Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture and Transfection
2.3. FGF5 Alternative Spliceosome Cloning and Plasmid Vector Construction
- Forward Primer: 5′-GCAUCGGUUUCCAUCUGCATT-3′;
- Reverse Primer: 5′-UGCAGAGUUAAACCGAUGCTT-3′;
- siRNA-NC Forward Primer: 5′-UUCUCCGAACGUGUCACGUTT-3′;
- siRNA-NC Reverse primer: 5′-ACGUGACACGUUCGGAGAATT-3′.
2.4. Bioinformatics Analysis of FGF5 Alternative Spliceosomes
2.5. Quantitative RT-PCR
2.6. Cell Proliferation Assay
2.7. Statistical Analysis
3. Results and Analysis
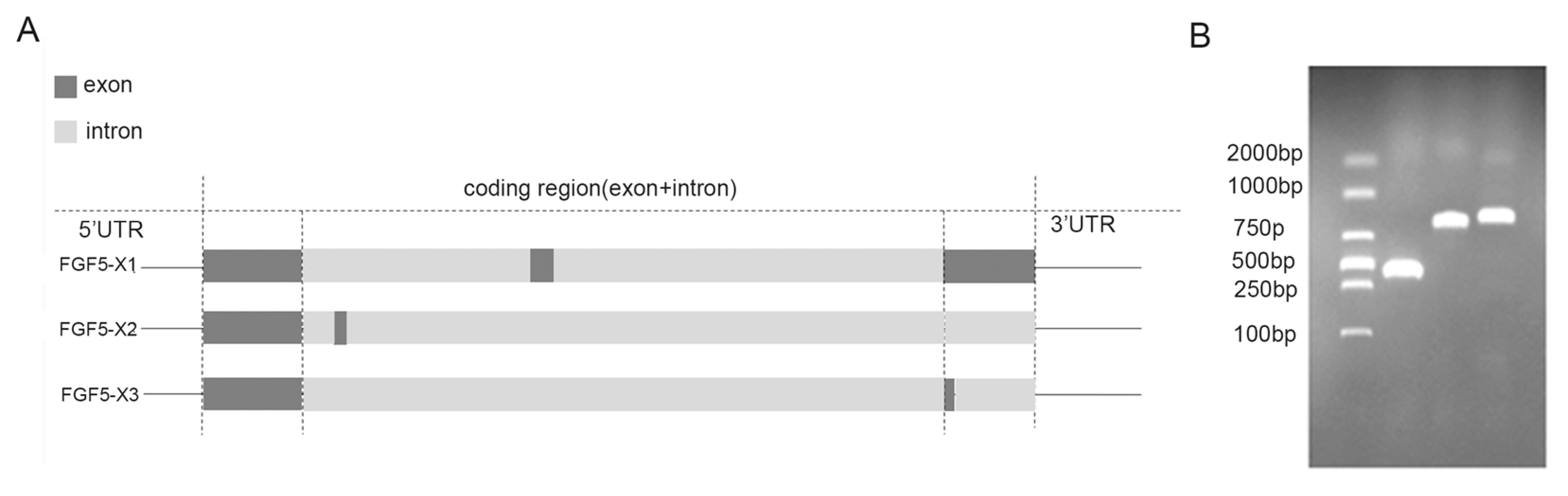
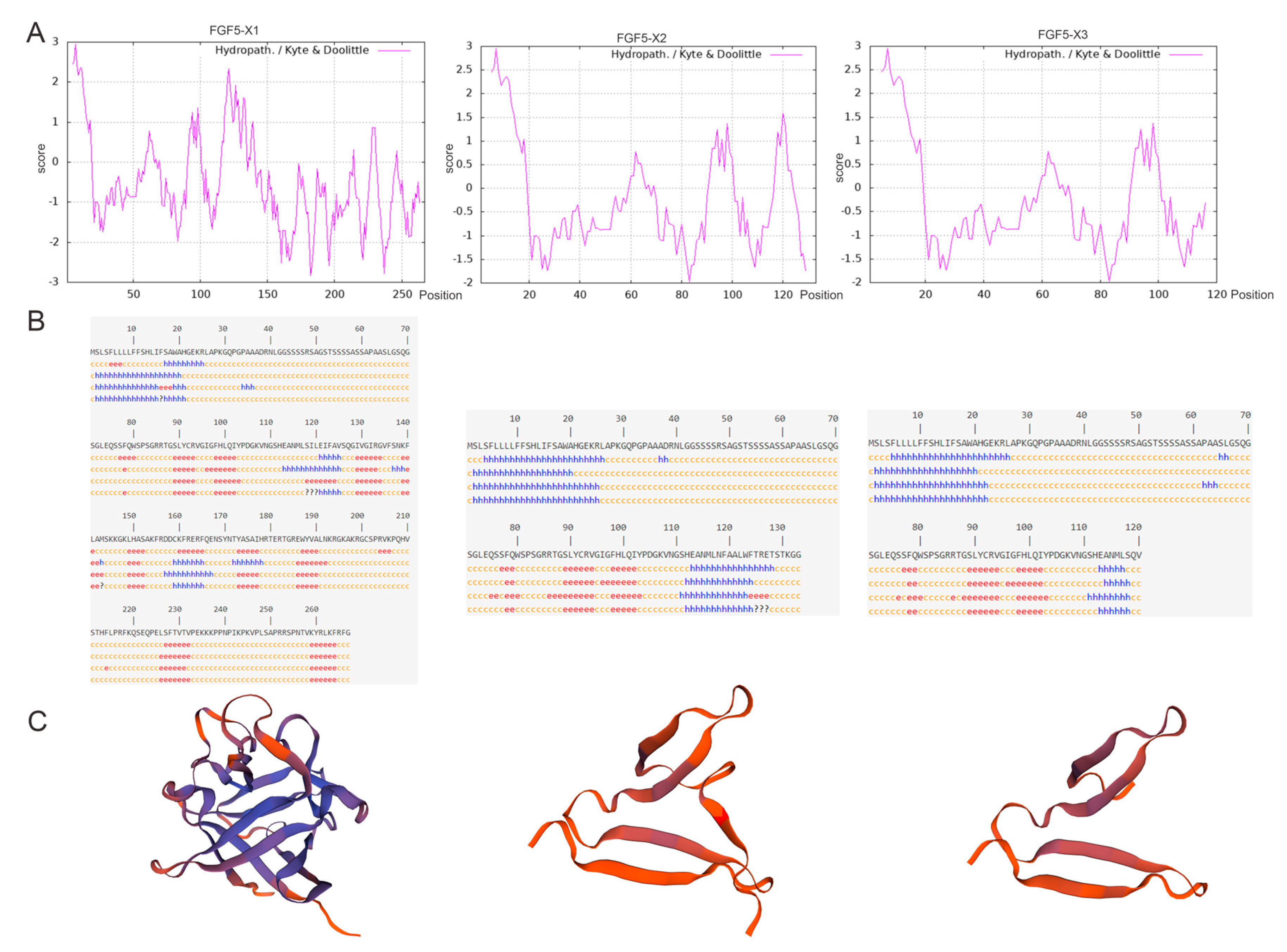
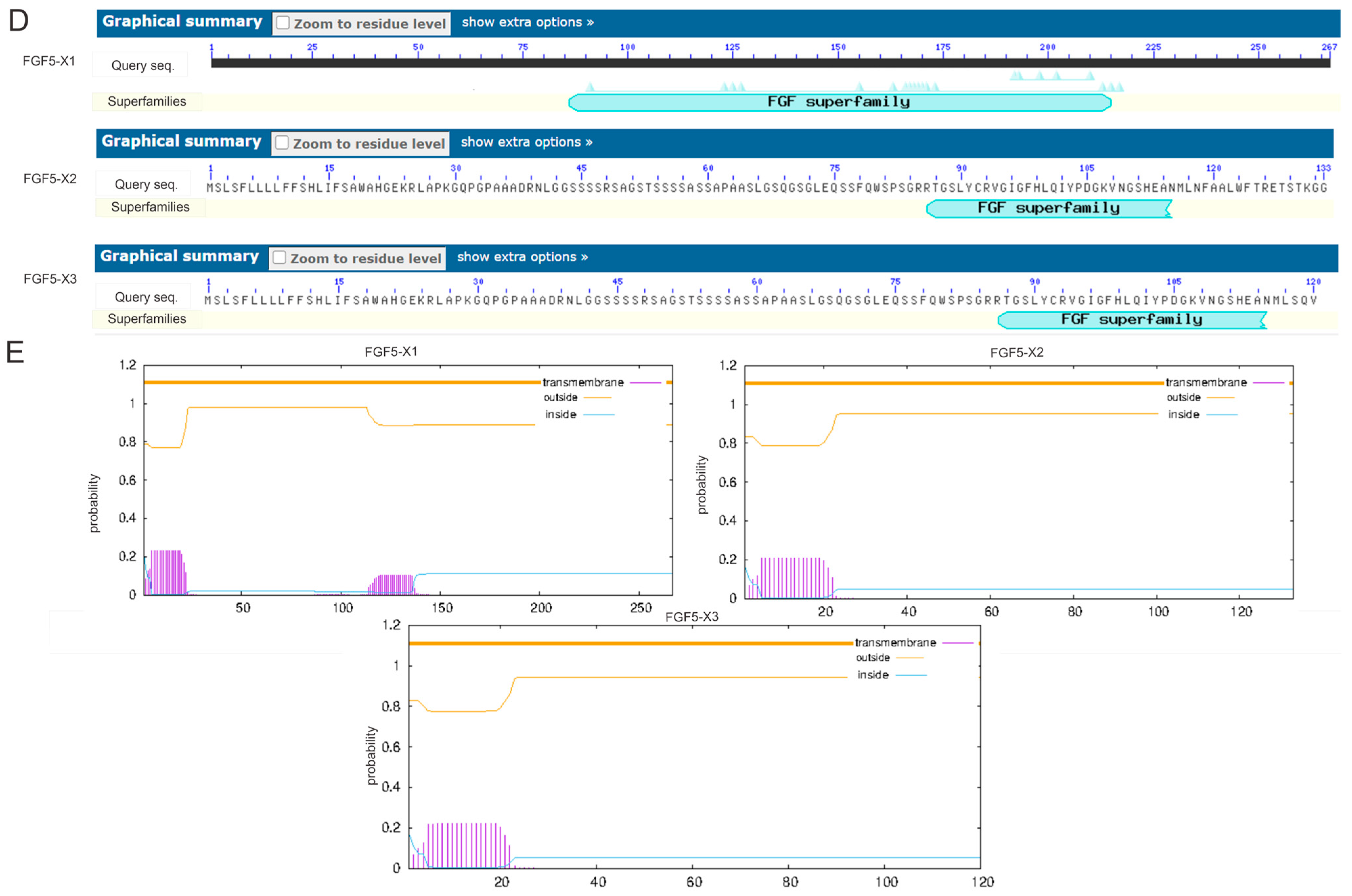
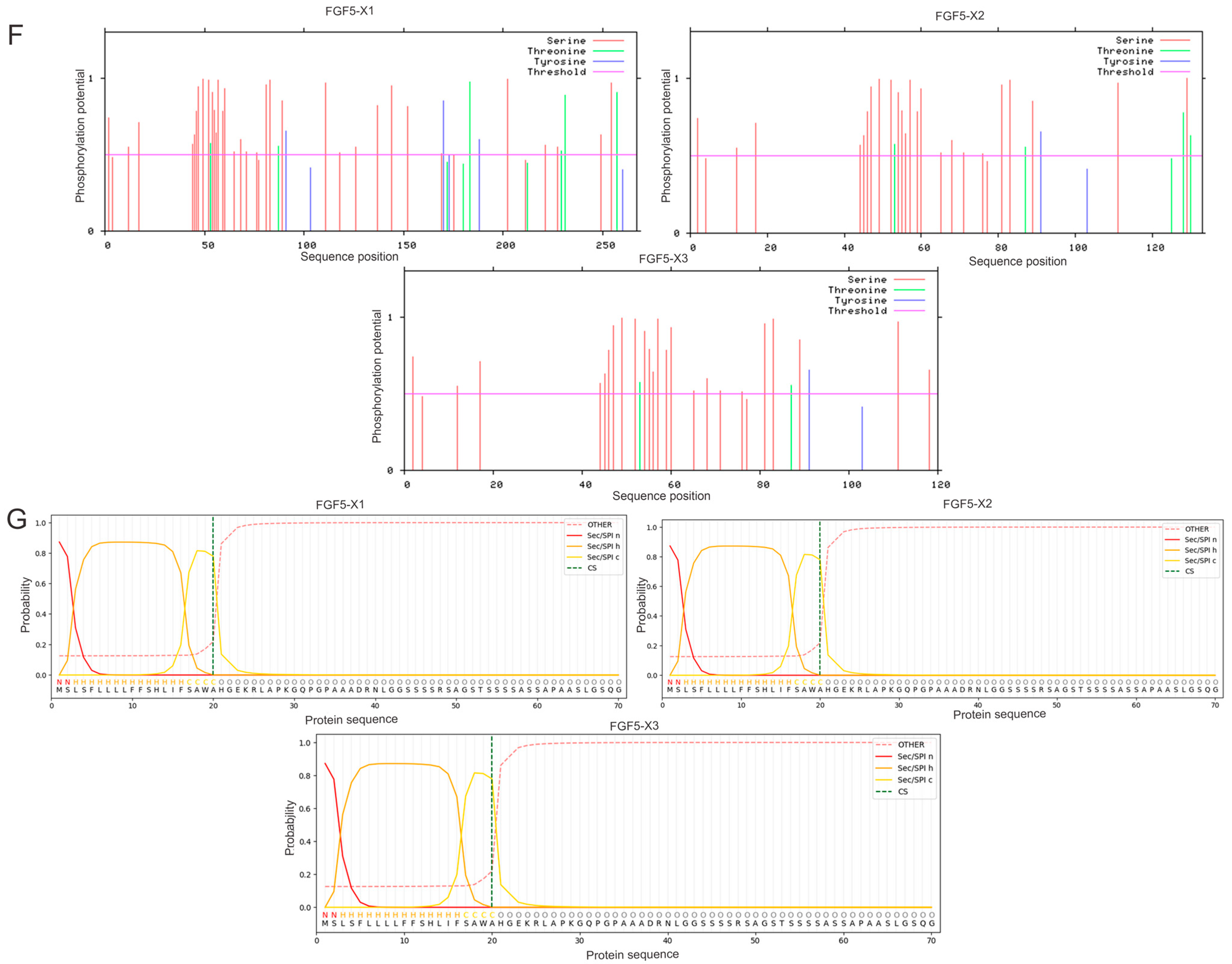
3.1. Bioinformatics Analysis of Alternative Spliceosomes of FGF5
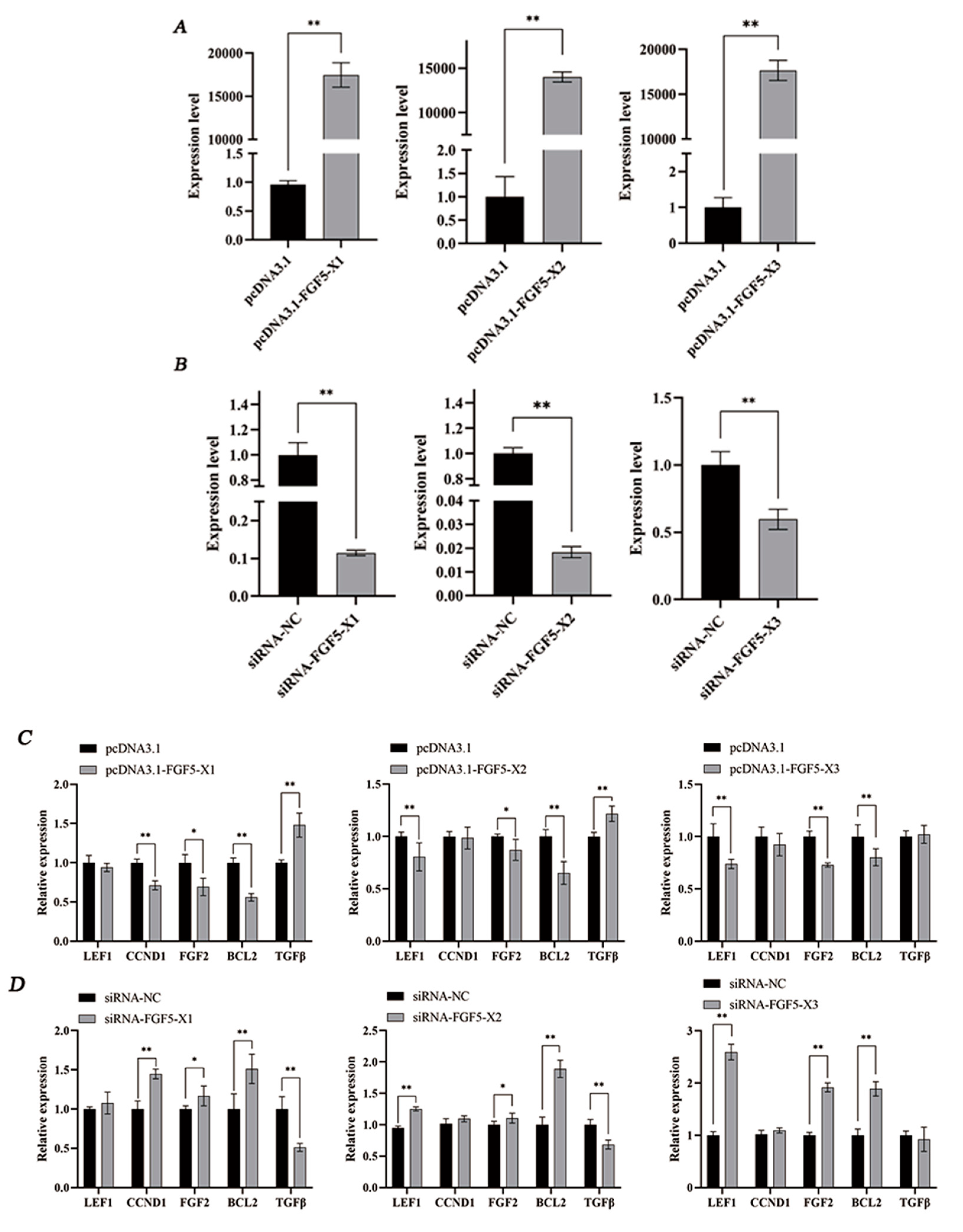
3.2. Regulation of HF Growth- and Development-Related Genes by FGF5 Alternative Spliceosomes
3.3. FGF5 Alternative Spliceosomes Inhibit DPC Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davidson, P.; Hardy, M.H. The development of mouse vibrissae in vivo and in vitro. J. Anat. 1952, 86, 342–356. [Google Scholar]
- Whiteley, H.J. The effect of the hair-growth cycle on the development and distribution of virus-induced lesions in the skin of the rabbit. J. Pathol. Bacteriol. 1956, 72, 1–13. [Google Scholar] [CrossRef]
- Philip, G.; Moore, M.; A Panaretto, B.; Carter, N.B. Epidermal Hyperplasia and Wool Follicle Regression in Sheep Infused with Epidermal Growth Factor. J. Investig. Dermatol. 1985, 84, 172–175. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F.; et al. Mesenchymal Stem Cells: Potential for Therapy and Treatment of Chronic Non-Healing Skin Wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Liu, M.; Yang, N.; Bao, Z.; Zhang, X.; Dai, Y.; Cai, J.; Chen, Y.; Wu, X. DNA methylation mediates lncRNA2919 regulation of hair follicle regeneration. Int. J. Mol. Sci. 2022, 23, 9481. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R.; Chuong, C.-M.; Randall, V.A.; Widelitz, R.B.; Wu, P.; Jiang, T.-X.; Paul, M.J.; George, N.T.; Zucker, I.; et al. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Johansson, O.; Paus, R.; Eichmüller, S. Hair cycle-dependent plasticity of skin and hair follicle innervation in normal murine skin. J. Comp. Neurol. 1997, 386, 379–395. [Google Scholar] [CrossRef]
