon, B.; Bromfield, E.G. Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline. J. Clin. Med. 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kołodziejczyk, J.; Blixt, O.; Olejnik, B.; Zimmer, M.; Ferens-Sieczkowska, M. Application of lectin microarrays for the analysis of seminal plasma glycome. Andrologia 2018, 50, e13018. [Google Scholar] [CrossRef] [PubMed]
- Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 5, CD007411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Y.S.; Chen, Y.L.; Li, W.Y.; Yang, Y.Y.; Lin, S.J.; Wu, C.H.; Yang, J.I.; Wang, T.E.; Yu, J.; Tsai, P.S. Antioxidant Nanoparticles Restore Cisplatin-Induced Male Fertility Defects by Promoting MDC1-53bp1-Associated Non-Homologous DNA Repair Mechanism and Sperm Intracellular Calcium Influx. Int. J. Nanomed. 2023, 18, 4313–4327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iovine, C.; Mottola, F.; Santonastaso, M.; Finelli, R.; Agarwal, A.; Rocco, L. In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol. Reprod. Dev. 2021, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Palmieri, I.; Carannante, M.; Barretta, A.; Roychoudhury, S.; Rocco, L. Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes 2024, 15, 539. https://doi.org/10.3390/genes15050539
Mottola F, Palmieri I, Carannante M, Barretta A, Roychoudhury S, Rocco L. Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes. 2024; 15(5):539. https://doi.org/10.3390/genes15050539
Chicago/Turabian StyleMottola, Filomena, Ilaria Palmieri, Maria Carannante, Angela Barretta, Shubhadeep Roychoudhury, and Lucia Rocco. 2024. "Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives" Genes 15, no. 5: 539. https://doi.org/10.3390/genes15050539
APA StyleMottola, F., Palmieri, I., Carannante, M., Barretta, A., Roychoudhury, S., & Rocco, L. (2024). Oxidative Stress Biomarkers in Male Infertility: Established Methodologies and Future Perspectives. Genes, 15(5), 539. https://doi.org/10.3390/genes15050539







