Abstract
The rapid genetic evolution of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the coronavirus disease 2019 (COVID-19) pandemic has greatly challenged public health authorities worldwide, including in Mauritania. Despite the presence of the virus in Mauritania, only one study described its genomic variation during the course of the epidemic. The purpose of the present study was to document the genomic pattern of SARS-CoV-2 variants from clinical isolates during the COVID-19 outbreak in Mauritania, from September to November 2021. The whole genomes from 54 SARS-CoV-2 strains detected in nasopharyngeal swabs with a cycle threshold value ≤ 30 were successfully sequenced using next-generation sequencing (NGS) and the Illumina protocol. The mean genome coverage (±standard deviation) was 96.8% (±3.7). The most commonly identified clade was 21J (57.4%), followed by 21D (16.7%), 20A (11.1%), and 20B (9.2%). At the level of lineages, the majority of the samples were Delta variants with the sub-lineage AY.34 (or B.1.617.2.34). Among the 54 SARS-CoV-2 isolates that were successfully sequenced, 33 (61.1%) came from vaccinated individuals, and 21 (38.9%) were from unvaccinated individuals. Several SARS-CoV-2 variants were present in Mauritania between September and November 2021. As Mauritania, like many West African countries, is resource-limited regarding viral genome sequencing facilities, establishment of mutualized sub-regional sequencing platforms will be necessary to ensure continuous monitoring of mutations in viral genomes and track potential reduction in COVID-19 vaccine efficacy, increased transmissibility, and disease severity.
1. Introduction
Coronavirus disease 2019 (COVID-19) is an acute respiratory tract infection due to the emerging coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded RNA virus of positive polarity. COVID-19 was first reported in December 2019 in the city of Wuhan, Hubei Province, China, in patients with pneumonia. From there, it spread quickly across China and then to the rest of the world [1]. Over three years (i.e., from 20 January 2020 to 5 May 2023), COVID-19 was considered by the World Health Organization (WHO) as a public health emergency of global scope [2]. As of 17 December 2023, over 772 million confirmed cases and nearly seven million deaths have been reported globally [3]. Africa represents 1.2% of positive cases and 2.5% of deaths worldwide [3].
According to the WHO, 65% of Africans were infected with SARS-CoV-2 between 2020 and 2021 [4]. According to the same source, exposure to SARS-CoV-2 in Africa rose sharply from 3% (with a margin of error ranging from 1% to 9.2%) in June 2020 to 65% (margin of error between 56.3% and 73%) in September 2021, equivalent to 800 million infections, while only 8.2 million cases were diagnosed and officially reported over the same period. The level of exposure to the virus has risen sharply following the appearance of the Beta and Delta variants. Seroprevalence varied widely within and between countries in Africa. Seroprevalence was higher in more densely populated urban centers than in rural areas. Seroprevalence also varied between age groups, with children aged 0–9 years old being less infected than adults. Exposure to the virus also differed from one sub-region to another on the continent, being higher in East, West, and Central Africa. In addition, Africa has reported a fewer number of severe cases compared to other regions of the world. One of the major factors underlying this trend is likely to be the relatively high proportion of children and young people in African countries although other possible protective factors, such as genetics and environment, may also be involved [5].
The genome of SARS-CoV-2 is undergoing rapid evolution that has given rise to several variants as the result of mutations. For instance, more than 25,000 mutations have already been reported in the SARS-CoV-2 genome since the emergence of the virus in the human populations [6]. Among these mutations, those that occur in the gene encoding the Spike (S) glycoprotein are the most important for viral transmission and pathogenicity because it serves as a ligand and is essential for virus entry through interaction with the human angiotensin-converting enzyme 2 (hACE2) receptor on the host cells [7]. More than 4700 distinct mutations in the S protein gene were reported during the first two years of the pandemic [8]. Since its emergence in 2020, numerous virus variants at the origin of different COVID-19 epidemic waves have been identified, including Alpha (B.1.1.7, first reported in the United Kingdom), Delta (B.1.617.2, first reported from India), and Omicron (B.1.1.529, first reported from South Africa) variants characterized by their high level of human-to-human transmissibility, disease severity, and immune escape [9,10,11,12]. For instance, the Omicron variant was shown to spread easily, even among vaccinated populations [13].
In the West African country of Mauritania, the first COVID-19 case was detected on 13 March 2020 in a 40-year-old Australian expatriate traveling from abroad [14]. Since that time, the country experienced successive epidemic waves affecting all regions characterized by the emergence of variants of concern detected as early as in September 2020 [15,16,17]. As of 19 December 2023, there have been a total of 63,764 confirmed cases and 997 (1.56%) deaths due to COVID-19 in Mauritania [18].
Although COVID-19 is present in Mauritania, the limited number of studies conducted on the disease were mainly focused on its epidemiological characteristics [14,15] and the capacities of the local healthcare system to cope with the pandemic [19]. Studies addressing the dynamic of the SARS-CoV-2 variants circulating in the country during the course of the epidemic are scarce [16,17]. The objective of this study was to determine the genomic characteristics of SARS-CoV-2 clinical isolates during an episode of the COVID-19 epidemic in Mauritania.