- Ule, J.; Blencowe, B.J. Alternative splicing regulatory networks: Functions, mechanisms, and evolution. Mol. Cell 2019, 76, 329–345. [Google Scholar] [CrossRef]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef]
- Fedor, M.J. Alternative Splicing Minireview Series: Combinatorial Control Facilitates Splicing Regulation of Gene Expression and Enhances Genome Diversity. J. Biol. Chem. 2008, 283, 1209–1210. [Google Scholar] [CrossRef]
- Mardon, H.J.; Sebastio, G.; Baralle, F.E. A role for exon sequences in alternative splicing of the human fibronection gene. Nucleic Acids Res. 1987, 15, 7725–7733. [Google Scholar] [CrossRef]
- Song, H.; Wang, L.; Chen, D.; Li, F. The Function of Pre-mRNA Alternative Splicing in Mammal Spermatogenesis. Int. J. Biol. Sci. 2020, 16, 38–48. [Google Scholar] [CrossRef]
- Spivak-Kroizman, T.; Lemmon, M.A.; Dikic, I.; Ladbury, J.E.; Pinchasi, D.; Huang, J.; Jaye, M.; Crumley, G.; Schlessinger, J.; Lax, I. Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 1994, 79, 1015–1024. [Google Scholar] [CrossRef]
- Yamashita, T.; Yoshioka, M.; Itoh, N. Identification of a Novel Fibroblast Growth Factor, FGF-23, Preferentially Expressed in the Ventrolateral Thalamic Nucleus of the Brain. Biochem. Biophys. Res. Commun. 2000, 277, 494–498. [Google Scholar] [CrossRef]
- Hattori, Y.; Yamasaki, M.; Itoh, N. The rat FGF-5 mRNA variant generated by alternative splicing encodes a novel truncated form of FGF-5. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1996, 1306, 31–33. [Google Scholar] [CrossRef]
- Niu, J.; Liu, N.; Zhang, J.; Sun, S.; Feng, H.; Qiao, H.-B.; Sun, Q. Expression and clinical significance of fibroblast growth factor 1 in gastric adenocarcinoma. OncoTargets Ther. 2015, 8, 615–621. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Lee, S.; Jeon, E.; Kang, W.; Kim, K.-H.; Kim, H.-W.; Jang, J.-H. Fibroblast growth factor 2-functionalized collagen matrices for skeletal muscle tissue engineering. Biotechnol. Lett. 2011, 34, 771–778. [Google Scholar] [CrossRef]
