A TMEM63A Nonsense Heterozygous Variant Linked to Infantile Transient Hypomyelinating Leukodystrophy Type 19?
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data Collection
2.2. DNA Sequence Analysis
3. Results
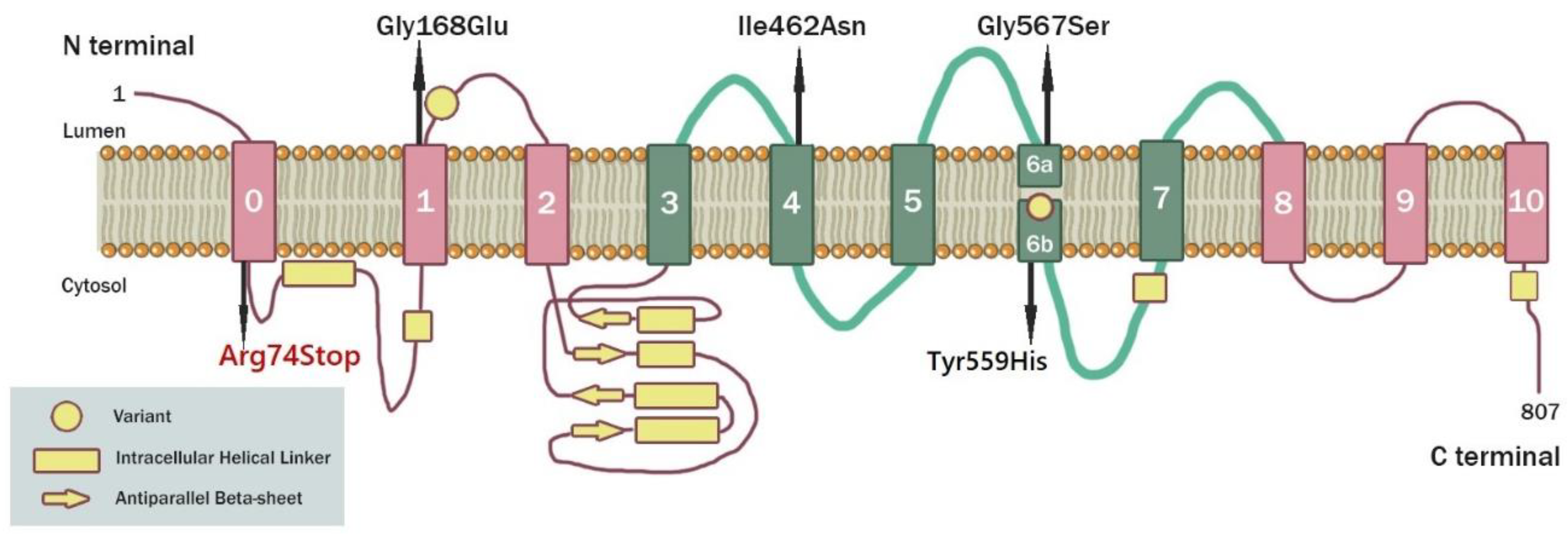
3.1. Case Report
3.2. Molecular Genetic Analysis
3.3. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Knaap, M.S.; Schiffmann, R.; Mochel, F.; Wolf, N.I. Diagnosis, prognosis, and treatment of leukodystrophies. Lancet Neurol. 2019, 18, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.I.; Ffrench-Constant, C.; van der Knaap, M.S. Hypomyelinating leukodystrophies-unravelling myelin biology. Nat. Rev. Neurol. 2021, 17, 88–103. [Google Scholar] [CrossRef]
- Tomassy, G.S.; Dershowitz, L.B.; Arlotta, P. Diversity Matters: A Revised Guide to Myelination. Trends Cell Biol. 2016, 26, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Vanderver, A.; Hussey, H.; Schmidt, J.L.; Pastor, W.; Hoffman, H.J. Relative incidence of inherited white matter disorders in childhood to acquired pediatric demyelinating disorders. Semin. Pediatr. Neurol. 2012, 19, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Chanoumidou, K.; Mozafari, S.; Baron-Van Evercooren, A.; Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 2020, 68, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Amado, D.A.; Davidson, B.L. Gene therapy for ALS: A review. Mol. Ther. 2021, 29, 3345–3358. [Google Scholar] [CrossRef]
- Meschkat, M.; Steyer, A.M.; Weil, M.T.; Kusch, K.; Jahn, O.; Piepkorn, L.; Agüi-Gonzalez, P.; Phan, N.T.N.; Ruhwedel, T.; Sadowski, B.; et al. White matter integrity in mice requires continuous myelin synthesis at the inner tongue. Nat. Commun. 2022, 13, 1163. [Google Scholar] [CrossRef]
- Kassmann, C.M. Myelin peroxisomes-essential organelles for the maintenance of white matter in the nervous system. Biochimie 2014, 98, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cali, J.J.; Hsieh, C.L.; Francke, U.; Russell, D.W. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 1991, 266, 7779–7783. [Google Scholar] [CrossRef]
- Weigel, M.; Wang, L.; Fu, M.M. Microtubule organization and dynamics in oligodendrocytes, astrocytes, and microglia. Dev. Neurobiol. 2021, 81, 310–320. [Google Scholar] [CrossRef]
- Merheb, E.; Cui, M.H.; DuBois, J.C.; Branch, C.A.; Gulinello, M.; Shafit-Zagardo, B.; Moir, R.D.; Willis, I.M. Defective myelination in an RNA polymerase III mutant leukodystrophic mouse. Proc. Natl. Acad. Sci. USA 2021, 118, e2024378118. [Google Scholar] [CrossRef] [PubMed]