2. Materials and Methods
2.1. Study Site
Mauritania is a country in West Africa, located between 15 and 27 degrees north latitude and 5 and 17 degrees west longitude. It is bordered to the north by Western Sahara and Algeria; to the east by Mali; and to the south by Mali and Senegal. To the west, it borders the North Atlantic Ocean with a 600 km coastline, stretching from Ndiago in the south to Nouadhibou in the north. It has a surface area of 1,030,700 km2 and a population of around 4,500,000 [20].
2.2. Sample Collection
Mauritania was struck by the third wave of COVID-19 epidemic in the second half of 2021. Due to inadequate laboratory facilities in many health centers and hospitals, samples from symptomatic patients suspected to be COVID-19-positive were collected and sent to the Mauritanian National Institute of Public Health Research (“Institut National de Recherche en Santé Publique”, INRSP), where virological diagnosis was centralized and undertaken by reverse transcription-polymerase chain reaction (RT-PCR). Among the available samples during this epidemic, a total of 103 positive nasopharyngeal swab samples collected from September to November 2021 were selected by simple random sampling from the banked nasopharyngeal samples at the INRSP. Socio-demographic and clinical information of individuals from whom nasopharyngeal swabs were collected was also obtained from the INRSP patient records. All patient data were anonymized. The present study was reviewed and approved by the Ethics Committee of the University of Nouakchott under reference no. 2020-010.
2.3. Viral RNA Extraction and Ampslification
Viral RNA was extracted from nasopharyngeal samples using the MagMax Viral/Pathogen nucleic acid isolation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions. The quality and concentrations of all RNA extracts were measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific). Extracted viral RNAs were immediately used for viral detection and, if positive, sequenced or stored at −20 °C until use.
Real-time RT-PCR was performed to detect SARS-CoV-2 RNA using a commercial kit for real-time fluorescent RT-PCR for the detection of SARS-CoV-2 (BGI Health (HK) Co. Ltd., Shenzhen, China) following the manufacturer’s instructions. The BGI kit detects SARS-CoV-2 in a one-step strategy using the ORF1ab probe. Quantitative real-time RT-PCR reaction was performed using a LightCycler® 480 Instrument II (Roche, Basel, Switzerland). RT-PCR was performed with a single thermal cycling program using 30 µL of mix (containing the enzyme and primers) and 10 µL of eluted RNA. The RT-PCR program consisted of an initial step at 50 °C for 20 min and denaturation step at 94 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 30 s. Only positive samples with cycle threshold (Ct) values ≤ 30 were retained for sequencing.
2.4. SARS-CoV-2 Whole Genome Sequencing
Libraries were prepared following the Illumina COVIDSeq protocol (Illumina Inc., San Diego, CA, USA) as previously described [21]. This protocol comprises four steps: cDNA preparation, target amplification, library preparation and pooling, and sequencing. First, the first strand of cDNA was synthesized using reverse transcriptase and random hexamer primer. In this step, the extracted RNA (8.5 µL) was first annealed using the elution prime fragment 3HC (8.5 µL) and then transcribed into cDNA using reverse transcriptase HT (1 µL).
In the second step, the synthesized cDNA was amplified by performing two multiplex PCR amplifications using two non-overlapping COVIDSeq primer pools 1 and 2 (CPP1 and CPP2, respectively). The sequences of these specific primer pools (ARTIC v3 SARS-CoV-2 primer sets) were retrieved from an open source platform [22]. The thermal cycler was programmed as follows: after the initial denaturation step (98 °C for 3 min), 35 cycles of denaturation (98 °C for 30 s) and annealing/extension (65 °C).
In the third step, the PCR products were tagged using 4 µL of Enrichment BLT HT beads for library preparation, followed by adapter ligation with 10 µL of the Illumina IDT® PCR indexes sets 1, 2, 3, and 4. The labeled PCR products were amplified by another PCR amplification: initialization (72 °C for 3 min), initial denaturation (98 °C for 3 min), seven cycles of amplification (denaturation at 98 °C for 20 s, hydridization at 60 °C for 30 s, and extension at 72 °C for 1 min), and the final extension (72 °C for 3 min). Each 96-well PCR plate included a positive control (COVIDSeq HT, CPC HT) and a negative control. An individual library was derived from 96 PCR-amplified products in a 96-well PCR plate; 5 µL of each PCR-amplified product were pooled and mixed in a 1.5-mL microfuge tube to constitute an individual library. Individual libraries were quantified using the Qubit 2.0 fluorometer (Invitrogen, Villebon-sur-Yvette, France) and pooled in equimolar concentration as recommended by Illumina. The pooled PCR products were diluted (1:10) in the resuspension buffer, and the concentration was adjusted to 4 nM.
In the fourth and last step, the normalized library pools (25 µL of each pooled samples adjusted to the concentration of 4 nM; volume of the final library, 2.25 µL) were diluted to a final concentration of 0.5 nM, as recommended by Illumina, denatured, neutralized (mixture of 4 µL of 0.2 N NaOH and 5 µL of 400 mM of Tris-HCl), and mixed with 63 µL of ExAmp mix for sequencing using Nova Seq system (Illumina Inc.).