- Kurniawan, D.W.; Booijink, R.; Pater, L.; Wols, I.; Vrynas, A.; Storm, G.; Prakash, J.; Bansal, R. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J. Control. Release 2020, 328, 640–652. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, L.; Zhang, M.; Huang, J.; Zhang, J.; Chang, Y. FGF9 Alleviates the Fatty Liver Phenotype by Regulating Hepatic Lipid Metabolism. Front. Pharmacol. 2022, 13, 850128. [Google Scholar] [CrossRef]
- Borello, U.; Cobos, I.; E Long, J.; Murre, C.; Rubenstein, J.L. FGF15 promotes neurogenesis and opposes FGF8 function during neocortical development. Neural Dev. 2008, 3, 17. [Google Scholar] [CrossRef]
- Meier, K.; Nanney, L.B. Emerging new drugs for wound repair. Expert Opin. Emerg. Drugs 2006, 11, 23–37. [Google Scholar] [CrossRef]
- Suzuki, S.; Kato, T.; Takimoto, H.; Masui, S.; Oshima, H.; Ozawa, K.; Suzuki, S.; Imamura, T. Localization of rat FGF-5 protein in skin macrophage-like cells and FGF-5S protein in hair follicle: Possible involvement of twoFgf-5 gene products in hair growth cycle regulation. J. Investig. Dermatol. 1998, 111, 963–972. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- He, X.; Chao, Y.; Zhou, G.; Chen, Y. Fibroblast growth factor 5-short (FGF5s) inhibits the activity of FGF5 in primary and secondary hair follicle dermal papilla cells of cashmere goats. Gene 2016, 575, 393–398. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Saif, R.; Azhar, A.; Khan, E.; Choudhry, S.; Hussain, T.; Babar, M.E.; Awan, A.R.; Tayyab, M.; Wasim, M. In silico Characterization of Hspb1 Mutant Protein in Canine and Feline Cancers. Pak. J. Biochem. Mol. Biol. 2016, 49, 18–28. [Google Scholar]
- Kouza, M.; Faraggi, E.; Kolinski, A.; Kloczkowski, A. The GOR method of protein secondary structure prediction and its application as a protein aggregation prediction tool. Predict. Protein Second. Struct. 2017, 1484, 7–24. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Möller, S.; Croning, M.D.R.; Apweiler, R. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 2001, 17, 646–653. [Google Scholar] [CrossRef]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Shie, W.-Y.; Cheng, S.-J.; Chen, K.-C.; Tang, C.-C.; Peng, H.-H.; Ko, H.-H.; Hou, H.-H.; Chou, H.-Y.E. Fibroblast growth factor 5 expression predicts the progression of oral squamous cell carcinoma. J. Formos. Med. Assoc. 2024, 123, 390–399. [Google Scholar] [CrossRef]