- Meservey, L.M.; Topkar, V.V.; Fu, M.M. mRNA transport and local translation in glia. Trends Cell Biol. 2021, 31, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.I.; Gutierrez Salazar, M.; Guerrero, K.; Thiffault, I.; Salomons, G.S.; Gauquelin, L.; Tran, L.T.; Forget, D.; Gauthier, M.-S.; Waisfisz, Q.; et al. Bi-allelic mutations in EPRS, encoding the glutamyl-prolyl-aminoacyl-tRNA synthetase, cause a hypomyelinating leukodystrophy. Am. J. Hum. Genet. 2018, 102, 676–684. [Google Scholar] [CrossRef]
- Van der Knaap, M.S.; Boor, I.; Estévez, R. Megalencephalic leukoencephalopathy with subcortical cysts: Chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet. Neurol. 2012, 11, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M.; Jacque, C.; Nguyen-Legros, J.; Bornhofen, J.H.; Araoz, C.A.; Daudu, O.; Baumann, N. Pelizaeus-merzbacher disease: Biochemical analysis of isolated myelin (electron-microscopy; protein, lipid and unsubstituted fatty acids analysis). Eur. Neurol. 1978, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yapijakis, C.; Kalogera, S.; Angelopoulou, A.; Paraskevas, G.; Kapaki, E. Craniofacial and neurological phenotype in a case of oculodentodigital syndrome. Adv. Exp. Med. Biol. 2021, 1339, 325–329. [Google Scholar] [PubMed]
- Elpidorou, M.; Poulter, J.A.; Szymanska, K.; Baron, W.; Junger, K.; Boldt, K.; Ueffing, M.; Green, L.; Livingston, J.H.; Sheridan, E.G.; et al. Missense mutation of MAL causes a rare leukodystrophy similar to Pelizaeus-Merzbacher disease. Eur. J. Hum. Genet. 2022, 30, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Woodward, L.J.; Anderson, P.J.; Austin, N.C.; Howard, K.; Inder, T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006, 355, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Marelli, A.J.; Mackie, A.S.; Ionescu-Ittu, R.; Rahme, E.; Pilote, L. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 2007, 115, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Adams-Chapman, I.; Fanaroff, A.A.; Hintz, S.R.; Vohr, B.; Higgins, R.D.; National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004, 292, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Helman, G.; Murthy, S.E.; Ji, H.; Crawford, J.; Kubisiak, T.; Bent, S.J.; Xiao, J.; Taft, R.J.; Coombs, A.; et al. Heterozygous variants in the mechanosensitive ion channel TMEM63A result in transient hypomyelination of infancy. Am. J. Hum. Genet. 2019, 105, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Tonduti, D.; Mura, E.; Masnada, S.; Bertini, E.; Aiello, C.; Zini, D.; Parmeggiani, L.; Cantalupo, G.; Talenti, G.; Veggiotti, P.; et al. Spinal cord involvement and paroxysmal events in ‘infantile onset transient hypomyelination’ due to TMEM63A mutation. J. Hum. Genet. 2021, 66, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ji, H.; Kubisiak, T.; Wu, Y.; Xiao, J.; Gu, Q.; Yang, Y.; Xie, H.; Ji, T.; Gao, K.; et al. Genetic analysis of 20 patients with hypomyelinating leukodystrophy by trio-based whole-exome sequencing. J. Hum. Genet. 