2.5. Data Analysis
Dragen Bcl Convert v3.9.3 and FreeBayes v1.3.5 pipelines were used to process base and variant callings, respectively [23,24]. The bwa-mem2 tool v2.2.1 was used for mapping, based on the genome of Wuhan-Hu-1 isolate (GenBank accession no. NC_045512.2) [25,26]. The resulting sequence was cleaned using SAMtools program v1.13 [27,28]. Consensus genomes were built with the BCFtools program v1.13 [29].
All nucleotide and deduced amino acid sequences were aligned against the Wuhan-Hu-1 isolate genome as reference, and sequence modifications were detected using the Nextclade tool [30,31,32]. Phylogenetic clades and lineages were assigned using the Nextclade web application [30,31,32] and Pangolin tool [33,34], respectively. Phylogenetic trees were obtained using Nextstrain [30].
Qualitative data were grouped and analyzed using Fisher’s exact test. MedCalc version 22.021, an open source software [35], was used to calculate the p-values. A two-tailed p-value < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Clinical Isolates
In this study, 103 nasopharyngeal swab samples tested positive by quantitative RT-PCR in the virology laboratory of the INRSP were transferred to the Institut Hospitalo-Universitaire—Méditerranée Infection (IHU-MI; Marseille, France) for whole genome sequencing. Of these samples, 81 (78.6%) were confirmed to be positive by quantitative RT-PCR. Of these 81 confirmed SARS-CoV-2 samples, 54 (66.7%) with Ct values ranging from 12 and 30 were successfully sequenced.
3.2. Patient Characteristics
The great majority of the patients whose viral samples were successfully sequenced (n = 54) were men (n = 46; 85.2%); eight were women (14.8%). Their mean (±standard deviation) and median ages were 36.8 (±13.6) and 40 years, respectively. Among 54 SARS-CoV-2 isolates that were sequenced, 21 (38.9%) were sampled from unvaccinated individuals, and 33 (61.1%) were from vaccinated individuals. The vaccinated individuals included 17 (51.5%) patients who received the Sinopharm COVID-19 vaccine (Beijing Bio-Institute of Biological Products), 14 (42.4%) who were vaccinated with the first dose of Vaxzevria vaccine (Oxford/AstraZeneca, Cambridge, UK), and 2 (6.1%) who were vaccinated with the Janssen COVID-19 vaccine (Janssen Pharmaceutical Co./Johnson & Johnson, Titusville, NJ, USA). The INRSP patient records indicated that 26 of 54 (48%) sequenced samples were from hospitalized patients, while the others (n = 28; 52%) were from outpatients with no history of travel and classified as community transmission. Minimal clinical data were available in the INRSP patient records. The patients’ original hospital records and outpatient registry records were not available for consultation.
3.3. SARS-CoV-2 Genome Sequence Analysis and Variants
SARS-CoV-2 clinical isolates were sequenced using the Illumina protocol. High-resolution genotyping results were obtained. All samples had a genome sequence coverage between 78.8% and 99.3%, with a mean of 96.8% and a mean depth of 1767.7 x (range, 1049 to 2610) (Table S1: Genomic features of Mauritanian clinical isolates of SARS-CoV-2). The genomic sequences were submitted to GenBank (accession numbers OR353179–OR353230).
Of 54 SARS-CoV-2 isolates, 36 (66.9%) were variants of concern (VOC), including 33 (61.2%) Delta variant, 1 (1.9%) Alpha variant, 1 (1.9%) 20A variant, and 1 (1.9%) 20B variant (B.1.1.528) (Table 1). In addition, 15 (27.8%) were variants of interest (VOI) (11 [20.4%] Eta and 4 [7.4%] 20B), while 3 (5.5%) were variants under monitoring (VUM) (20A). Overall, there were 10 different lineages belonging to 6 different clades with AY.34 (or B.1.617.2.34) being predominant (31/54; 57.4%), followed by B.1.525 (11/54; 20.4%), B.1.620 (3/54; 5.5%), and B.1.1.318 (3/54; 5.5%). For six additional variants, only a single isolate was found in the present study. Clade 19A, which was predominant during the first COVID-19 wave, was not detected among the sequenced SARS-CoV-2 isolates. The Delta (21J) variant was identified in 7 of 8 (87.5%) women. It was also predominant in males (26/46; 56.5%). Data analysis did not show any statistically significant association (p > 0.05) between the virus variants or clades and the vaccination status of the patients (Table 2).

Table 1.
Genotypes identified among SARS-CoV-2 clinical isolates during the COVID-19 epidemic in Mauritania (September–November 2021).

Table 2.
Relationship between virus variants or clades and the vaccination status of the Mauritanian patients.
3.4. Characteristic Mutations in the Spike Protein of Delta and Eta Variants
The NGS allowed the analysis of mutations and their deduced amino acid substitutions of all SARS-CoV-2 variants (Table S1: Genomic features of Mauritanian clinical isolates of SARS-CoV-2). As the most notable mutations that are suspected to affect transmissibility and pathogenicity of SARS-CoV-2 variants are those found in the spike glycoprotein (S protein), only variations observed in the deduced amino acid sequence of the S protein of the two predominant Delta and Eta variants in our samples were analyzed. The results of this analysis are summarized in Table 3.

Table 3.