- Kehler, J.S.; David, V.A.; Schäffer, A.A.; Bajema, K.; Eizirik, E.; Ryugo, D.K.; Hannah, S.S.; O’Brien, S.J.; Menotti-Raymond, M. Four Independent Mutations in the Feline Fibroblast Growth Factor 5 Gene Determine the Long-Haired Phenotype in Domestic Cats. J. Hered. 2007, 98, 555–566. [Google Scholar] [CrossRef]
- Dierks, C.; Mömke, S.; Philipp, U.; Distl, O. Allelic heterogeneity of FGF5 mutations causes the long-hair phenotype in dogs. Anim. Genet. 2013, 44, 425–431. [Google Scholar] [CrossRef]
- Legrand, R.; Tiret, L.; Abitbol, M. Two recessive mutations in FGF5 are associated with the long-hair phenotype in donkeys. Genet. Sel. Evol. 2014, 46, 65. [Google Scholar] [CrossRef]
- Wang, X.; Cai, B.; Zhou, J.; Zhu, H.; Niu, Y.; Ma, B.; Yu, H.; Lei, A.; Yan, H.; Shen, Q.; et al. Disruption of FGF5 in Cashmere Goats Using CRISPR/Cas9 Results in More Secondary Hair Follicles and Longer Fibers. PLoS ONE 2016, 11, e0164640. [Google Scholar] [CrossRef]
- Ito, C.; Saitoh, Y.; Fujita, Y.; Yamazaki, Y.; Imamura, T.; Oka, S.; Suzuki, S. Decapeptide with fibroblast growth factor (FGF)-5 partial sequence inhibits hair growth suppressing activity of FGF-5. J. Cell. Physiol. 2003, 197, 272–283. [Google Scholar] [CrossRef]
- Housley, D.J.E.; Venta, P.J. The long and the short of it: Evidence that FGF5 is a major determinant of canine ‘hair’-itability. Anim. Genet. 2006, 37, 309–315. [Google Scholar] [CrossRef]
- Fatima, N.; Jia, L.; Liu, B.; Li, L.; Bai, L.; Wang, W.; Zhao, S.; Wang, R.; Liu, E. A homozygous missense mutation in the fibroblast growth factor 5 gene is associated with the long-hair trait in Angora rabbits. BMC Genom. 2023, 24, 298. [Google Scholar] [CrossRef]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growthfactor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 1996, 205, 379–386. [Google Scholar] [CrossRef]
- Chow, L.T.; Gelinas, R.E.; Broker, T.R.; Roberts, R.J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell 1977, 12, 1–8. [Google Scholar] [CrossRef]
- Martinez-Montiel, N.; Rosas-Murrieta, N.H.; Ruiz, M.A.; Monjaraz-Guzman, E.; Martinez-Contreras, R. Alternative splicing as a target for cancer treatment. Int. J. Mol. Sci. 2018, 19, 545. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, W.; Liu, Z.; He, X.; Hong, Q.; Lan, R.; Liu, Y.; Chu, M. Identification of WNT4 alternative splicing patterns and effects on proliferation of granulosa cells in goat. Int. J. Biol. Macromol. 2022, 223, 1230–1242. [Google Scholar] [CrossRef]