2021, 66, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.I.; Vanderver, A.; van Spaendonk, R.M.; Schiffmann, R.; Brais, B.; Bugiani, M.; Sistermans, E.; Catsman-Berrevoets, C.; Kros, J.M.; Pinto, P.S.; et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology 2014, 83, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Hobson, G.M.; Garbern, J.Y. Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders. Semin. Neurol. 2012, 32, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Dubin, A.E.; Whitwam, T.; Jojoa-Cruz, S.; Caha-lan, S.M.; Mousavi SA, R.; Ward, A.B.; Patapoutian, A. OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels. eLife 2018, 7, e41844. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yan, X.; Liu, Y.; Zhang, P.; Ni, X. Co-expression of mouse TMEM63A, TMEM63B and TMEM63C confers hyperosmolarity activated ion currents in HEK293 cells. Cell Biochem. Funct. 2016, 34, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Rawson, S.; Shen, Z.; Tamilselvan, E.; Smith, H.E.; Halford, J.; Shen, C.; Murthy, S.E.; Ulbrich, M.H.; Sotomayor, M.; et al. TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels. Neuron 2023, 111, 3195–3210.e7. [Google Scholar] [CrossRef]
- Chen, X.; Wang, N.; Liu, J.-W.; Zeng, B.; Chen, G.-L. TMEM63 mechanosensitive ion channels: Activation mechanisms, biological functions and human genetic disorders. Biochem. Biophys. Res. Commun. 2023, 683, 149111. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siori, D.; Vlachakis, D.; Makrythanasis, P.; Traeger-Synodinos, J.; Veltra, D.; Kampouraki, A.; Chrousos, G.P. A TMEM63A Nonsense Heterozygous Variant Linked to Infantile Transient Hypomyelinating Leukodystrophy Type 19? Genes 2024, 15, 525. https://doi.org/10.3390/genes15050525
Siori D, Vlachakis D, Makrythanasis P, Traeger-Synodinos J, Veltra D, Kampouraki A, Chrousos GP. A TMEM63A Nonsense Heterozygous Variant Linked to Infantile Transient Hypomyelinating Leukodystrophy Type 19? Genes. 2024; 15(5):525. https://doi.org/10.3390/genes15050525
Chicago/Turabian StyleSiori, Dimitra, Dimitrios Vlachakis, Periklis Makrythanasis, Joanne Traeger-Synodinos, Danai Veltra, Afrodite Kampouraki, and George P. Chrousos. 2024. "A TMEM63A Nonsense Heterozygous Variant Linked to Infantile Transient Hypomyelinating Leukodystrophy Type 19?" Genes 15, no. 5: 525. https://doi.org/10.3390/genes15050525
APA StyleSiori, D., Vlachakis, D., Makrythanasis, P., Traeger-Synodinos, J., Veltra, D., Kampouraki, A., & Chrousos, G. P. (2024). A TMEM63A Nonsense Heterozygous Variant Linked to Infantile Transient Hypomyelinating Leukodystrophy Type 19? Genes, 15(5), 525. https://doi.org/10.3390/genes15050525