Notable mutations in the spike protein of both Delta and Eta variants among SARS-CoV-2 clinical isolates during the COVID-19 epidemic in Mauritania (September-November 2021).
Comparison with the Wuhan-Hu-1 reference strain genome showed that 29 of 33 Delta variant isolates harbored the characteristic S protein mutations T19R, L452R, T478K, D614G, P681R, and D950N (Table 2). One isolate (MAURCOVID-024) did not have the amino acid substitutions T19R and T478K, and three isolates (MAURCOVID-015, MAURCOVID-047, and MAURCOVID-018) did not have the mutation at position 452. Regarding the Eta variant, all defining single nucleotide polymorphisms (E484K, F888L, Q52R, and Q677H) in the S protein of this variant were observed among the Mauritanian SARS-CoV-2 isolates, except for the isolate MAURCOVID-016 in which both E484K and F888L amino acid substitutions were not present. It is worth noting that all Delta and Eta variant isolates harbored the D614G amino acid substitution in their respective S protein.
3.5. Phylogeny of SARS-CoV-2 Isolates
To better understand the genetic relationship among 54 Mauritanian SARS-CoV-2 clinical isolates, the phylogenetic tree of these clinical isolates was constructed based on date (Figure 1A,B) and mutation (Figure 2). The 54 sequences obtained in the present study were grouped into six distinct clades indicating the genetic divergence among SARS-CoV-2 isolates and therefore, possible distinct introduction of the virus in the country.
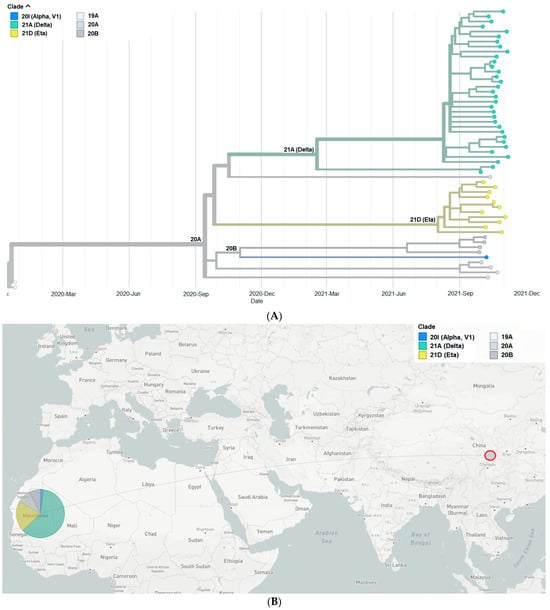
Figure 1.
(A) Phylogenetic tree constructed based on the dates of emergence of different SARS-CoV-2 variants. (B) Map showing China, where the first SARS-CoV-2 case was detected, and Mauritania, the study site. The pie chart indicates the proportion of SARS-CoV-2 clades among the Mauritanian samples.
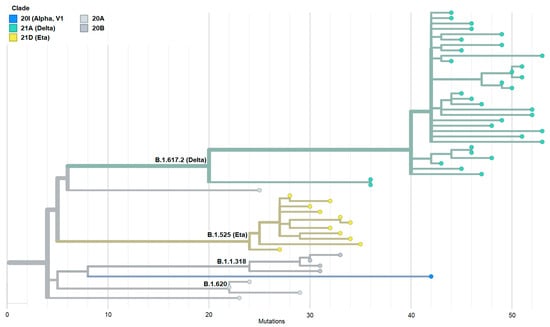
Figure 2.
Phylogenetic tree constructed based on the emergence of SARS-CoV-2 variants with different mutations. This figure shows that it is the Delta variant that is in the majority with the highest number of mutations.
4. Discussion
SARS-CoV-2, the etiologic agent of the COVID-19 pandemic, is the most devastating coronavirus of the 21st century to date. Within three years, this respiratory illness caused millions of deaths and enormous economic loss worldwide. The SARS-CoV-2 detection strategy in Mauritania has initially been based on serological tests (i.e., rapid antigen tests). But, very rapidly, as in most countries in the world, a PCR-based approach was introduced as gold standard to accurately detect viral RNA [14]. However, following the emergence of SARS-CoV-2 variants of concern associated with severe clinical signs and symptoms, whole genome sequencing using NGS technique has become the method of choice to timely detect the emergence and spread of new variants with novel mutations and understand the transmission dynamics of the virus worldwide, including in Mauritania [16,17,41]. To document COVID-19 dynamics in Mauritania, a set of SARS-CoV-2 clinical isolates collected from Mauritanian COVID-19-positive patients were analyzed for their genomic profile during the period from September to November 2021, corresponding to the third wave of the COVID-19 epidemic in most West African countries [42]. Starting from 103 quantitative RT-PCR positive nasopharyngeal samples, diagnosed at the INRSP, which was the reference center during the pandemic for SARS-CoV-2 testing in Mauritania, the positivity of 83 of 103 samples was confirmed at the IHU–Méditerranée Infection in Marseille, France. Of 83 samples, 54 with a Ct value ≤ 30 were successfully sequenced. The decrease in the number of positive samples when re-tested at IHU–Méditerranée Infection may have resulted from the degradation of viral RNA during storage and transportation.