- Ast, G. How did alternative splicing evolve? Nat. Rev. Genet. 2004, 5, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lin, H.; Wang, L.; Guo, K.; Jing, R.; Li, X.; Chen, Y.; Hu, Z.; Gao, S.; Xu, N. Suppression of FGF5 and FGF18 Expression by Cholesterol-Modified siRNAs Promotes Hair Growth in Mice. Front. Pharmacol. 2021, 12, 666860. [Google Scholar] [CrossRef]
- Corrigan, D.J. Differential Roles of PRDM16 Isoforms in Normal and Malignant Hematopoiesis; Columbia University: New York, NY, USA, 2018. [Google Scholar]
- Webster, G.M.; Haresign, W. Seasonal changes in LH and prolactin concentrations in ewes of two breeds. Reproduction 1983, 67, 465–471. [Google Scholar] [CrossRef]
- Li, W.; Zeng, W.; Jin, X.; Xu, H.; Fang, X.; Ma, Z.; Cao, G.; Li, R.; Ma, L. High-altitude stress orchestrates mRNA expression and alternative splicing of ovarian follicle development genes in tibetan sheep. Animals 2022, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, H.; Lian, Z. Bioinformatics analysis of evolutionary characteristics and biochemical structure of FGF5 Gene in sheep. Gene 2019, 702, 123–132. [Google Scholar] [CrossRef]
- Jia, Y.; Lin, J.; Zeng, W.; Zhang, C. Effect of prostaglandin on luteinizing hormone-stimulated proliferation of theca externa cells from chicken prehierarchical follicles. Prostaglandins Other Lipid Mediat. 2010, 92, 77–84. [Google Scholar] [CrossRef]
- Bièche, I.; Olivi, M.; Noguès, C.; Vidaud, M.; Lidereau, R. Prognostic value of CCND1 gene status in sporadic breast tumours, as determined by real-time quantitative PCR assays. Br. J. Cancer 2002, 86, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lyle, S.; Liu, Y.; Solky, B.; Cotsarelis, G. Differential expression of cyclin D1 in the human hair follicle. Am. J. Pathol. 2003, 163, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Whoriskey, W.; Park, M.Y.; Bronson, R.T.; Medema, R.H.; Li, T.; Weinberg, R.A.; Sicinski, P. Rescue of cyclin D1 deficiency by knock in cyclin E. Cell 1999, 97, 767–777. [Google Scholar] [CrossRef]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Liang, J.; Cao, R.; Wang, X.; Zhang, Y.; Wang, P.; Gao, H.; Li, C.; Yang, F.; Zeng, R.; Wei, P.; et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2016, 27, 329–351. [Google Scholar] [CrossRef]
- Monni, O.; Franssila, K.; Joensuu, H.; Knuutila, S. BCL2 overexpression in diffuse large B-cell lymphoma. Leuk. Lymphoma 1999, 34, 45–52. [Google Scholar] [CrossRef]
- Van Nguyen, C.; Nguyen, Q.T.; Vu, H.T.N.; Phung, H.T.; Pham, K.H.; Le, R.D. Combined p53 and Bcl2 Immunophenotypes in Prognosis of Vietnamese Invasive Breast Carcinoma: A Single Institutional Retrospective Analysis. Technol. Cancer Res. Treat. 2020, 19, 376–385. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Rossiter, H.; Lindner, G.; Peters, E.M.; Kupper, T.S.; Paus, R. Hair follicle apoptosis and Bcl-2. J. Investig. Dermatol. Symp. Proc. 1999, 4, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; He, J.; Wang, J.; Chen, X.; Yang, R. Regulation of signaling pathways in hair follicle stem cells. Burn. Trauma 2022, 10, tkac022. [Google Scholar] [CrossRef]
- Lebowitz, E.R.; Orbach, M.; Marin, C.E.; Salmaso, N.; Vaccarino, F.M.; Silverman, W.K. Fibroblast Growth Factor 2 Implicated in Childhood Anxiety and Depression Symptoms. J. Affect. Disord. 2021, 282, 611–616. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, W.; Yang, J.-H.; Chang, P.-Y.; Walterscheid, J.P.; Chen, H.-H.; Marcelli, M.; Tang, D.; Lee, Y.-T.; Liao, W.S.; et al. Electronegative LDL Impairs Vascular Endothelial Cell Integrity in Diabetes by Disrupting Fibroblast Growth Factor 2 (FGF2) Autoregulation. Diabetes 2008, 57, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ni, X.; Huang, X.; Yao, J.; He, Q.; Wang, K.; Duan, T. Potential Role of Hyperglycemia in Fetoplacental Endothelial Dysfunction in Gestational Diabetes Mellitus. Cell. Physiol. Biochem. 2016, 39, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, F.N.d.L.; Levy, M.J.; Wailemann, R.A.M.; Lopes, M.; Silva, M.L.; Sardiu, M.E.; Garcia, B.A.; Motta, M.C.M.; Oliveira, C.C.; Armelin, H.A.; et al. The antiproliferative effect of FGF2 in K-Ras-driven tumor cells involves modulation of rRNA and the nucleolus. J. Cell Sci. 2023, 136, jcs260989. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Hafner, S.; Moratille, S.; Vaigot, P.; Mine, S.; Rigaud, O.; Martin, M.T. FGF2 mediates DNA repair in epidermoid carcinoma cells exposed to ionizing radiation. Int. J. Radiat. Biol. 2012, 88, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Ramyar, L.; Murphy, P.R.; Li, A.W.; Ezzat, S. The endogenous fibroblast growth factor-2 antisense gene product regulates pituitary cell growth and hormone production. Mol. Endocrinol. 2001, 15, 589–599. [Google Scholar] [CrossRef]
- Ropiquet, F.; Giri, D.; Lamb, D.J.; Ittmann, M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J. Urol. 1999, 162, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Cerliani, J.P.; Guillardoy, T.; Giulianelli, S.; Vaque, J.P.; Gutkind, J.S.; Vanzulli, S.I.; Martins, R.; Zeitlin, E.; Lamb, C.A.; Lanari, C. Interaction between FGFR-2, STAT5, and progesterone receptors in breast cancer. Cancer Res. 2011, 71, 3720–3731. [Google Scholar] [CrossRef]
- Jeong, W.; Bae, H.; Lim, W.; Bazer, F.W.; Lee, H.; Song, G. The functional effects and mechanisms by which fibroblast growth factor 2 (FGF2) controls bovine mammary epithelial cells: Implications for the development and functionality of the bovine mammary gland1. J. Anim. Sci. 2017, 95, 5365–5377. [Google Scholar] [CrossRef]
- Koledova, Z.; Sumbal, J. FGF signaling in mammary gland fibroblasts regulates multiple fibroblast functions and mammary epithelial morphogenesis. Development 2019, 146, dev185306. [Google Scholar] [CrossRef]
- Désiré, L.; Courtois, Y.; Jeanny, J.C. Endogenous and Exogenous Fibroblast Growth Factor 2 Support Survival of Chick Retinal Neurons by Control of Neuronal Neuronal bcl-xL and bcl-2 Expression Through a Fibroblast Berowth Factor Receptor 1-and Erk-Dependent Pathway. J. Neurochem. 2000, 75, 151–163. [Google Scholar] [CrossRef]
- Paus, R.; Foitzik, K.; Welker, P.; Bulfone-Paus, S.; Eichmüller, S. Transforming growth factor-β receptor type I and Type II expression during murine hair follicle development and cycling. J. Investig. Dermatol. 1997, 109, 518–526. [Google Scholar] [CrossRef] [PubMed]

| Name | Primers (5′→3′) | Version |
|---|---|---|
| FGF5-X1 | F: gggagacccaagctggctagcATGAGCTTGTCCTTCCTCCTCC | XM_008267686.3 |