Among the Mauritanian SARS-CoV-2 isolates characterized in the present study, the Delta variant (lineage B.1.617.2) predominated, accounting for 61.2% of sequenced genomes (Figure 1A). Similar findings were reported by Abdelmalick et al. in SARS-CoV-2 strains isolated from 13 PCR-positive symptomatic patients sampled five months before our sampling (i.e., 3 March 2021 to 31 May 2021) [17]. However, comparison with data from other West African countries shows that the predominant lineage in the third epidemic wave in West Africa was sub-lineage B.1.617.2.36 (45% of all sequences) and that lineage B.1.617.2 only represented 6% of the collected samples [42].
The Delta variant was first detected in India on 5 October 2020. By 22 November 2021, it had spread to over 179 countries [43]. The Delta variant is among five VOCs designated by the WHO. This variant marked the third wave of the COVID-19 pandemic by its remarkable efficacy in transmission and its enhanced capacity for immune escape [44]. Several characteristic mutations are suspected to allow the Delta variant to be one of the most transmissible variants, of which the most notable gene mutations are those found in the S proteins. The mutations in S protein gene found in the present study in the Mauritanian Delta variant isolates were T19R, L452R, T478K, D614G, P681R, and D950N [45]. The viral S protein is necessary for attachment to the surface of the host cells to enable entry into the cells. The S protein is also the protein that is targeted by the host immune system for eradication of the virus [45].
The second most abundant variant among the sequenced SARS-CoV-2 isolates was the Eta variant and its lineage B.1.525, found in 16.6% of the isolates. This variant was first detected in Nigeria (West Africa). During the same period, this lineage was reported in Mali in 30% of 162 SARS-CoV-2 isolates [46]. It is worth noting that during the third wave of COVID-19 in Mali, the Eta variant was predominant from March to May 2021; then the relative proportion of Delta variant increased from June 2021 to October 2021, which corresponds to our study period. In Senegal, the Eta variant was also present but at a lower proportion (5.26%) [47]. The presence of the Eta variant in Mauritania at such a relatively high proportion is not surprising regarding the geographical proximity with Mali and Senegal and the porosity of the borders despite the measures of travel restriction applied during the pandemic. Spike mutations that are commonly reported in Eta variant include Q52R, E484K, D614G, Q677H, and F888L, of which E484K and F888L in the S2 domain of the S protein are characteristics of the variant [48]. All these mutations were found in the Mauritanian isolates. Some of these mutations have been reported to help the virus to resist the neutralizing antibodies response (E484K) or alter virus transmissibility (Q677H) in the earlier SARS-CoV-2 strains [40].
Two other variants, namely 20A (lineage B.1.620) and 20B (lineage B.1.1.318), were also detected among the sequenced SARS-CoV-2 isolates in this study, each with a frequency of 11.1%. The lineage B.1.629 originated from central Africa, most likely from Cameroon [49], while the lineage B.1.1.318 has multiple origins as it was reported from Europe and parts of Africa [50].
Furthermore, our phylogenetic analysis perfectly illustrates the three distinct groups defined by the WHO, namely the VOC (Delta), the VOI (Eta), and the VUM (20B). The Delta variant, which appeared slightly more predominant than the others, had more mutations in the majority of isolates (61.1%). This Delta variant has been shown to be highly transmissible and has spread worldwide among fully vaccinated and unvaccinated individuals [51]. Indeed, among the five COVID-19 epidemic waves that Mauritania has experienced, the third one, which was mainly due to the Delta variant, was the deadliest wave with a total of 517 deaths, representing 52% of the total number of deaths (i.e., 997) attributable to COVID-19 in Mauritania during the pandemic.
An interesting finding of this study was that 61.1% of SARS-CoV-2 isolates were retrieved from individuals who had received different vaccines (i.e., 25.9% after receiving the first dose of AstraZeneca, 31.5% ‘protected’ with the Sinopharm vaccine, and 3.7% after vaccination with the Johnson & Johnson vaccine). This finding suggests that vaccination against COVID-19 could not fully prevent SARS-CoV-2 infection. However, due to insufficient clinical and hospitalization data on patients included in the present study, it cannot be deduced from our results whether vaccinated patients had milder infections than in unvaccinated patients. Nonetheless, a recent meta-analysis that assessed the efficacy of various COVID-19 vaccines in preventing SARS-CoV-2 infections of different severities showed that the efficacy of COVID-19 vaccines is higher to prevent severe infection and death than to prevent a milder infection and that vaccine efficacy wanes over time but can be enhanced by a booster [52].
As elsewhere in the world, Mauritania had to face important challenges during the COVID-19 pandemic. One of them was the capacity to accurately diagnose SARS-CoV-2 infections and rapidly characterize the newly emerging variants. Therefore, implementation of the NGS techniques during the COVID-19 epidemic has been of great interest in detecting and assessing the protective level of a particular vaccine [53] and, most importantly, in guiding the countries in making rapid and informed public health decisions.
Our study has several limitations. SARS-CoV-2 isolates were collected over only three months. It is possible that, during the study period, other variants were present but not sampled. Therefore, we are aware that the present finding may document only part of the SARS-CoV-2 genetic diversity during the study period. Moreover, our study was focused on, and limited essentially to, the analysis of SARS-CoV-2 genome. The patients’ full hospital records were not available for consultation, precluding us from analyzing our sequence data in the light of clinical presentation, co-morbidity, treatment, outcome, and short- and long-term complications. In addition, the total number of samples sequenced and analyzed in the present study was relatively small. The limited number of sequences did not allow for a more robust statistical analysis of a possible relationship between the subsets of different anti-COVID-19 vaccines and different clades and variants of SARS-CoV-2 found in Mauritania. Nonetheless, the present study is one of the few studies that have characterized the whole genomes of Mauritanian SARS-CoV-2 isolates by NGS. Further monitoring and analysis of a higher number of viral samples will be required as the current pandemic seems to have taken hold in the world and may even have stabilized [3].
5. Conclusions
Several variants of SARS-CoV-2 were present in Mauritania between September and November 2021. As Mauritania, like most West African countries, is resource limited to undertake viral genome sequencing, especially by NGS, an establishment of a mutualized sub-regional sequencing platform will be necessary to ensure continuous monitoring of SARS-CoV-2 variants as an essential step to track the potential reduction in COVID-19 vaccine efficacy, increased viral transmissibility, and disease severity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes15030361/s1, Table S1: Genomic features of Mauritanian clinical isolates of SARS-CoV-2: mutations, amino acid substitutions and deletions, coverage, clade, and Pangolin lineage.
Author Contributions
Conceptualization, J.D., N.P.M., M.B., S.M.A., A.E.B., M.A.B. and P.-E.F.; methodology, J.D., N.P.M., M.B. and S.M.A.; validation, M.A.B. and N.P.M.; formal analysis, N.P.M. and M.B.; investigation, J.D.; data curation, J.D., A.E.B., N.P.M. and M.B.; writing—original draft preparation, J.D. and A.O.M.S.B.; writing—review and editing, L.B., N.P.M., A.O.M.S.B. and P.-E.F.; supervision, A.O.M.S.B. and P.-E.F.; project administration, A.O.M.S.B. and P.-E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. J.D. received a post-doctoral fellowship grant from the French government through the Service de Coopération et d’Action Culturelle (SCAC), French Embassy in Nouakchott, Mauritania.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and reviewed and approved by the Ethics Committee of the University of Nouakchott (protocol reference no. 2020-010).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Only nasopharyngeal swab samples from patients who gave their written informed consent were used in this study.
Data Availability Statement
Genomic sequence data of the Mauritanian isolates of SARS-CoV-2 characterized and presented in this study are available in GenBank (accession numbers OR353179–OR353230).
Acknowledgments
The authors thank the technical and administrative personnel of the INRSP (Nouakchott, Mauritania) and IHU–Méditerranée Infection (Marseille, France) for facilitating the realization of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AA, amino acid; COVID-19, coronavirus disease 2019; CPP, COVIDSeq primer pool; Ct, cycle threshold; hACE2, human angiotension-converting enzyme 2; HLA, human leukocyte antigen; IHU-MI, Institut Hospitalo-Universitaire–Méditerranée Infection; INRSP, Institut National de Recherche en Santé Publique (National Institute of Public Health Research); NGS, next-generation sequencing; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RBD, receptor-binding domain; RT-PCR, reverse transcription-polymerase chain reaction; SD, standard deviation; S glycoprotein, spike glycoprotein; VOC, variants of concern; VOI, variants of interest; VUM, variants under monitoring; WHO, World Health Organization.
References
- Zhu, H.; Wei, L.; Niu, P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Policy 2020, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 3 March 2024).
- WHO. COVID-19 Epidemiological Update: 22 December 2023 (Edition 162). Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---22-december-2023 (accessed on 25 January 2024).
- WHO. Over Two-Thirds of Africans Exposed to Virus Which Causes COVID-19: WHO Study. WHO Regional Office for Africa, 2022. Available online: https://www.afro.who.int/news/over-two-thirds-africans-exposed-virus-which-causes-covid-19-who-study (accessed on 3 March 2024).
- Lalaoui, R.; Bakour, S.; Raoult, D.; Verger, P.; Sokhna, C.; Devaux, C.; Pradines, B.; Rolain, J.M. What could explain the late emergence of COVID-19 in Africa? New Microbes New Infect. 2020, 38, 100760. [Google Scholar] [CrossRef]
- China National Center for Bioinformation. RCoV19—Variation Annotation. 2024. Available online: https://ngdc.cncb.ac.cn/ncov/variation/annotation (accessed on 11 February 2024).