| R: ggtttaaacgggccctctagaTCATCCAAAGCGAAACTTGAGTC | ||
| FGF5-X2 | F: gggagacccaagctggctagcATGAGCTTGTCCTTCCTCCTCC | XM_051819654.1 |
| R: ggtttaaacgggccctctagaTCATCCTCCCTTAGTGCTGGTT | ||
| FGF5-X3 | F: gggagacccaagctggctagcATGAGCTTGTCCTTCCTCCTCC | XM_008267687.3 |
| R: ggtttaaacgggccctctagaCTAAACTTGGCTTAACATATTGGCTT |
| Genes | Primer Sequence (5′→3′) |
|---|---|
| FGF5-X1 | F: TTGGAAATATTTGCTGTGTCTCAGG |
| R: TCGCTAAAAATTTGTTGCTGAAAAC | |
| FGF5-X2 | F: CGTCCCTCCTCCCTTACAGA |
| R: GTCATCCTCCCTTAGTGCTGGG | |
| FGF5-X3 | F: GGTAGCACCTCGTCTTCCTC |
| R: TCGTGGGAGCCATTGACTTT | |
| GAPDH | F: CACCAGGGGCTGCTTTTAACTCT |
| R: CTTCCCGTTCTCAGCCTTGACC | |
| LEF1 | F: GATCCTAGGCAGAAGGTGGC |
| R: GCAGCTGTCATTCTTGGACC | |
| CCND1 | F: ACACGGACGTGGATTGTCTC |
| R: GGTCCAGTTCATCCTCCGAC | |
| FGF2 | F: GTGTGTGCAAACCGTTACCTT |
| R: TCGTTTCAGTGCCACATACCAG | |
| BCL2 | F: AACATCGCCCTGTGGATGAC |
| R: GGCCGTACAGTTCCACGAA | |
| TGFβ | F: CAGGTCCTGCGGAAGTCAA |
| R: CTGGAACGGGCTCAACATCTA |
| Alternative Spliceosome | Amino Acid Quantity | Theoretical Isoelectric Point | Total Atom | Amino Acid Sequence Length | Fat Solubility Index |
|---|---|---|---|---|---|
| FGF5-X1 | 267 | 10.66 | 4138 | 267 | 64.72 |
| FGF5-X2 | 133 | 10.01 | 1920 | 133 | 64.74 |
| FGF5-X3 | 120 | 9.85 | 1720 | 120 | 69.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Zhao, B.; Li, J.; Zhang, X.; Yao, S.; Bao, Z.; Cai, J.; Yang, J.; Chen, Y.; Wu, X. Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits. Genes 2024, 15, 409. https://doi.org/10.3390/genes15040409
Sun S, Zhao B, Li J, Zhang X, Yao S, Bao Z, Cai J, Yang J, Chen Y, Wu X. Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits. Genes. 2024; 15(4):409. https://doi.org/10.3390/genes15040409
Chicago/Turabian StyleSun, Shaoning, Bohao Zhao, Jiali Li, Xiyu Zhang, Shuyu Yao, Zhiyuan Bao, Jiawei Cai, Jie Yang, Yang Chen, and Xinsheng Wu. 2024. "Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits" Genes 15, no. 4: 409. https://doi.org/10.3390/genes15040409
APA StyleSun, S., Zhao, B., Li, J., Zhang, X., Yao, S., Bao, Z., Cai, J., Yang, J., Chen, Y., & Wu, X. (2024). Regulation of Hair Follicle Growth and Development by Different Alternative Spliceosomes of FGF5 in Rabbits. Genes, 15(4), 409. https://doi.org/10.3390/genes15040409