- Sgorlon, G.; Roca, T.P.; Passos-Silva, A.M.; Custódio, M.G.F.; Queiroz, J.A.D.S.; da Silva, A.L.F.; Teixeira, K.S.; Batista, F.S.; Salcedo, J.M.V.; Rampazzo, R.C.P.; et al. SARS-CoV-2 spike protein mutations in different variants: A comparison between vaccinated and unvaccinated population in western Amazonia. Bioinform. Biol. Insights 2023, 17, 11779322231186477. [Google Scholar] [CrossRef]
- Guruprasad, K. Mutations in human SARS-CoV-2 spike proteins, potential drug binding and epitope sites for COVID-19 therapeutics development. Curr. Res. Struct. Biol. 2022, 4, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- DeGrace, M.M.; Ghedin, E.; Frieman, M.B.; Krammer, F.; Grifoni, A.; Alisoltani, A.; Alter, G.; Amara, R.R.; Baric, R.S.; Barouch, D.H.; et al. Defining the risk of SARS-CoV-2 variants on immune protection. Nature 2022, 605, 640–652. [Google Scholar] [CrossRef]
- Zhao, S.; Lou, J.; Cao, L.; Chong, K.C.; Zee, B.C.Y.; Chan, P.K.S.; Wang, M.H. Differences in the case fatality risks associated with SARS-CoV-2 Delta and non-Delta variants in relation to vaccine coverage: An early ecological study in the United Kingdom. Infect. Genet. Evol. 2022, 97, 105162. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.; Poduri, R. Omicron, a new SARS-CoV-2 variant: Assessing the impact on severity and vaccines efficacy. Hum. Vaccin. Immunother. 2022, 18, 2034458. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Zhang, S.; Huang, N.; Zhao, T.; Lu, Q.B.; Cui, F. Differences in incidence and fatality of COVID-19 by SARS-CoV-2 Omicron variant versus Delta variant in relation to vaccine coverage: A world-wide review. J. Med. Virol. 2023, 95, e28118. [Google Scholar] [CrossRef]
- El Vally, A.; Bollahi, M.A.; Ould Ahmedou Salem, M.S.; Deida, J.; Parola, P.; Basco, L.; El Bara, A.; Ouldabdallahi, M.; Ould Mohamed Salem Boukhary, A. Retrospective overview of a COVID-19 outbreak in Mauritania. New Microbes New Infect. 2020, 38, 100788. [Google Scholar] [CrossRef]
- Ahmed, M.L.C.B.; Zehaf, S.; El Alem, M.M.; Elbara, A.; Ely Mahmoud, M.M.; Mohamed Abdellahi, M.V.; Heukelbach, J. COVID-19 outbreak in Mauritania: Epidemiology and health system response. J. Infect. Dev. Ctries. 2021, 15, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Laghdaf, S.M.; El Baraa, A.; Mohamed Beydjeu, A. Characterization of a cluster of COVID-19 cases linked to the Omicron variant, in Mauritania. Tunis. Med. 2022, 100, 217–221. [Google Scholar]
- Abdelmalick, A.; Sehli, S.; Idrissi Azami, A.; Habib, N.; Al Idrissi, N.; Belyamani, L.; Houmeida, A.; Ghazal, H. Genomic evidence of multiple introductions of SARS-CoV-2 in Mauritania. Bioinform. Biol. Insights. 2023, 17, 11779322231167927. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mauritania: The Current COVID-19 Situation. Available online: https://www.who.int/countries/mrt/ (accessed on 25 January 2024).
- Accoe, K.; Criel, B.; Ag Ahmed, M.A.; Buitrago, V.T.; Marchal, B. Conditions for health system resilience in the response to the COVID-19 pandemic in Mauritania. BMJ Glob. Health 2023, 8, e013943. [Google Scholar] [CrossRef]
- Agence Nationale de la Statistique et de l’Analyse Démographique et Economique. 2022. Available online: https://ansade.mr/fr/ (accessed on 3 March 2024).
- Papa Mze, N.; Kacel, I.; Beye, M.; Tola, R.; Sarr, M.; Basco, L.; Bogreau, H.; Colson, P.; Fournier, P.E. High throughput SARS-CoV-2 genome sequencing from 384 respiratory samples using the Illumina COVIDSeq protocol. Genes 2023, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- ARTIC Network. Real-Time Molecular Epidemiology for Outbreak Response. Available online: https://artic.network (accessed on 11 February 2024).
- Garrison, E.; Marth, G. Freebayes, a Haplotype-Based Variant Detector: User Manual and Guide. MIT License, 2010. Available online: https://github.com/freebayes/freebayes (accessed on 29 January 2024).
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:1207.3907v2. [Google Scholar] [CrossRef]
- Li, H. BWA-MEM2 (Sequence Alignment Using Burrows-Wheeler Transform), v2.2.1. MIT License, 2019. Available online: https://github.com/bwa-mem2/bwa-mem2 (accessed on 12 September 2022).
- Vasimuddin, M.; Misra, S.; Li, H.; Aluru, S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 20–24 May 2019; pp. 314–324. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Genome Research Ltd. Samtools. Available online: https://github.com/samtools/samtools (accessed on 12 September 2022).
- Li, H.; Handsaker, B.; Danecek, P.; Samtools Team. BCFtools(1) Manual Page. Available online: https://samtools.github.io/bcftools/bcftools.html (accessed on 12 September 2022).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef]
- Bedford, T.; Neher, R. Nextclade Web 2.12.0, Nextclade CLI 2.12.0. Copyright 2020–2024. Available online: https://clades.nextstrain.org (accessed on 12 September 2022).
- Aksamentov, I.; Roemer, C.; Hodcroft, E.B.; Neher, R.A. Nextclade: Clade assignment, mutation calling and quality control for viral genomes. J. Open Source Softw. 2021, 6, 3773. [Google Scholar] [CrossRef]
- Centre for Genomic Pathogen Surveillance. Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN). Available online: https://cov-lineages.org/pangolin.html (accessed on 12 September 2022).
- Rambaut, A.; Holmes, E.C.; O’Toole, A.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef]
- MedCalc Software Ltd. Fisher Exact Probability Calculator, Version 22.021. Available online: https://www.medcalc.org/calc/fisher.php (accessed on 4 March 2024).
- WHO. Updated Working Definitions and Primary Actions for SARS-CoV-2 Variants, 4 October 2023; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/m/item/updated-working-definitions-and-primary-actions-for--sars-cov-2-variants (accessed on 29 January 2024).
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Annavajhala, M.K.; Mohri, H.; Wang, P.; Nair, M.; Zucker, J.E.; Sheng, Z.; Gomez-Simmonds, A.; Kelley, A.L.; Tagliavia, M.; Huang, Y.; et al. Emergence and expansion of SARS-CoV-2 B.1.526 after identification in New York. Nature 2021, 597, 703–708. [Google Scholar] [CrossRef] [PubMed]
- United Kingdom Health Security Agency (UKHSA). SARS-CoV-2 Variant Surveillance and Assessment: Technical Briefing 55. UK Government, 2023. Available online: https://www.gov.uk/gouvernment/publications/investigations-of-sars-cov-2-variants-technical-briefings/sars-cov-2-variant-surveillance-and-assessment-technical-briefing-55 (accessed on 18 February 2024).
- Carpenter, R.E.; Tamrakar, V.K.; Almas, S.; Sharma, A.; Sharma, R. SARS-CoV-2 Next Generation Sequencing (NGS) data from clinical isolates from the East Texas Region of the United States. Data Brief. 2023, 49, 109312. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, A.J.S.; Beye, M.; Sow, A.; Lo, G.; Padane, A.; Sokhna, C.; Kane, C.T.; Colson, P.; Fenollar, F.; Mboup, S.; et al. COVID-19 in 16 West African countries: An assessment of the epidemiology and genetic diversity of SARS-CoV-2 after four epidemic waves. Am. J. Trop. Med. Hyg. 2023, 109, 861–873. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The global epidemic of the SARS-CoV-2 Delta variant, key spike mutations and immune escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef]
- Li, M.; Lou, F.; Fan, H. SARS-CoV-2 Variants of Concern Delta: A great challenge to prevention and control of COVID-19. Signal Transduct. Target. Ther. 2021, 6, 349. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta variant B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. [Google Scholar] [CrossRef]
- Koné, A.; Diallo, D.; Kané, F.; Diarra, B.; Coulibaly, T.A.; Sameroff, S.C.; Diarra, H.B.; Diakité, M.T.; Camara, F.; Maiga, O.; et al. Dynamics of SARS-CoV-2 variants characterized during different COVID-19 waves in Mali. IJID Reg. 2023, 6, 24–28. [Google Scholar] [CrossRef]
- Padane, A.; Mbow, M.; Mboup, A.; Diedhiou, C.K.; Gueye, K.; Lo, C.I.; Ndiour, S.; Leye, N.; Ndoye, A.S.; Selbé Ndiaye, A.J.; et al. Rapidly rising cases with Omicron in Senegal. New Microbes New Infect. 2022, 45, 100959. [Google Scholar] [CrossRef]
- Yadav, P.D.; Nyayanit, D.A.; Gupta, N.; Shastri, J.; Sahay, R.R.; Patil, D.Y.; Shete, A.M.; Razdan, A.; Agrawal, S.; Kumar, A.; et al. Detection and isolation of SARS-CoV-2 Eta variant from the international travelers and local residents of India. J. Med. Virol. 2022, 94, 3404–3409. [Google Scholar] [CrossRef]
- Park, A.K.; Kim, I.H.; Man Kim, H.; Lee, H.; Lee, N.J.; Kim, J.A.; Woo, S.; Lee, C.Y.; Lee, J.; Oh, S.J.; et al. SARS-CoV-2 B.1.619 and B.1.620 lineages, South Korea, 2021. Emerg. Infect. Dis. 2022, 28, 415–419. [Google Scholar] [CrossRef]
- Manouana, G.P.; Nzamba Maloum, M.; Bikangui, R.; Oye Bingono, S.O.; Ondo Nguema, G.; Honkpehedji, J.Y.; Rossatanga, E.G.; Zoa-Assoumou, S.; Pallerla, S.R.; Rachakonda, S.; et al. Emergence of B.1.1.318 SARS-CoV-2 viral lineage and high incidence of alpha B.1.1.7 variant of concern in the Republic of Gabon. Int. J. Infect. Dis. 2022, 114, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 22, 183–195, Erratum in Lancet Infect. Dis. 2021, 21, e363. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.R.; Jiang, Y.W.; Li, F.X.; Liu, D.; Lin, T.F.; Zhao, Z.Y.; Wei, C.; Jin, Q.Y.; Li, X.M.; Jia, Y.X.; et al. Efficacy of SARS-CoV-2 vaccines and the dose-response relationship with three major antibodies: A systematic review and meta-analysis of randomised controlled trials. Lancet Microbe. 2023, 4, e236–e246. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ye, Q. The variants of SARS-CoV-2 and the challenges of vaccines. J. Med. Virol. 2022, 94, 1366–1372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).